Chemical Principles for Allied Health - Delta...
Transcript of Chemical Principles for Allied Health - Delta...

Chemical Principles for Allied Health
Mark RobertsonProfessor of Biology
Delta College
First StepsQuick Roll Call – I will add any extra folks in our next meeting (if you complete the first pre-assignment)!
Read Through My Online Resources (in D-2-L or online) at: http://websites.delta.edu/mgrobert/
Print and Complete Pre-Assignment #1
Check out C-112 (main campus) or Main Desk (Ricker) for a study room!
Find a partner (or 2) and form a study group (FaceBook groups work great for this!)
Matter and EnergyMatter
has weight (or mass w/out gravity)takes up space (so we measure volume)exists in 3 states (solid, liquid, gas)
Density weight per volume (measure as g/cm3 or g/ml, so water = 1g/cm3)
Energy kinetic (energy of motion)potential (stored energy; in our food, fat pads, stretched rubber band, etc.)
First Law of Thermodynamics energy is neither created nor destroyed

Some Basic DefinitionsAtom (made of 3 subatomic particles)
p+ (positive protons)e- (negative electrons)n (neutral neutrons)
Elements (from periodic table)building blocks used for chemical compounds (ex: C for carbon)
Molecules2 or more atoms joined (ex: O2, NH3, CO2)
Compoundscombination of at least 2 elements in constant proportions (ex: H2O or C6H12O6)
Mixturescombination of at least 2 substances (ex: air)
Periodic TableAtomic Number
gives number of protonsalso equals electrons as every element is neutral
Elemental Namehonor goes to discoverer
Symbol on Chartoften based on the original Latin nameearly discoveries have single letter
Atomic Weight (AW) versus Mass Number (MN)AW listed as amu’s (atomic mass units); 1amu equals 1/12 the weight of a carbon atomMN is basically the weight of nucleus (protons plus neutrons), so: weight-protons=neutrons
8Oxygen
O16 amu

_ protons_ neutrons
__4px__4py__4pz__4s
__3px__3py__3pz__3s
__2px__2py__2pz__2s
__1s
Nucleus fill in number of protons and neutrons first
Electron Shellsdraw shell profile
Placing Electrons space out electrons one per orbital at firstnext, place shared electron in the orbital if more are still needed in the shellfill in lower shells first
Blank spots left determine number of bonds the element will make
Diagramming an Atom
Reactants form Products ex: 2(H2) + O2 ---> 2H2OReaction type is based on interaction of the compounds used and their specific propertiesDehydration synthesis or Condensation reaction (makes water when small molecules linked to make large one)
2(C6H12O6) ---> C12H22O11 + H2O (in sugar beets) glucose sucrose
Hydrolysis or Decomposition Reaction (uses water to break up large molecule into smaller ones)
C12H22O11 + H2O ---> 2(C6H12O6) (liver releasing sugars) sucrose glucose
Chemical Reactions in Cells
Recall that elements are electrically neutral and thus have protons equal electronsIn ions, the number of electrons is altered, thus changing the charge:
Cations are positive ions that lose electrons; ex: Na+Anions are negative ions that gain electrons; ex: Cl-
In isotopes, the protons and electrons stay balanced, but the nucleus either gains or loses neutrons. The most stable isotope is listed on the periodic table as atomic weight
Heavy Isotopes -> gain neutrons (ex: C-14)Light Isotopes -> lose neutrons (ex: U-235)
Ions and Isotopes

Hydrogen BondsVery weak attraction between water molecules (or other polar molecules) with outer hydrogen atomsActs like a magnetic force between molecules, but easy to disrupt the bondUsed to form shapes in DNA, 3-D proteins, surface tension
Ionic BondsFormed when a stronger anion steals electrons from a weaker cation (to form salts)More stable than hydrogen bonds, but dissolves in water (so great for forming electrolytes in humans)
Examples include NaCl, CaCl2, NaF, MgBr2, etc. Covalent Bonds
Occurs when electrons are shared between atomsStrongest and most stable form of a bondStability leads to use for building cell components like C6H12O6, proteins, lipids, etc.
Bonding Between Atoms
Salts dissolve in water to form cations and anionsIf water dissociates, forms H+ (hydrogen) and OH- (hydroxyl) ions
Basesmolecules that absorb hydrogen ions
Acidsmolecules that release hydrogen ions
Buffersmolecules that absorb both acids and bases to hold pH constantexamples include blood & breastmilk
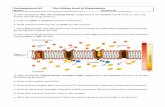
Electrolytes and pH Scale
Possible Journal Article Research
Topics?
Acid/Base Imbalances in Blood?
Electrolyte Imbalances in Athletes?
Metabolic (Energy) Imbalances in Patients?
Disorders due to Mineral Deficiencies?