Pseudomonas Bacteriophage Phi6 as a Model for Virus Emergence
Transcript of Pseudomonas Bacteriophage Phi6 as a Model for Virus Emergence

City University of New York (CUNY) City University of New York (CUNY)
CUNY Academic Works CUNY Academic Works
Dissertations, Theses, and Capstone Projects CUNY Graduate Center
9-2015
Pseudomonas Bacteriophage Phi6 as a Model for Virus Pseudomonas Bacteriophage Phi6 as a Model for Virus
Emergence Emergence
Brian Elwood Ford Graduate Center, City University of New York
How does access to this work benefit you? Let us know!
More information about this work at: https://academicworks.cuny.edu/gc_etds/924
Discover additional works at: https://academicworks.cuny.edu
This work is made publicly available by the City University of New York (CUNY). Contact: [email protected]

PSEUDOMONAS BACTERIOPHAGE Φ6 AS A MODEL FOR VIRUS EMERGENCE by
BRIAN E. FORD
Academic dissertation submitted to the Graduate Faculty in Biology in partial fulfillment of the requirements for the degree of Doctor of Philosophy, The City University of New York
2015

ii
© 2015 BRIAN E. FORD
All Rights Reserved.

iii
This manuscript has been read and accepted for the Graduate Faculty in Biology in satisfaction of the dissertation
requirement for the degree of Doctor of Philosophy.
Date Chair of Examining Committee Dr. John J. Dennehy
Date Executive Officer
Dr. Laurel Eckhardt
Dr. John J. Dennehy, Queens College
Dr. Stéphane Boissinot, NYU Abu Dhabi
Dr. Nathalia Glickman Holtzman, Queens College
Dr. Paul Gottlieb, City College of New York
Dr. José A. Fernández-‐Romero, Population Council Supervisory Committee
THE CITY UNIVERSITY OF NEW YORK

iv
Abstract
Bacteriophage Φ6 as a Model for Virus Emergence
by
Brian E. Ford
Mentor: John J. Dennehy
The Pseudomonas bacteriophage Φ6 has a long and well-‐established history as a model
organism. Here we describe a set of experiments to extend this model system in to concepts
previously unclaimed. Chapter 1 presents a brief background of the ecology of viruses that
infect microorganisms. Chapter 2 examines genetic mutations allowing for host range
expansion. Chapter 3 presents a novel paired strain assay to study how a non genetic host
acquired factor affects fitness of these enveloped viruses on subsequent hosts. Chapter 4 is an
extension of this system to include how the bacteria host is affected in virus-‐host coevolution.

v
ABSTRACT ............................................................................................................................. IV
LIST OF TABLES ...................................................................................................................... XI
LIST OF FIGURES ................................................................................................................... XII
INTRODUCTION ................................................................................................................... XIV
CHAPTER 1: EVOLUTIONARY ECOLOGY OF THE VIRUSES OF MICROORGANISMS ..................... 1
ABSTRACT ...................................................................................................................................... 2
INTRODUCTION ............................................................................................................................... 3
VOM ABUNDANCE, BIODIVERSITY, AND BIOGEOGRAPHY ......................................................................... 3
POPULATION DYNAMICS OF VOMS ..................................................................................................... 6
Factors affecting rates of VoM population growth. ............................................................. 7
Predator-‐prey dynamics. ..................................................................................................... 11
VOM IMPACT ON HOST POPULATIONS AND COMMUNITIES. .................................................................... 12
VoM-‐host coevolution and its limitations. .......................................................................... 12
VoMs as drivers of host biodiversity. .................................................................................. 15
VOM ECOLOGICAL ADAPTATION ...................................................................................................... 16
Generation time as an optimizable trait. ............................................................................ 16
Coinfection, competition and cheating. .............................................................................. 18
Productivity versus latency. ................................................................................................. 19
VOM GENOME EVOLUTION ............................................................................................................. 21
VoM genomes. ..................................................................................................................... 22
Horizontal gene transfer and genome mosaicism. ............................................................. 23

vi
VOM IMPACT ON HOST GENETICS .................................................................................................... 24
VOMS AS ECOSYSTEM MODIFIERS .................................................................................................... 26
CLOSING REMARKS ........................................................................................................................ 28
ACKNOWLEDGEMENTS .................................................................................................................... 28
FIGURE LEGENDS ........................................................................................................................... 29
REFERENCES ................................................................................................................................. 34
CHAPTER 2: FREQUENCY AND FITNESS CONSEQUENCES OF BACTERIOPHAGE Φ6 HOST RANGE
MUTATIONS .......................................................................................................................... 49
ABSTRACT .................................................................................................................................... 50
INTRODUCTION ............................................................................................................................. 51
METHODS AND MATERIALS ............................................................................................................. 53
Study Organisms and Culture Conditions ............................................................................. 53
Virus Stock Preparation ........................................................................................................ 54
Host Range Mutant Frequency ............................................................................................. 54
Host Range Mutant Isolation ............................................................................................... 55
RNA Extraction and Sequencing ........................................................................................... 55
Mutant Characterization ...................................................................................................... 56
Plaque Size Estimates ........................................................................................................... 56
Mutant Fitness on Native and Novel Hosts .......................................................................... 57
Attachment Rate Assays ....................................................................................................... 57
RESULTS ...................................................................................................................................... 58
Mutation Frequency ............................................................................................................. 58

vii
Mutation Substitution Frequency ......................................................................................... 59
Phenotypic Change Analysis ................................................................................................. 60
P3 3D Structure Prediction ................................................................................................... 61
Plaque Size ............................................................................................................................ 62
Mutant Fitness on Original and Novel Hosts ........................................................................ 63
Attachment Rate .................................................................................................................. 64
Data Availability ................................................................................................................... 65
DISCUSSION ................................................................................................................................. 65
Φ6 Host Range Mutation Frequency .................................................................................... 65
Mutation Hotspots ............................................................................................................... 69
Structural Speculations ......................................................................................................... 70
Fitness on Native and Novel Hosts ....................................................................................... 71
Attachment to Native and Novel Hosts ................................................................................ 72
Implications for Disease Emergence ..................................................................................... 73
ACKNOWLEDGEMENTS .................................................................................................................... 74
FIGURE LEGENDS ........................................................................................................................... 75
FIGURES ...................................................................................................................................... 77
REFERENCES ................................................................................................................................. 84
CHAPTER 3: HOST-‐ACQUIRED FACTOR IMPACTS FITNESS ON SUBSEQUENT HOSTS IN AN RNA
VIRUS .................................................................................................................................... 88
ABSTRACT .................................................................................................................................... 89
INTRODUCTION ............................................................................................................................. 90

viii
MATERIALS AND METHODS ............................................................................................................. 91
Bacterial Host Strain Determination and Selection .............................................................. 91
Paired Bacteriophage Strain Preparation ............................................................................. 92
Fitness Assay Conditions ....................................................................................................... 93
Statistical Analysis ................................................................................................................ 94
RESULTS ...................................................................................................................................... 94
Φ6 ......................................................................................................................................... 94
Φ9 ......................................................................................................................................... 95
Φ13 ....................................................................................................................................... 95
Φ14 ....................................................................................................................................... 95
DISCUSSION ................................................................................................................................. 97
AUTHOR CONTRIBUTIONS ............................................................................................................. 101
CONFLICT OF INTEREST STATEMENT ................................................................................................. 101
ACKNOWLEDGEMENTS .................................................................................................................. 101
CAPTIONS .................................................................................................................................. 102
SUPPLEMENTAL FIGURES ............................................................................................................... 107
REFERENCES ............................................................................................................................... 112
CHAPTER 4: BENEFITS OF STICKING TOGETHER: FITNESS REDUCED IN VIRUS-‐CHALLENGED
PSEUDOMONAS SPECIES FOLLOWING SHIFT TO PLANKTONIC PHENOTYPE .......................... 114
ABSTRACT .................................................................................................................................. 115
INTRODUCTION ........................................................................................................................... 116
MATERIALS AND METHODS ........................................................................................................... 117

ix
Bacteria Culture Preparation .............................................................................................. 117
Selection for planktonic (non clumping) strain ................................................................... 117
Isolation and Verification of Non Clumping Phenotype ...................................................... 117
Heat Shock Buoyancy Test .................................................................................................. 118
Determination of Cell Number in Clumps ........................................................................... 118
Colony Size Determination ................................................................................................. 119
TEM Analysis of Cell Morphology ....................................................................................... 119
Bacteriophage Strain Preparation ...................................................................................... 120
Growth Curve Optical Density to Cell Number Correlation ................................................. 120
Flask Fitness Analysis .......................................................................................................... 121
High-‐Throughput Microplate Fitness Analysis .................................................................... 122
Statistics ............................................................................................................................. 122
DNA Preparation for Next Generation Sequencing ............................................................ 123
RESULTS .................................................................................................................................... 123
1.1 Growth .......................................................................................................................... 123
1.2 Morphology .................................................................................................................. 125
1.3 Virus Susceptibility ........................................................................................................ 126
1.4 Comparisons ................................................................................................................. 127
1.5 Genotyping ................................................................................................................... 129
DISCUSSION ............................................................................................................................... 129
AUTHOR CONTRIBUTIONS ............................................................................................................. 131
ACKNOWLEDGEMENTS .................................................................................................................. 132

