A Critical Review of LDL Cholesterol and HDL - Sun Diagnostics
UPDATE ON THE PCKS9 INHIBITION TO LOWER LDL CHOLESTEROL
-
Upload
praveen-nagula -
Category
Education
-
view
497 -
download
0
Transcript of UPDATE ON THE PCKS9 INHIBITION TO LOWER LDL CHOLESTEROL
Background
For patients who are eligible for statin therapy but are considered
to be statin intolerant,inhibition of proprotein convertase
subtilism/kexin type9 (PCKS9) has been proposed as an
alternative mechanism of action for lowering LDL cholesterol.
3
Brown MS, et al. Proc Natl Acad Sci U S A. 1979;76:3330-3337.Steinberg D, et al. Proc Natl Acad Sci U S A. 2009;106:9546-9547.Goldstein JL, et al. Arterioscler Thromb Vasc Biol. 2009;29:431-438.
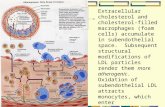
LDL receptor
AMG 145, a fully human monoclonal antibody that binds PCSK9,
was well tolerated and lowered LDL in phase Ia and Ib studies (Dias CS, JACC published online Oct 17, 2012. http://dx.doi.org/10.1016/j.jacc.2012.08.986)
Qian YW, et al. J Lipid Res. 2007;48:1488-1498.Horton JD, et al. J Lipid Res. 2009;50:S172-S177.Zhang DW, et al. J Biol Chem. 2007;282:18602-18612.
PCSK9 Regulates the Surface Expression of LDLRs by Targeting LDLRs for Lysosomal Degradation
“Proprotein Convertase Subtilisin/Kexin type 9.”
5The PCSK9 protein appears to control the number of low-density lipoprotein receptors
• Evolocumab,a monoclonal antibody that inhibits PCKS9 and is
administered subcutaneously,was recently tested in several
manufacturer – funded phase 3 trials..
• The bottom line findings of them are….
6
DESCARTES trial
Durable Effect of PCSK9 antibody CompARed wiTh placEbo Study
(NCT01516879)
About 900 pts with hyperlipidemia
Started on 4-12 weeks of background lipid lowering therapy
according to risk categories from the Adult Treatment Panel III of
the National Cholesterol Education Program.
Patients with an LDL cholesterol ≥75 mg/dl, assigned randomly in a
2:1 ratio to receive evolocumab(420 mg) or placebo every weeks.
7
Background lipid-lowering therapy
• diet alone
• diet plus atorvastatin,10 mg daily,
• Atorvastatin ,80 mg daily,
• Atorvastatin ,80 mg daily plus ezetimibe, 10 mg daily,
Time period of 4 to 12 weeks.
8
Results
At 52 weeks, mean LDL reduction was 57% lower with
Evolocumab than with placebo, with significant advantages (49-
62%) across the various background therapy subgroups.
Also significant reduction of Apo B, non–HDL, lp(a) and TGs.
The most common adverse events were nasopharyngitis, upper
respiratory tract infection, influenza, and back pain.
9
CONCLUSIONS
At 52 weeks, evolocumab added to diet alone, to low-dose
atorvastatin, or to high-dose atorvastatin with or without
ezetimibe significantly reduced LDL cholesterol levels in
patients with a range of cardiovascular risks.
10
GAUSS -2 trial
• GAUSS-2 (Goal Achievement after Utilizing an Anti-PCSK9
Antibody in Statin Intolerant Subjects)
• Evolocumab vs Ezetimibe in 307 statin intolerant patients.
• Evolocumab reduced LDL cholesterol by 53 -56% (compared
to Ezetimibe it was 37-39%advantage),the advantage was
dependent on the dosing intensity and schedule.
11
MENDEL -2 trial
• 614 Statin naive patients.
• Evolocumab reduced LDL levels by 55% to 57% compared
with placebo ( a 38% to 40% advantage over ezetimibe)
12
LAPLACE TIMI 57 trial
• Achievement of LDL C target goals in high risk groups.
• In 282 subjects,at high risk according to NCEP-ATP III criteria, the
proportion of subjects achieving the recommended LDL-C goal
of <70 mg/dl across treatment arms were compared.
• Other outcomes included the triple goals of LDL-C <70 mg/dl, non–
high-density lipoprotein cholesterol (HDL-C) <100 mg/dl, and
apolipoprotein B (ApoB) <80 mg/dl.
16
Objectives
To compare 12 weeks of AMG 145 (given SC Q2 or Q4
weeks) vs placebo in stable patients with
hypercholesterolemia on a statin ± ezetimibe:
– Primary: % change in LDL-C*
– Secondary: changes in other lipoproteins
pharmacokinetics/pharmacodynamics
tolerability and safety
* measured using ultracentrifugation in a central core laboratory
Study Design
Kohli P, et al. Clin Cardiol. 2012;35:385-391.