x
CAPTIONS .................................................................................................................................. 132
FIGURES .................................................................................................................................... 134
SUPPLEMENTARY TABLES AND FIGURES ............................................................................................ 139
REFERENCES ............................................................................................................................... 140
CONCLUSION ....................................................................................................................... 143
(INTRODUCTION) REFERENCES ............................................................................................ 145

xi
List of Tables
Chapter 2
TABLE 1. PHAGE Φ6 MUTATIONS ALLOWING INFECTION OF NOVEL HOST ERA ............................................. 80
TABLE 2. HOST RANGE SUBSTITUTION HOTSPOTS IN Φ6 P3 PROTEIN .......................................................... 81
TABLE 3. AMINO ACID SUBSTITUTIONS ASSOCIATED WITH Φ6 HOST RANGE EXPANSION ON ERA ...................... 82
TABLE 4 . PHENOTYPIC CHARACTERISTICS OF BACTERIOPHAGE Φ MUTANTS. ................................................. 83
Chapter 3
TABLE 1. BACTERIAL STRAINS ............................................................................................................... 92
TABLE 2. RELATIVE VIRUS PRODUCTIVITY ON MISMATCHED HOSTS ............................................................. 96
SUPPLEMENTARY TABLE ST1. CYSTOVIRIDAE HOST RANGE ON SELECT HOST STRAINS ................................... 108
SUPPLEMENTARY TABLE ST2. ONE-‐WAY ANOVA TABLES FOR THE EFFECT OF VIRUS LIPID MEMBRANE COMPOSITION
ON FITNESS AT 15 HOURS AS MEASURED BY AREA UNDER CURVE (AUC), TIME TO INFECTION (X-‐MAX) AND
MAXIMUM BACTERIAL GROWTH (Y-‐MAX). FITNESS IS MEASURED FOR EACH PAIRED STRAIN ON BOTH BACTERIAL
HOSTS. .................................................................................................................................. 109
SUPPLEMENTARY TABLE ST3. ANOVA POST-‐HOC TUKEY’S MULTIPLE COMPARISON TEST ............................. 110
Chapter 4
TABLE 1. HEAT SHOCK GROWTH VERIFICATION ..................................................................................... 124
SUPPLEMENTARY TABLE 1. MICROPLATE FITNESS (BACTERIA VS BACTERIA + PHAGE) UNPAIRED T-‐TEST SUMMARY
............................................................................................................................................ 139

xii
List of Figures
Introduction
FIGURE 1. PSEUDOMONAS BACTERIOPHAGE Φ6. ..................................................................................... XIV
FIGURE 2: SCHEMATIC OF THE PSEUDOMONAS PHAGE Φ6 LIFE CYCLE. ......................................................... XV
Chapter 1
FIG. 1 POPULATION GROWTH OVER TIME. .............................................................................................. 31
FIG. 2 VIRUSES OF MICROORGANISMS SIGNIFICANTLY IMPACT THE CYCLING OF ORGANIC MATTER AND NUTRIENTS IN
THE OCEAN. .............................................................................................................................. 32
FIG. 3 HORIZONTAL GENE TRANSFER ACROSS DOMAINS. .......................................................................... 33
Chapter 2
FIG. 1 – SPATIAL MODELS OF Φ6 P3 PROTEIN MUTANTS ............................................................................ 77
FIG. 2 – MUTANT ABSOLUTE FITNESS ON CANONICAL AND NOVEL HOSTS ....................................................... 78
FIG. 3 – MEAN ERA ATTACHMENT RATE (K) IS PLOTTED AGAINST PHAGE Φ6 FITNESS ON ERA .......................... 79
Chapter 3
FIGURE 1. EXPERIMENTAL DESIGN ...................................................................................................... 104
FIGURE 2. PAIRED FITNESS ASSAYS ..................................................................................................... 105
FIGURE 3. HOST LEGACY EFFECT INFECTION DYNAMICS ........................................................................... 106
SUPPLEMENTARY FIGURE 1. PAIRED FITNESS ASSAY SUMMARY 6H (~TWO INFECTION CYCLES) ...................... 107
Chapter 4
FIGURE 1. PLANKTONIC SELECTION SCHEME ......................................................................................... 134
FIGURE 2. PHENOTYPE, CELLULAR AND COLONY MORPHOLOGIES .............................................................. 135

xiii
FIGURE 3. BACTERIAL GROWTH WITH SURFACTANT ................................................................................ 136
FIGURE 4. COLONY FORMING UNIT AREA COMPARISON .......................................................................... 137
FIGURE 5. SUSCEPTIBILITY TO BACTERIOPHAGE INFECTION ....................................................................... 138
SUPPLEMENTARY FIGURE 1. GROWTH CURVE AND PLATING NUMBER ........................................................ 139
Conclusion
FIGURE 1. PSEUDOMONAS PSEUDOALCALIGENES EAST RIVER ISOLATE A (ERA). ........................................... 143

xiv
Introduction
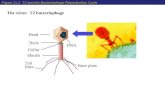
Isolated in 1973 [1], the Pseudomonas bacteriophage Φ6 has an extremely established and well
characterized history as a model system. The Cystoviridae family reference strain Φ6 (Figure 1)
has many properties making it an ideal candidate to carry out the following studies.
Figure 1. Pseudomonas bacteriophage Φ6. Electron micrographs of (A) Φ6 (magnification = 30K x) and (B) Φ6 attached to pilus of PP (magnification = 40K x). Samples were negatively stained with a 1% UA solution.
Φ6 has a segmented dsRNA linear tripartite genome with its segments designated small (2.9kb),
medium (4.0kb) and large (6.4kb). [2,3] Studies have demonstrated a lack of recombination
between the segments. [4,5] The Cystoviridae family has nine (Φ6, Φ7, Φ8, Φ9, Φ10, Φ11,
Φ12, Φ13 and Φ14*) canonical members and one member (Φ2954) awaiting classification.
Family members Φ7, Φ9, Φ10 and Φ11 are classified as close relatives while Φ8, Φ12, Φ13 and
Φ14 are considered distant relatives to Φ6 based on the latter group’s adsorption and infection
via the lipopolysaccharide (LPS) rather than the pilus[2,6] (Figure 2). Previous studies have
* Φ14 appears as a member of the Cystoviridae family in the 2011 9th report of the International Committee on Taxonomy of Viruses, but not in the online NCBI database.
A B
cba Brian E. Ford, The Dennehy Lab

xv
characterized Φ8 as the most distantly related relative of Φ6 in the Cystovirus family[7,8] and
recent evidence suggests that segmented RNA genomes are packaged into capsids more
efficiently than are nonsegmented versions. [4,9]
Figure 2: Schematic of the Pseudomonas phage Φ6 life cycle. (A) Adsorption. (B) Envelope fusion. (C) Peptidoglycan digestion. (D–E) Endocytotic uptake of nucleocapsid. (F) Early transcription. (G) Polymerase complex assembly. (H) ssRNA packaging and replication. (I) Late transcription. (J) Nucleocapsid shell assembly. (K) Translocation of the viral envelope and assembly of spikes. (L) Host cell lysis and release of mature virions. From Virus Taxonomy: Ninth Report of the International Committee on Taxonomy of Viruses (Elsevier: 2011). Reprinted with permission.
While the Pseudomonas bacteriophage Φ6 is most closely related to the Reoviridae (family in
which the medically relevant rotaviruses fall), it is also related to both the hepatitis C virus
(HCV) and the flaviviruses. [6,10] Well known flaviviruses include West Nile virus (WNV),
Dengue virus (DENV) and Yellow fever virus. A similarly segmented virus, the influenza virus, is
carefully monitored for reassortment as that results in antigenic shift and opens the potential

xvi
for pandemic outbreaks. Understanding of the niche breadth of a pathogen can help to
accurately predict its potential for host emergence. [9,11]
Here, we extend this study system to include: 1) genetic mutations in the P3 attachment
protein permitting host range expansion to a novel host, supported by a predicted 3D model
(Chapter 2); 2) a novel paired strain assay examining a non genetic host factor’s impact on
subsequent infections (Chapter 3); and 3) characterization of a lab-‐evolved host strain
phenotype with improved growth characteristics but increased susceptibility to bacteriophage
infection.

1
Chapter 1: Evolutionary Ecology of the Viruses of Microorganisms
Brian E. Ford1,2, Marko Baloh1 and John J. Dennehy1,2,3
1Department of Biology, Queens College, Queens, NY, 11367
2The Graduate Center of the City University of New York, New York City, NY, 10016
3Corresponding Author: [email protected]
Running title: Virus Evolutionary Ecology
Accepted for publication January 2014 in Viruses of Microorganisms: Diversity, Molecular
Biology and Applications. Hyman P & Abedon ST (eds.). Poole, UK: Caister Academic Press.

2
Abstract
With estimated numbers greater than 1031, viruses are the most abundant organisms on
the planet, and are found in all habitats: aquatic, atmospheric and terrestrial. No cellular
organisms – whether animal, plant or microbe – are free from viral parasitism. Consequently,
the effects and influences of viruses are pervasive, directly or indirectly impacting all organisms,
populations, communities and ecosystems. Here we consider the evolutionary ecology of the
viruses of microorganisms (hereafter VoMs) which, due to the abundance of their hosts,
outnumber all other types of viruses. Subfields of evolutionary ecology include life history
evolution, population biology, biogeography, and community ecology. Like blind men describing
an elephant, each approach only describes a facet of VoM evolutionary ecology. Here we
describe some of the approaches used to describe VoM evolutionary ecology in hopes that a
synthesis will allow some perception of the whole.

3
Introduction
Whether or not one describes viruses as organisms, living entities, or instead simply as
infectious agents, viruses nonetheless both evolve (evolutionary biology) and interact with
other organisms (ecology). In this chapter we consider the evolutionary biology and ecology of
the viruses of microorganisms or VoMs, which include bacteriophages (the viruses of bacteria),
archaeal viruses, viruses of protists, and various mycoviruses (viruses of fungi). In addition, we
emphasize the “hybrid” discipline of evolutionary ecology, which covers the subdisciplines of
life history evolution, population biology, biogeography, and community ecology. To our
knowledge, this is the first publication to review the evolutionary ecology of VoMs as a group,
though previous reviews consider these subjects as they apply to bacteriophages or archaeal
viruses (Brussow and Kutter 2005; Abedon, 2008; Abedon 2009; Prangishvili 2013). For further
discussion especially of VoM ecology, see the chapters (this volume) by Weinbauer on aquatic
VoMs and Williamson on soil VoMs.
VoM Abundance, Biodiversity, and Biogeography
The viruses of microorganisms may be the most numerous organisms on Earth (Bergh et
al., 1989; Suttle, 2005; Angly et al., 2006). In aquatic and soil habitats, viral concentrations
higher than 108 per milliliter or gram have been reported (Fuhrman, 1999; Wommack and
Colwell, 2000; Ashelford et al., 2003; Suttle, 2005; Williamson et al., 2005) [see also Williamson,
this volume]. Even the Earth's atmosphere has been shown to harbor relatively high
concentrations of viruses (107 -‐ 108 m-‐3) (Whon et al., 2012).

4
Given that VoMs exist in almost unimaginable numbers, we might expect a
correspondingly high level of diversity. Studying VoM diversity, however, is complicated by the
fact that, unlike cellular organisms, VoMs do not have universal ribosomal DNA sequences that
facilitate the identification of discrete species and determine the phylogenetic relationships
between these species (Rowher and Edwards, 2002). Thus, sampling VoM biodiversity was
approached in a piecemeal fashion (i.e., laboratory culturing or direct observation) until the
advent of direct environmental genomic sampling (i.e., metagenomics [see Vega Thurber, this
volume]). Metagenomic estimates of VoM diversity entail the isolation and high-‐throughput
sequencing of all viral nucleic acids in an environmental isolate (e.g., water, soil, tissue, feces).
To ensure that only viral genetic material is sequenced, environmental isolates are ultra-‐filtered
and nucleases are used to digest non-‐capsid protected prokaryotic and eukaryotic nucleic acids
(Breitbart et al., 2004a; Delwart, 2007; Thurber et al., 2009). Partial genetic fragments obtained
from random sequence reads are computationally aligned and assembled into contigs
(consensus sequences based on overlapping partial fragments). A contig spectrum is generated
for a virome by counting the number of sequences that fall into each contig (Allen et al., 2013).
Abundant VoM types are inferred by the presence of a large number of sequences mapping to
any particular contig.
By comparing the genomic sequences newly obtained from a community with those in
existing genetic sequence databases, VoM genetic richness and diversity can be estimated.
Previously unknown VoM sequences (i.e., sequences not matching those in existing genetic
databases) can be identified and compared among different communities (Edwards and
Rohwer, 2005; Suttle, 2007). The percentage of unknown VoM sequences in recent studies has

5
ranged from 60% to 97% (Angly et al., 2006; Desnues et al., 2008; Hurwitz and Sullivan, 2013).
As in population mark-‐recapture studies (Pradel, 1996), a high proportion of novel types
recovered from population resampling indicates that a large number of new types remain
undiscovered.
Some of the first metagenomic estimates of VoM diversity were conducted on marine
and human virus communities by Rohwer, Breitbart, and colleagues (Breitbart et al., 2003;
Breitbart et al., 2002). For the marine communities, mathematical models predicted the
existence of 374 to 7,114 viral types in the oceans off the California coast (Breitbart et al.,
2002). The human virome has been estimated to contain at least 1,250 distinct virus types,
most of which are VoMs (Breitbart et al., 2003). In retrospect, large as they may be, these
numbers may be significant underestimates (Allen et al., 2011; Angly et al., 2006; Culley et al.,
2006; Hurwitz and Sullivan, 2013; Kristensen et al., 2010; Mokili et al., 2012; Rosario and
Breitbart, 2011; Rosario et al., 2009). For one, these estimates do not include RNA virus
diversity, which is harder to analyze due to the difficulties in sequencing RNA. Distinct VoM
types in the biosphere may number in the millions (Allen et al., 2013).
Interestingly, analyses of VoM genetic material collected from geographically-‐distinct
environments (i.e., freshwater, marine, terrestrial) show that similar VoM genetic sequences
can be found in widely separated ecosystems, indicating that VoMs (or at least their genes) are
in constant motion through the biosphere (Breitbart et al., 2004a; Breitbart et al., 2004b;
Breitbart and Rohwer, 2005; Hambly and Suttle, 2005; Kunin et al., 2008; O'Keefe et al., 2010;
Sano et al., 2004; Short and Suttle, 2005). Nonetheless, host habitat requirements may play a
strong role in the distribution of VoMs. For example, an investigation of hot spring microbial

6
communities found that genetically distinct viral populations were associated with each local
geothermal region (Held and Whitaker, 2009). This ties into Baas Becking's idea that
"everything is everywhere, but, the environment selects" (Baas Becking, 1934). In this context,
the presence of hosts is the key aspect of the “environment” that determines whether a virus is
present in a specific habitat (Angly et al., 2006).
Habitat specialization and isolation can have far-‐reaching effects on VoM evolution
because the distribution of populations across space, and the connections between them,
govern population diversification. Since population genetic differentiation is usually a function
of gene flow between populations, connected populations will tend to follow more similar
evolutionary trajectories, whereas isolated populations will tend to diverge as they become
locally adapted (Kawecki and Ebert, 2004). We expect that VoM distributions will be highly
contingent on chance historical events and the specific details of their ecology. Overall, there
appears to be a tremendous diversity of VoMs, and they tend to be broadly, but unevenly,
distributed across the biosphere (Thurber, 2009).
Population Dynamics of VoMs
The number of VoMs present in a habitat ultimately depends on the conditions that
assist or impede VoM reproduction. Since most VoMs move between hosts as particles (except
fungal viruses that are transmitted intracellularly during cell division (Ghabrial and Suzuki
2009)), their macroscale movement depends on the physical properties of the medium and the
forces acting upon it (i.e., bulk flow of water/air). At the microscale, the laws of random
diffusion by Brownian motion govern VoM movement. Infection is contingent on chance

7
collisions between VoMs and hosts, the probabilities of which may be quite low on a virus-‐by-‐
virus basis. Abedon estimated that if a virus were the size of the Titanic, then a milliliter of fluid
would be analogous to the volume of the Earth (Abedon, 2011). If it were not for the enormous
population sizes of VoMs and their hosts, then infection events would be improbable.
There is a growing appreciation of how VoM morphology may affect mobility in
different environments, thus increasing the chances of encountering a host. For example, rod-‐
shaped viruses may diffuse faster than spherical viruses in tissues or gels (Lee et al., 2013). We
would expect also that very large virus particles such as those of protists that have been
discovered [see La Scola, this volume] would diffuse at slower rates than small, icosahedral
single-‐stranded bacteriophages [see Lehman, this volume]. In addition, Barr et al. (2013) have
documented the potential of bacteriophage particles to adhere reversibly to mucus. We expect
that further investigations of the relationship between the ecological milieu of VoM's and their
morphologies would be profitable.
Factors affecting rates of VoM population growth. Because of VoM random diffusion,
most models assume that VoM population dynamics follow mass action principles where host
infection rates are directly proportional to the concentrations of VoMs and their hosts. That is,
the greater the concentration of VoMs and hosts in a fluid, the greater the probability of
collisions leading to infection. Growth of a population of size N can be described by a
differential equation that accounts for births (b) and deaths (d) over time:
!"!#= 𝑏𝑏 − 𝑑𝑑 𝑁𝑁 (1)
In evolutionary biology, births minus deaths, b – d, over short intervals is termed the per capita
growth rate, r, which often is used as an estimate of absolute fitness, a.k.a., reproductive