78 centers5 countries
934 screened 631 random. 629 treated( *2 subjects assigned placebo Q4W received no study drug)
Screening and Placebo Run-in
Period
Subcutaneous injection of 6 mL
placebo
Fasting LDL-C 5-10 days before
randomization
Maximum 6 weeks
Day 1Visits: Week 2 Week 8 Week 12Week 6Week 4 Week 10 Week 14
70 mg AMG 145 SC Q2W 79 Subjects
105 mg AMG 145 SC Q2W 79 Subjects
140 mg AMG 145 SC Q2W 78 Subjects
Placebo SC Q2W 78 Subjects
Q2W:
280 mg AMG 145 SC Q4W 79 Subjects
350 mg AMG 145 SC Q4W 79 Subjects
420 mg AMG 145 SC Q4W 80 Subjects
Placebo SC Q4W 77 Subjects
Q4W:
Primary EndpointAssessed
• Results During the dosing interval, more than 90% of subjects in
both of the top dose groups every 2 weeks and every 4 weeks attained
this lipid target over the dosing interval, with similar success rates for
the triple lipid goal.
Conclusions PCSK9 inhibition with AMG 145 enables high-risk
patients to achieve established lipid goals. If this therapy demonstrates
efficacy for reducing cardiovascular events with a favorable safety
profile in ongoing phase 3 trials, we believe it will have major public
health implications.
19
The LAPLACE-2 Study
20
LDL-C Assessment with PCSK9 MonoclonaL Antibody Inhibition
Combined With Statin ThErapy – 2 (NCT01763866)
Design:
A 12-week, randomized, double-blind, placebo- and ezetimibe-
controlled, phase III study
Objective:
To evaluate the efficacy and safety of evolocumab administered
biweekly (140 mg) or monthly (420 mg) in combination with a statin
in hypercholesterolemic patients
The RUTHERFORD-2 Study
22
RedUcTion of LDL-C with PCSK9 Inhibition in HEteRozygous
Familial HyperchOlesteRolemia Disorder (NCT20110117)
Design:
A 12-week, randomized, double-blind, placebo-controlled,
multicenter phase 3 study
Objective:
To evaluate the efficacy and safety of evolocumab (AMG 145)
140 mg Q2W and 420 mg QM administered subcutaneously in a
large cohort of HeFH patients unable to achieve an LDL-C < 100
mg/dL despite statin therapy with or without ezetimibe
RUTHERFORD-2: Conclusions
• Evolocumab was well tolerated, with no notable difference
in the AE profile compared with placebo.
– The rate of nasopharyngitis and muscle-related adverse
events (AEs) was higher in the evolocumab group.
• The imbalance in the overall set of muscle-related AEs was not due to
significant imbalances in any individual muscle-related event (i.e., creatine
kinase).
• Evolocumab may offer a new and effective treatment option
to further reduce LDL-C in HeFH patients.
23
FOURIER phase 3
• Further Cardiovascular Outcomes Research With
PCSK9 Inhibition in Subjects With Elevated Risk
(FOURIER)
• 22,310 pts higher risk group
• Above statins
• Any benefit in ACS
• First received: January 8, 2013
• Last updated: May 7, 2014
• Last verified: May 2014
24
Comment
• Evolocumab significantly lowered LDL cholesterol and had a
generally favorable side effect profile in several different patient
populations with varying background therapies.
• Still cannot be recommended as primary therapy,as these LDL
reductions whether assosciated with improvements in cardiovascular
outcomes is not proven(need long term studies).
25
TAKE HOME MESSAGE
• Hypercholesterolemia management beyond statins has taken a
considerable development….results are satisfactory..
• The results applicability to the long term benefits needs further
studies.
• Statin intolerant patients have better options for treatment in the
near future.
• Evolocumab is near recognition for FDA approval once the
results of FOURIER trial are released.
27
DES OUTCOMES :
SORT OUT III TRIAL
The Winner of the Sprint does not necessarily Win the Marathon
Lancet 2014,March 14(e pub ahead of print)28
Background
• Limitations of early DES such as late stent thrombosis and evidence
of delayed neointimal healing have prompted the development of
DESs with new platform materials, thinner stent status, more
biocompatible or more biodegradable surface polymer coatings, and
less potent antiproliferative drugs.
29
The winner of sprint…..
• In SORT OUT III trial ,risks for both definite stent thrombosis and
target vessel revascularisation (TVR) were significantly higher with
the second generation zotarolimus eluting stent (ZES) than with the
first generation sirolimus eluting stent (SES) during the first year
after implantation.
30
5 yr clinical outcomes …
• 99.7 % of the 2232 patients who were randomly assigned to
receive ZESs or SES s were followed up clinically (Danish
national registry).
SES s ZES s P value
MACE AT 5 YEARS 15.6% 17.0% 0.40
Outcomes between 1 and 5 years
12.3% 9.9% 0.07
Definite Stent thrombosis
1.8% 0.1%
TVR 7.0% 4.1%
31
Not won the marathon…
• The superiority of the SES to the ZES at 1 yr follow up was lost at
5 years because of higher rates of late stent thrombosis and TVR
with the first generation stent.
32