8
capacity (Fisher, 1930). Population growth in this model shows a characteristic concave-‐up
curve, commonly termed exponential growth (Fig. 1A).
Each of the major VoM life history patterns—lytic, lysogenic (or equivalent), and chronic
[see Hyman and Abedon, this volume]—will exhibit exponential growth, but at varying rates.
Lytic VoMs, given sufficient environmental densities of host cells, will have growth rates greatly
exceeding hosts. By contrast, chronic and especially lysogenic VoMs (existing as prophages or
proviruses) will grow at rates more closely matching those of their hosts. The major distinction
between these modes of life is the degree of host exploitation effected by VoMs. Lytic viruses
are parasitic, while proviruses and chronic viruses have the potential to be mutualistic with
their hosts. For instance, it has long been known that bacteriophage lysogens can carry genes
that in many cases are likely to be beneficial to host fitness (Andersson and Banfield, 2008).
Examples include the CTXφ phage-‐encoded cholera toxin (Waldor and Mekalanos, 1996) and
the bor gene of phage λ, which provides serum resistance to Escherichia coli (Barondess and
Beckwith, 1990; 1995). In addition, prophages often confer immunity to coinfection to their
hosts, and may enable hosts to survive environmentally unfavorable periods (Brussow et al.,
2004; Paul, 2008).
The exponential growth model shown in Equation (1) is deterministic since the only
variables are initial population size and reproduction rate. In reality, VoM population dynamics
depend on many other factors, including stochastic influences. An additional complication not
addressed in Equation (1) is that VoM per capita growth rate can depend on the ratio of
adsorbed VoMs to susceptible hosts found within a system (the multiplicity of infection or
MOI). For many bacteriophage, growth rates are lower at high MOIs (i.e., negative density-‐

9
dependence) because infected bacteria cannot support additional phage infections while
bacteria that are not yet phage infected are not a rapidly renewed resource. Specifically, at
MOIs << 1, uninfected hosts can readily replenish themselves via replication, whereas at MOIs
>> 1 all hosts will become infected which, for lytic infections, results in a block on ability of a
system to replenish uninfected cells. Thus, the total number of hosts available for infection over
time can be greater when MOIs are low, but ultimately more host infections over the same
period means higher phage growth rates along with higher resulting MOIs.
Surprisingly, Pseudomonas bacteriophage ɸ6 shows positive density-‐dependence –
greater phage growth rates at higher MOIs under certain conditions (Dennehy et al., 2006).
While the mechanism for this growth pattern is not entirely clear, it seems to stem from an
enhancement of infection when multiple viruses attack the same cell (Joseph et al., 2009). Allee
first described positive density-‐dependence of population growth with reference to growth
limitations in small populations due to limited access to mates (Allee, 1931). Several theoretical
studies suggest that positive density-‐dependence may be an important factor in host-‐parasite
evolutionary ecology (Gerla et al., 2013; Regoes et al., 2000; Weitz and Dushoff, 2008).
However, while positive density-‐dependent growth has been experimentally observed in
several host-‐parasite systems, including bacteria-‐Daphnia (Ebert et al., 2000; Little and Ebert,
2000), microsporidian-‐mosquito (Agnew and Koella, 1999), prion-‐mouse (McLean and Bostock,
2000), ɸ6-‐Pseudomonas remains the only virus-‐host system where positive density-‐dependent
growth has been observed (Dennehy et al., 2006). Whether this is due to an intrinsic property
of this particular phage, or simply due to multiplicity reactivation or its genome being split into
different virion particles, is unknown.

10
Another complication is that host numbers are finite for reasons that are independent
of virus infection. Limited environmental resources ultimately circumscribe the number of hosts
that a habitat can support, which, more formally, is referred to as the carrying capacity. The
number of hosts in a habitat changes over time also due to factors such as host reproduction,
environmentally-‐induced host death (including VoM-‐induced host lysis), and acquisition of
resistance mechanisms to environmental antagonists, such as chemical and physical
antagonists but also predators and viruses, which can have the effect of reducing, for example,
host replication efficiency. As such, VoM population dynamics can be described by Verhulst-‐
Pearl logistic models, where the population size over time follows a sigmoid or S-‐shaped
function (Gause, 1934; Pearl, 1927; Pearl and Reed, 1920). This model can be formalized using
the differential equation:
!"!#= 𝑟𝑟𝑁𝑁(,-"
,) , (2)
where N is population size and K represents the maximal possible value of N (Fig. 1B).
Closer inspection of growth curves derived from this model reveals an inherent
asymmetry between host and VoM growth rates. This asymmetry results from the fact that
from hosts reproduce by binary fission while VoMs produce, e.g., 10-‐100 progeny per cycle of
reproduction over similar intervals. It is clear that only a few rounds of reproduction are
required for VoMs to outstrip their hosts. This observation supports reports of VoM
superabundance (~10:1) in marine (Chibani-‐Chennoufi et al., 2004) [see also Weinbauer, this
volume] and terrestrial (Ashelford et al., 2003) [see also Williamson, this volume] habitats.
Alternatively, the VoM growth advantage is dependent on host density, with a high VoM
advantage given high host densities (as a result of greater similarity between VoM and host

11
generation times) and a low or lower VoM advantage at low host densities (where VoM
generation times can be potentially much greater than those of hosts due to the substantial
extensions of the virion search stages of VoM life cycles). A major consequence of VoM
superabundance, when it occurs, can be extreme competition among VoMs for limited hosts,
exerting strong evolutionary pressure on VoMs to optimize infection mechanics and host
utilization.
Predator-‐prey dynamics. While interactions among VoMs play a role in shaping VoM life
history traits, the influence of interactions with their hosts are essential, both molecularly and
ecologically. In terms of ecology, a standard means of considering these virus-‐host interactions
is in terms of predator-‐prey dynamics. The first explicit models of predator-‐prey dynamics were
introduced independently by Lotka and by Volterra (Lotka, 1925; Volterra, 1926). To apply
Lotka and Volterra's model to VoMs, assume that in the absence of VoMs, hosts increase
exponentially as described in Eq. 1. By contrast, VoMs will decline in the absence of prey:
!/!#= −𝑟𝑟0𝑃𝑃 (3)
where P is the number of VoMs and r2 is the VoM instantaneous death rate. If VoMs and hosts
are introduced into a limited space, the host growth rate, r1, will be reduced by a factor
dependent on VoM density:
!"!#= 𝑟𝑟2 − 𝛼𝛼𝑃𝑃 𝑁𝑁 , (4)
where α is the adsorption rate and N is the number of hosts. The VoM population will increase
at a rate dependent on host density:
!/!#= 𝛼𝛼𝛼𝛼𝑁𝑁 − 𝑟𝑟0 𝑃𝑃 (5)

12
where β is the burst size or number of progeny produced per infection. Under these simplistic
conditions, VoM and host populations will oscillate in a systematic manner, showing a high
degree of stability (Fig. 1C ). Extensions of the basic Lotka-‐Volterra model have included
resource limitations on host density, the time lag due to VoM latent period and the acquisition
of host resistance (Bohannan and Lenski, 1997; Campbell, 1961; Kerr et al., 2008; Lenski, 1988;
Lenski and Levin, 1985; Levin et al., 1977; Stopar and Abedon, 2008).
VoM impact on host populations and communities.
Darwin suggested that “mutual relations” among organisms drives biological diversification
(Darwin, 1859). It is easy to imagine that the evolutionary arms race among VoMs and hosts can
splinter populations into myriad resistant and counter-‐resistant variants. Indeed, studies have
shown that diversification is a frequent occurrence in both static microcosms (Buckling and
Rainey, 2002; Buckling et al., 2006; Morgan et al., 2009) and chemostat communities (Chao et
al., 1977; Marston et al., 2012). For example, at least 4 cycles of adaptation-‐counteradaptation
between the cyanobacterium Synechococcus and the VoM RIM8 were observed during 6-‐month
chemostat experiments (Fig. 1D) (Marston et al., 2012). In these experiments, up to 13 newly-‐
evolved RIM8 and 11 Synechococcus variants appeared in coevolving populations. In this
section, we consider two associated phenomena stemming from these mutual relations. The
first is that of VoM-‐host coevolution—including limitations often seen on that coevolution,
particularly in experiments involving bacteriophages—and the second is the result that this
coevolution can have on host and indeed community diversity.
VoM-‐host coevolution and its limitations. The general findings of predator-‐prey models
are that 1) VoMs will limit host populations below the carrying capacity provided by available

13
resources, 2) host populations can fluctuate in a cyclic manner and 3) coexistence can occur
over long periods. Experimental support from coevolution studies for these theoretical
predictions is mixed. Frequently hosts have tended to stabilize at a density similar to that of
controls, and VoMs persisted at a relatively low density (see Dennehy, 2012, for review). In
other cases, VoMs were driven to extinction outright. The most likely interpretation of the
former results is that, although resistant hosts came to dominate the habitat, some VoMs were
able to persist due to the continued presence of a small number of sensitive hosts in a spatial or
numerical refuge (Chao et al., 1977; Schrag and Mittler, 1996). The main point here is that, due
to the rise of resistant hosts, demonstrations of repeated cycles of host-‐parasite coevolution in
experimental microcosms have been somewhat rare.
This lack of repeated cycles implies a lack of repeated VoM adaptation and host
counter-‐adaptation during VoM-‐host co-‐cultivations, or at least that such dynamics typically are
not dominating during VoM growth within microcosms. One reason for the relative lack of such
arms races between co-‐cultured VoMs and hosts may be due to a potential asymmetry in VoM
and host evolutionary potential. Because of their high mutation rates and large population
sizes, microbial host organisms are able to search the VoM-‐resistance sequence space relatively
rapidly. While VoMs can have even greater population sizes and rates of mutation, the larger
genomes of hosts versus VoMs may provide hosts with greater means to evolve VoM
resistance. By contrast, the relatively small VoM genomes may be less well equipped to
respond to challenges of host resistance. This is particularly acute when hosts can modify their
resistance to VoMs via mutational loss of information (e.g., gene inactivation). Due to their
limited genomic repertoire, VoMs often must mutationally change without substantially

14
impacting gene function, though VoMs may be more effective during VoM-‐host coevolution if
they target, for virion adsorption, host molecules that the host is unable to do without.
VoM genome sizes can be limited by the capacities of their capsids. Hosts, by contrast,
often can easily assimilate even relatively large blocks of new genetic material (e.g., as genomic
islands). Study of this asymmetry may be exacerbated by the long history of laboratory
culturing of the hosts and parasites typically used in experimental coevolution studies (e.g. E.
coli and its phages λ, T4, T7). These laboratory-‐adapted strains may lack evolutionary potential
for extensive coevolution because they have been selected under constrained conditions for
hundreds of generations. This idea is supported by observations of repeated rounds of
coevolution among hosts and parasites recently isolated from the wild (Barnet et al., 1981;
Buckling and Rainey, 2002; Marston et al., 2012).
How these laboratory studies relate to host-‐VoM evolutionary dynamics in the wild is
poorly understood. Wild populations are subject to much greater spatial, environmental, and
biotic heterogeneity, significantly impacting community structure. Moreover, VoM resistance
usually incurs fitness costs (Bohannan and Lenski, 2000; Buckling and Rainey, 2002; Chao et al.,
1977; Lenski, 1988; Levin et al., 1977; Schrag and Mittler, 1996). As a consequence, while
resistant hosts may predominate in the presence of VoMs, they will be outcompeted by VoM-‐
sensitive hosts in the absence of VoMs. This can ensure that any shifts in host susceptibility to
VoMs may be transient. As long as some VoMs can persist during the fallow period, including
non-‐locally, their local populations may be able to recover following a precipitous drop in
population size due to host resistance. VoM-‐host coevolution may be an important aspect of

15
VoM ecology where extrapolating from laboratory studies to dynamics in the wild may require
careful consideration.
VoMs as drivers of host biodiversity. A widely held notion in ecological theory is that
there is an inherent tradeoff between maximizing reproduction and minimizing predation
(Ludwig and Rowe, 1990; Pianka, 1976). This tension should promote the diversification of prey
populations into competitive and defensive specialists (Winter et al., 2010). Such frequency-‐
dependent selection among alternative variants is commonly referred to as “killing the winner”
(Thingstad, 2000; Weinbauer and Rassoulzadegan, 2004). When predators are absent,
competition specialists should dominate as they have a reproductive advantage over defensive
specialists. However, their burgeoning numbers will allow for the propagation of predators,
freeing up resources for survival-‐maximizing defensive specialists. Predators under these
conditions will consequently tend to decline in number, allowing the return of competition
specialists. These dynamics should encourage an increase in the microbial diversity of both prey
and predator species.
Given that chemostats are used to create minimally varying microhabitats, we might
expect coevolution in the wild to result in much greater diversification given the much higher
degree of environmental heterogeneity. This notion is supported by the geographic mosaic
theory of coevolution, which holds that populations respond to ever-‐changing patterns of
interactions across space and time (Thompson, 1994; Thompson, 2012). Here fragmentation of
the natural landscape allows local populations of one species to adapt to local populations of
other species in a genotype-‐by-‐genotype-‐by-‐environment interaction. As these interactions

16
play out across larger spatial and temporal scales, and as different subpopulations interact or
fail to interact, coevolutionary changes can result in speciation and increased biodiversity.
VoM Ecological Adaptation
Like all organisms, VoMs are a collection of features and traits shaped by natural
selection, but the story of evolution is not simply one of continual small improvements in each
trait. Rather, selective forces on organismal traits often can be in direct opposition.
Improvement in one trait, that is, can entail a reduction in performance of another. For
example, capsid thickness can be correlated with virus survival, but inversely correlated with
the virus multiplication rate (De Paepe and Taddei, 2006; García-‐Villada and Drake, 2013).
Natural selection, then, will favor a capsid thickness that represents the optimal tradeoff
between survival and reproduction such that the maximum number of surviving offspring are
produced over time for a given set of environmental circumstances. Since environments are
often changing, natural selection is continuously recalibrating the optimal balance between
specific traits.
Generation time as an optimizable trait. Nowhere is this issue of tradeoffs in the
optimization of different traits more apparent than in the fine-‐tuning of lysis timing. A general
assumption of VoM biology is that progeny are assembled at a linear rate (Wang et al., 1996).
Consequently, an early lysis time will result in fewer offspring produced than a later lysis time,
but the progeny of early lysers will have a head start in infecting new host cells. The optimal
lysis time therefore depends on the number and quality of hosts available for infection
(Abedon, 1989; Wang et al., 1996). If the number or quality of available hosts is low, then it may

17
be advantageous to delay lysis and produce more progeny. By contrast, high host densities will
favor shorter latent periods and faster lysis. Experimental work on bacteriophage lysis timing
has generally confirmed the predictions of optimality models (Abedon et al., 2003; Heineman
and Bull, 2007; Shao and Wang, 2008), but has revealed that the evolution of optimal
phenotypes is subject to genetic and physical constraints (Chantranupong and Heineman, 2012;
Heineman and Bull, 2007). Optimality theory provides a starting point to develop hypotheses
regarding the evolution of organismal traits, but consideration should be paid to the genetic
details and physiological constraints of the system under study.
A salient question is, when conditions change, how will VoMs adjust lysis timing, and by
extension, generation time, to optimize reproduction in the new environment? Natural
selection has several potential targets that could be manipulated to alter generation time: 1)
the adsorption rate could be adjusted, 2) the eclipse period (i.e., time from genome
penetration to production of first mature progeny) could be extended, or 3) the rise period (i.e.,
time from first mature progeny until lysis) could be altered (Chantranupong and Heineman,
2012). Time spent in the first period is a function of the attachment rate constant and host
concentration. Naively, one might expect this rate to be maximized. However, high attachments
rates may be deleterious because they may limit the number of hosts encountered per burst,
and therefore productivity per host (Gallet et al., 2011; Gallet et al., 2009). Furthermore,
mutations increasing attachment rates could have negative pleiotropic effects (Pepin et al.,
2006). Finally, high attachment rates could be disadvantageous when there are high levels of
stationary phase cells or cellular debris to which too effectively adsorbing viruses might non-‐
productively adsorb (Gallet et al. 2012). On the other hand, low attachment rates would

18
prolong search times and expose phages to increased risks of environmentally-‐induced
inactivation. These constraints suggest that attachment rate is probably not adjusted to
optimize lysis time.
During the eclipse period, the phage genome is being replicated, transcribed and
translated, and consequently the phage is in its most vulnerable state. Death of the host cell
would entail destruction of the phage thus we expect this period to be minimized across all
conditions. This leaves the rise period which we expect to be the most evolutionarily malleable.
Evidence from evolution experiments manipulating host density is consistent with this
conjecture. In these studies, mutations tend to occur in regions of genome associated with
genome replication and lysis, but not with attachment (Abedon et al., 2003; Heineman and Bull,
2007, but see Shao and Wang 2008).
Coinfection, competition and cheating. Describing the outcomes of competition
between organisms is an essential feature of evolutionary ecology. Similar to other organisms,
VoMs compete for the resources required to replicate additional copies of themselves. As with
any competitive interaction, natural selection favors VoMs that are more efficient at acquiring
and using host resources. Notably for VoMs, this competition occurs not only extracellularly as
VoMs compete for hosts, but also intracellularly as VoMs compete for host resources (Dennehy
et al., 2013; Preisig et al., 1998; Turner, 2005; Yau et al., 2011). In the case of coinfection or a
multiple/sequential infection (superinfection), or even reactivation of a latent infection, after a
VoM introduces its genome into a host cell, proteins translated from a VoM's genome enter a
common pool and are therefore susceptible to co-‐option by competing genotypes of VoMs
already present in the cell. That is, some virus types can package their genomes into capsids

19
containing proteins that they themselves did not cause to be produced. This parasitism enables
the "cheating" virus to specialize on other facets of their own reproduction, such as increasing
genome replication speed and packaging efficiency. An example of this type of competition
comes from coliphage P2 and its satellite P4 (Six and Klug, 1973). P4 lacks genes for head, tail
and lysis proteins, but is able to package its genome in the P2 capsid (Deho and Ghisotti, 2006).
As such, P4 is able to concentrate its resources on genome replication while parasitizing capsids
from P2.
Viruses or virus-‐like elements that require other "helper" viruses in order to reproduce
include satellite viruses (Deho and Ghisotti, 2006; Six and Klug, 1973), viroids, virusoids
(Symons, 1991), virophages (Fischer and Suttle, 2011; La Scola et al., 2008) [see Fischer, this
volume], and defective-‐interfering (DI) particles (Horiuchi, 1983; Huang, 1973; Roux et al.,
1991). As a rule, these types of parasitic viruses only arise when coinfection is common.
Consequently, there should be strong selection on the potentially parasitized virus to sequester
a host and limit coinfection. The outcome of intense ecological competition is reflected in the
numerous mechanisms of resistance to superinfection (Hyman and Abedon, 2010). For
example, some lambdoid phages bind outer membrane protein FhuA to infect E. coli (Uc-‐Mass
et al., 2004). Competing phages express a protein, Cor, that blocks FhuA-‐mediated ferrichrome
uptake, and prevents FhuA-‐mediated phage infection (Uc-‐Mass et al., 2004).
Productivity versus latency. Some VoMs can enter long-‐term associations with host
cells such as by integrating their genome into that of the host, which for bacteriophages has
been described as lysogeny but more generally can be described as a form of latent infections.
These VoMs, now existing as proviruses (and/or prophages in the case of bacteriophages) only

20
“replicate” when the host cell replicates. As a consequence, and if uninfected host cells are
otherwise fairly prevalent within an environment , then this life history strategy entails a
significant reduction in reproductive output compared to the lytic strategy (Abedon et al.,
2001). Since natural selection typically will maximize reproduction, lysogeny is likely the
product of a specific selective condition, namely where the probability of locating a new host is
relatively low (Abedon et al., 2001; Wang et al., 1996).
The best-‐studied lysis-‐lysogeny system is that of the bacteriophage λ [see Lehman, this
volume]. When growth conditions are favorable, an important regulator of λ gene expression,
CII, is rapidly degraded by host proteases, and the likelihood of the lytic pathway dominating is
high. By contrast, when growth conditions are poor, CII accumulates, increasing the probability
of entering the lysogenic state (Herskowitz and Hagen, 1980; Oppenheim et al., 2005 ).
Ecologically, this decision process is intuitive because the essential "life decision" a lysogen
must make is whether to accept a low rate of replication in exchange for shelter or instead to
potentially risk everything by infecting lytically for the potential payoff of greater replication in
new hosts.
There are at least two sources of information by which a lysogen can base this
"decision": host physiology and the presence of coinfecting viruses. A favorable host
physiological state may signal the likely presence of available hosts for progeny, whereas the
opposite condition may imply that suitable hosts are in short supply. Alternatively, the presence
of competing VoMs within a host implies that VoMs are common relative to hosts and
uninfected hosts may not be available. Indeed, coinfection can reinforce the lysis inhibition

21
pathway, another bacteriophage-‐associated lysis-‐delaying phenomenon, prolonging infection
state (Abedon, 1990; Abedon et al., 2003; Weitz et al., 2008).
Interestingly, a greater penalty probably arises from not retaining infections if no new
host cells are available or if all the other host cells are occupied versus choosing to stay in place
(retaining infections) when permissive hosts are available. In the former, a bad decision results
in VoM destruction, whereas in the latter, the cost is a reduced reproduction rate compared to
competing genotypes. This asymmetry should have interesting implications for the evolution of
control mechanisms dictating the lysis-‐lysogeny decision that occurs at the start of infections of
phages (or, more generally, viruses) that are able to choose between latent or productive
infection. Related to this, exit from lysogeny is often predicated by damage to the host DNA by
agents like UV light and mitomycin C (Cochran et al., 1998; Weinbauer and Suttle, 1999). As an
analogy, if one’s house were on fire, one would attempt escape regardless of the external
conditions. Since death in the former is assured, lysis pays off regardless of the probability of
survival outside the host.
VoM Genome Evolution
Microorganisms span the three domains of life and their VoMs reflect this breadth in
the variety of their genomes. Out of the seven virus classes within the Baltimore virus
classification system, six classes are known to parasitize all major phyla of microorganisms
while only one (Group 7, or non-‐retroviral dsDNA viruses that replicate through a ssRNA
intermediate) does not contain exemplars that infect microorganisms. Despite this great
diversity, not all virus classes are represented equally. Almost all of the nearly 100

22
characterized viruses infecting archaea hold their genetic information as dsDNA (Mochizuki et
al., 2012; Prangishvili 2013), while viruses infecting algae have genomes ranging from ssRNA to
dsDNA (Van Etten et al., 1991; Short, 2012). Viruses of fungi tend to be dsRNA (Göker et al.,
2011; Vainio et al., 2011), while representatives of at least four types can be found among the
bacteriophages (Calendar and Abedon, 2006). In this section we provide primers on VoM
genomics and genome evolution. See [Klumpp, this volume] for further discussion of VoM
genomics.
VoM genomes. Virus nucleic acid characteristics can have a large effect on genomic
attributes such as overall genome size and mutation rates. Exemplifying the upper limit of VoM
genome size are the recently discovered amoeba phages, such as Mimiviridae, Marseilleviridae
and Pandovirideae, which have dsDNA genomes larger than 2.5 Mb, surpassing even some
bacteria (La Scola et al., 2003; Monier et al., 2008; Colson et al., 2013; Philippe et al., 2013) [See
also La Scola, this volume]. By contrast, some ssRNA phages have genomes as small as 3.3 Kb
(Hatfull and Hendrix, 2011), a 758-‐fold difference. Mutation rates show tremendous variation
as well, with values ranging from 1.1 × 10–3 nucleotide substitutions per site per cell infection
(s/n/c) in the ssRNA phage Qβ to 9.8 × 10–8 s/n/c for the bacteriophage T2 (Sanjuán et al.,
2010). Generally, VoMs that store their genetic information as RNA or ssDNA tend to have
smaller genomes and higher mutation rates, while dsDNA VoMs have larger genomes and lower
mutation rates. These differences are believed to reflect differences in polymerase fidelity and
nucleic acid stability (Duffy et al., 2008).
In addition to size and stability, VoM genomes show considerable structural variation.
Both circular and linear permutations are common, and do not seem to correlate with any

23
obvious factors. In addition, ssDNA, ssRNA and dsRNA VoM genomes can be segmented (Iranzo
and Manrubia, 2012; Ojosnegros et al., 2011). Numerous hypotheses have been proposed to
explain viral segmentation, including increased capsid stability (Iranzo and Manrubia, 2012;
Ojosnegros et al., 2011), virus sex (Chao et al., 1992), and copying fidelity (Pressing and
Reanney, 1984). Though a consensus is not available, observations show that the reassortment
of virus segments can be an important factor in the generation of virus genetic diversity
(Marshall et al., 2013; O'Keefe et al., 2010).
Horizontal gene transfer and genome mosaicism. VoMs are extremely successful
mediators of gene shuffling (Fig. 3). Regardless of reproductive strategy (lytic vs. latent vs.
chronic) or type of genetic material (RNA versus DNA), many VoM genomes contain an
amalgam of genetic information from a variety of sources, commonly referred to genomic
mosaicism (Casjens and Thuman-‐Commike, 2011). For example, the tail genes of phages HK97
and λ show a high degree of sequence similarity, but the head genes are wholly dissimilar
(Juhala et al., 2000). The most parsimonious explanation for this observation is that the head
genes of HK97 and λ, but not the tail genes, were acquired from two evolutionarily divergent
sources.
The life history circumstances of VoMs may predispose them to genomic mosaicism
since this is generated through recombination or reassortment with foreign DNA. As obligate
parasites, VoMs reproduce inside cells containing separate and distinct genomes as well as
coinfecting viruses, transposons, plasmids and even transformed foreign DNA (Desnues et al.,
2012). Given this proximity, the accidental integration of foreign DNA into VoM genomes is not
surprising. While capsid size can limit the extent of integration of new genetic material,

24
recombination and then retention of similar sized genetic fragments may be relatively common.
In fact, it is frequently suggested that the modular architecture of VoM genomes is specifically
evolved to allow suites of genes dedicated to a common function to be traded among VoM
populations, creating a vast meta-‐gene pool (Campbell and Botstein, 1983; Hendrix et al.,
2000). Some authors even go so far as to suggest that VoMs and their hosts constitute a
superorganism, with access to a global gene pool (Weinbauer and Rassoulzadegan, 2004).
Consistent with expectations, this genomic mixing is more pronounced in generalists – that is,
VoMs capable of infecting greater diversities of cellular organisms – as they are more
promiscuous in host range than specialists (Born et al., 2011). A non-‐genetic form of mosaicism
may also arise in the form of phenotypic mixing, when coinfecting VoMs produce proteins that
interact (Loverdo and Lloyd-‐Smith, 2013).
VoM Impact on Host Genetics
Horizontal gene transfer and genome mosaicism is common among hosts of VoMs as
well. It has been best studied among bacteria (Baltrus, 2013; McDaniel, 2012), but is known to
occur in all domains (Bock, 2010; Hotopp, 2011). A majority of all sequenced bacterial genomes
contain prophages (Canchaya et al., 2003). These latent viruses comprise up to 20% of some
bacterial genomes (Klumpp et al., 2013) and a significant portion of the total planetary phage
genes is contained by prophages (Forterre and Prangishvili, 2013). There are, in addition,
numerous examples of phage-‐mediated gene transfer with significant implications for public
health and other fields (Brussow et al., 2004; Klumpp and Fuchs, 2007; Chen and Novick, 2009;
Imamovic et al., 2009; Saunders et al., 2001). For example, lysogenic conversion of

25
Corynebacterium diphtheriae by the Corynebacteriophage β renders the bacterium toxigenic
(Freeman, 1951). Antibiotic resistance genes are widespread in phage DNA obtained from
environmental isolates, and Colomer-‐Lluch and colleages generated resistant clones after
transfection with phage DNA (Colomer-‐Lluch et al., 2011). Clearly VoMs are important
components of microbial horizontal gene transfer, resulting in the gain, loss or reshuffling host
genes (Andersson, 2009; Filée et al., 2002; Rowher and Thurber, 2009; Krupovic et al., 2011).
A general category of virus-‐ and especially phage-‐encoded genes that appear to be new
additions to virus genomes and which often affect lysogenic conversion, that is, the expression
of genes by prophages during lysogenic cycles, have been termed morons. Morons, intriguingly
so named because they add “more DNA” to phage genomes, often contain their own separately
regulated promoters and terminators. Although nonessential, they may allow prophages to
escape deletion from host populations by providing to hosts beneficial genes such as virulence
factors, restriction endonucleases, superinfection exclusion proteins and toxin-‐antitoxin pairs
(Cumby et al., 2012). For example, morons encoding superoxide dismutases confer resistance
to Salmonella against reactive oxygen species produced by the mammalian immune system
(Figueroa-‐Bossi and Bossi, 1999).
Morons, once acquired by a bacterial host, can also form pathogenicity Islands (PAIs),
encoding virulence factors (Hensel and Schmidt, 2008). Present in pathogenic, but not related
non-‐pathogenic strains, these presumptive phage-‐ or plasmid-‐mediated (pre-‐)virulence factors
increase bacterial fitness by providing adhesins, toxins, invasins and protein secretion systems
used in host infection (Hacker and Kaper, 2000). Recent theoretical modeling predicts that
genes contributed by viruses can increase the evolvability of their hosts, allowing them to

26
better explore their adaptive landscape (Williams, 2013). In doing so, there is no doubt that
VoMs play a role in shaping microbial communities over time (Koskella and Meaden, 2013). In
some cases, coevolution with VoM can even induce a host to change its genetic code. For
example, Taylor and colleagues reported several yeast strains using a modified genetic code
where CUG encoded serine (Taylor et al., 2013). Interestingly these authors also found a
Totivirus which possessed only one CUG codon and was able to infect these yeast strains.
Presumably this Totivirus adapted to the yeast by avoiding the modified codon (Taylor et al.,
2013).
VoMs as Ecosystem Modifiers
Microorganisms, such as heterotrophic bacteria and phytoplankton, form the
foundation of the food web as they convert solar energy and dissolved nutrients into
particulate organic matter usable by higher organisms. Although research in this area is
nascent, recent work indicates that more than 90% of marine biomass is composed of
microorganisms. Amazingly, VoM lysis reportedly liberates 20-‐55% of this biomass each day
(Suttle, 1994; Suttle, 2007; Winget et al., 2011; Wommack and Colwell, 2000). The destruction
of primary producers has the effect of reducing the total available resources to grazers at the
next trophic level, reducing their growth, with cascading effects at all higher trophic levels.
Through host cell lysis, however, VoMs also can convert organic matter from a particulate form
to a dissolved form consumable only by osmotrophs (organisms that acquire nutrition by taking
advantage of an osmotic gradient, that is by direct uptake of dissolved organic molecules) at
the very bottom of the food chain (Thingstad et al., 2008; Weinbauer et al., 2011). This semi-‐

27
closed “viral shunt” may reduce the flow of energy between the primary producers and
osmotrophs and the higher trophic levels (Bratbak et al., 1992; Fuhrman, 1999; Suttle, 2007).
Indeed, as much as 25% of the carbon fixed through photosynthesis may cycle through the viral
shunt (Wilhelm and Suttle, 1999).
Due to the viral shunt, VoMs may significantly impact 'the biological pump', a process
that sequesters CO2 and other nutrients into the deep ocean as a result of the death and sinking
of surface water microorganisms (Suttle, 2007). This effect plays a role in the global carbon
cycle and any alterations of this mechanism could influence climate change via a modification
of the efficiency of this sequestration process, though the exact nature of this impact is still
unclear (Danovaro et al., 2011). The current debate on the ultimate role of VoM-‐mediated lysis
of marine biomass on a global scale centers on several possible outcomes (Brussaard et al.,
2008). First, VoMs may short-‐circuit the biological pump by returning nutrients back to the
dissolved phase, which is hypothesized to limit their transport to the deep ocean (Poorvin et al.,
2004). Second, VoMs might accelerate the biological pump cycling by promoting the sinking of
infected host cells to the deep ocean (Lawrence and Suttle, 2004). One way this might occur is
the failure of flagella-‐driven motility in some infected microbes, disabling their ability to
prevent sinking (Lawrence and Suttle, 2004). Finally, VoM-‐mediated lysis may drive the flow of
nutrients to the deep sea via the aggregation and sinking of lysed cellular debris (e.g., marine
snow) (Danovaro et al., 2008; Mari et al., 2005). These nutrients may form the basis of the food
web at depths where the available sunlight is insufficient to allow photosynthesis.
We expect that, due to the high degrees of organismal and environmental
heterogeneity, the activity of the viral shunt and the biological pump will exhibit considerable

28
temporal and spatial variation. Net movement of nutrients among trophic levels and habitats
thereby should show significant context dependency. A major challenge for microbial ecologists
will be to parse major trends in biogeochemical dynamics across widespread ecosystems.
Closing Remarks
Viruses of microorganisms are a spectacularly diverse, influential and pervasive group of
organisms, but our understanding of their biology is not proportionate to their impact. They
thus may be described as the dark matter of the biosphere (Filée et al., 2005); the hidden world
that directly or indirectly influences every living organism. Much of the world's genetic
information perhaps also is contained within their genomes. VoMs too are masters of shuttling
genes, manipulating genomes, and either generating or otherwise increasing biodiversity. They
alter biogeographical cycles, communities, ecosystems, and may even influence global climate
and its change. Understanding how these influences interact to produce our living world
requires the application of ecology in the context of evolutionary history. Further study of the
evolution and ecology of VoMs can only increase our appreciation of the rich and wonderful
fabric of life.
Acknowledgements
The authors are grateful for rewarding discussions with James Carpino, Gregory Lallos
and past and present members of the Dennehy Laboratory. Funding was provided by the
National Science Foundation Faculty Early Career Award (#1148879) to JJD.

29
Figure Legends
Fig. 1 – Population growth over time is depicted for three functions (Panels A-‐C) and for
experimental data (Panel D). In panel A, the characteristic concave-‐up curve of
exponential growth is shown (solid line). The shape of this function changes with
increasing (dotted line) or decreasing (dashed line) growth rates. In Panel B, the
incorporation of resource limitation into the population growth model tempers growth
rates at higher densities, giving a distinctive S-‐shaped curve (solid line). Increasing
(dotted line) or decreasing dashed line) growth rate will shift the curve to the right or
left, but they both approach the same maxima, the carrying capacity. Reducing
resources will have the effect of reducing maximal carrying capacity (dash-‐dot line).
Panel C shows Lotka-‐Volterra style predator (dashed line) and prey (solid line). Predator
numbers usually correspond to prey numbers with a slight lag and a reduced magnitude.
VoMs behave differently in that their numbers usually exceed that of their “prey.” Panel
D (modified from Marston et al., 2012) shows the population dynamics of a bacterium,
Synechococcus (black line), and a virus, RIM8 (grey line), in a chemostat (top third). For
reference, the dashed line is bacterial abundance in the control, virus-‐free chemostat.
The middle and bottom panels show host and virus phenotypes found at six time points.
Host range mutants are numbered in their order of infectivity (e.g., ϕ1–ϕ12), with
higher numbers indicating the ability to infect a greater number of host phenotypes.
Host phenotypes are labeled by their ability to resist infection by each host range
mutant. For example, S (sensitive to RIM8) is the ancestral host, and R0–2 is resistant to
ϕ0, ϕ1, and ϕ2. Dashed lines are hypothetical evolutionary histories based on the most

30
parsimonious interpretation of the data. Reprinted with permission from Marston et al.,
2012.
Fig. 2 – Viruses of microorganisms significantly impact the cycling of organic matter and
nutrients in the ocean. The shuttling of resources among trophic levels is interrupted by
viruses (virus shunt). In addition, viruses impact the biological pump between the
surface and deep ocean.
Fig. 3 –Horizontal Gene Transfer Across Domains. Phage-‐dominate (dotted lines) and phage-‐
influenced (dashed lines) are emphasized. Reprinted with permission from Abedon,
2009.

31
Fig. 1 Population growth over time.

32
Fig. 2 Viruses of microorganisms significantly impact the cycling of organic matter and nutrients in the ocean.

33
Fig. 3 Horizontal Gene Transfer Across Domains.

34
References
Abedon, S.T. (1989). Selection for bacteriophage latent period length by bacterial density -‐ a theoretical examination. Microbial Ecology 18, 79-‐88. Abedon, S.T. (1990). Selection for lysis inhibition in bacteriophage. Journal of Theoretical Biology 146, 501-‐511. Abedon, S.T. Bacteriophage Ecology. (2008). Advances in Molecular and Cellular Microbiology (Book 15). (Cambridge, UK: Cambridge University Press). Abedon, S.T. (2009). Phage evolution and ecology. Advances in Applied Mircobiology 67, 1-‐45. Abedon, S.T. (2011). Envisaging bacteria as phage targets. Bacteriophage 1, 228-‐230. Abedon, S.T., Herschler, T.D., and Stopar, D. (2001). Bacteriophage latent-‐period evolution as a response to resource availability. Applied and Environmental Microbiology 67, 4233-‐4241. Abedon, S.T., Hyman, P., and Thomas, C. (2003). Experimental examination of bacteriophage latent-‐period evolution as a response to bacterial availability. Applied and Environmental Microbiology 69, 7499-‐7506. Agnew, P., and Koella, J.C. (1999). Life history interactions with environmental conditions in a host-‐parasite relationship and the parasite's mode of transmission. Evolutionary Ecology 13, 67-‐89. Allee, W.C. 1931. Animal Aggregations. A Study in General Sociology. (Chicago: University of Chicago Press). Allen, H.K., Bunge, J., Foster, J.A., Bayles, D.O., and Stanton, T.B. (2013). Estimation of viral richness from shotgun metagenomes using a frequency count approach. Microbiome 1, 2-‐7. Andersson, A.F., and Banfield, J.F. (2008). Virus population dynamics and acquired virus resistance in natural microbial communities. Science 320, 1047-‐1050. Andersson, J.O. (2009). Gene transfer and diversification of microbial eukaryotes. Annual Review of Microbiology 63, 177-‐93. Angly, F.E., Felts, B., Breitbart, M., Salamon, P., Edwards, R.A., Carlson, C., Chan, A.M., Haynes, M., Kelley, S., Liu, H., et al. (2006). The marine viromes of four oceanic regions. PLoS Biol 4, e368.

35
Ashelford, K.E., Day, M.J., and Fry, J.C. (2003). Elevated abundance of bacteriophage infecting bacteria in soil. Applied and Environmental Microbiology 69, 285-‐289. Baas Becking, L.G.M. (1934). Geobiologie of inleiding tot de milieukunde (The Hague, the Netherlands: W.P. Van Stockum & Zoon). Baltrus, D.A. (2013). Exploring the costs of horizontal gene transfer. Trends in Ecology and Evolution 28, 489-‐495. Barnet, Y.M., Daft, M.J., and Stewart, W.D.P. (1981). Cyanobacteria -‐ cyanophage interactions in continuous culture. Journal of Applied Bacteriology 51, 541-‐552. Barondess, J.J., and Beckwith, J. (1990). A bacterial virulence determinant encoded by lysogenic coliphage lambda. Nature 346, 871-‐874. Barondess, J.J., and Beckwith, J. (1995). Bor gene of phage lambda, involved in serum resistance, encodes a widely conserved outer-‐membrane lipoprotein. Journal of Bacteriology 177, 1247-‐1253 Bergh, O., Borsheim, K.Y., Bratbak, G., and Heldal, M. (1989). High abundance of viruses found in aquatic environments. Nature 340, 467-‐468. Bock, Ralph (2010). The give-‐and-‐take of DNA: horizontal gene transfer in plants. Trends in Plant Science, 15, 11-‐22 Bohannan, B.J.M., and Lenski, R.E. (1997). Effect of resource enrichment on a chemostat community of bacteria and bacteriophage. Ecology 78, 2303-‐2315. Bohannan, B.J.M., and Lenski, R. (2000). Linking genetic change to community evolution: insights from studies of bacteria and bacteriophage. Ecology Letters, 362-‐377. Born, Y., Fieseler, L., Marazzi, J., Lurz, R., Duffy, B., and Loessner, M.J. (2011). Novel virulent and broad-‐host-‐range Erwinia amylovora bacteriophages reveal a high degree of mosaicism and a relationship to Enterobacteriaceae phages. Applied and Environmental Microbiology 77, 5945-‐5954. Bratbak, G., Heldal, M., Thingstad, T.F., Riemann, B., and Haslund, O.H. (1992). Incorporation of viruses into the budget of microbial C-‐transfer -‐ a first approach. Marine Ecology Progress Series 83, 273-‐280. Breitbart, M., Felts, B., Kelley, S., Mahaffy, J.M., Nulton, J., Salamon, P., and Rohwer, F. (2004a). Diversity and population structure of a near-‐shore marine-‐sediment viral community. Proceedings of the Royal Society B-‐Biological Sciences 271, 565-‐574.

36
Breitbart, M., Hewson, I., Felts, B., Mahaffy, J.M., Nulton, J., Salamon, P., and Rohwer, F. (2003). Metagenomic analyses of an uncultured viral community from human feces. Journal of Bacteriology 185, 6220-‐6223. Breitbart, M., Miyake, J.H., and Rohwer, F. (2004b). Global distribution of nearly identical phage-‐encoded DNA sequences. FEMS Microbiology Letters, 249–256. Breitbart, M., and Rohwer, F. (2005). Here a virus, there a virus, everywhere the same virus? Trends in Microbiology 13, 278-‐284. Breitbart, M., Salamon, P., Andresen, B., Mahaffy, J.M., Segall, A.M., Mead, D., Azam, F., and Rohwer, F. (2002). Genomic analysis of uncultured marine viral communities. Proceedings of the National Academy of Sciences of the United States of America 99, 14250-‐14255. Brussaard, C.P.D., Wilhelm, S.W., Thingstad, F., Weinbauer, M.G., Bratbak, G., Heldal, M., Kimmance, S.A., Middelboe, M., Nagasaki, K., Paul, J.H., et al. (2008). Global-‐scale processes with a nanoscale drive: the role of marine viruses. ISME Journal 2, 575-‐578. Brussow, H., Canchaya, C., and Hardt, W.D. (2004). Phages and the evolution of bacterial pathogens: from genomic rearrangements to lysogenic conversion. Microbiology and Molecular Biology Reviews 68, 560-‐602. Brussow, H., and Cutter, K. 2005. Bacteriophage ecology. In Bacteriophages, E. Kutter and A. Sulakvelidze, eds. (Boca Raton, FL: CRC Press), pp. 129-‐164. Buckling, A., and Rainey, P.B. (2002). Antagonistic coevolution between a bacterium and a bacteriophage. Proceedings of the Royal Society of London Series B-‐Biological Sciences 269, 931-‐936. Buckling, A., Wei, Y., Massey, R.C., Brockhurst, M.A., and Hochberg, M.E. (2006). Antagonistic coevolution with parasites increases the cost of host deleterious mutations. Proceedings of the Royal Society B-‐Biological Sciences 273, 45-‐49. Calendar, R., and Abedon, S.T. (2006). The Bacteriophages 2/e. (Oxford: Oxford University Press). Campbell, A. (1961). Conditions for the existence of bacteriophage. Evolution 15, 153. Campbell, A., and Botstein, D. (1983). Evolution of the lambdoid phages. In Lambda II, R.W. Hendrix, ed. (Cold Spring Harbor: Cold Spring Harbor Laboratory Press), pp. pp. 365–380. Canchaya, C., Proux, C., Fournous, G., Bruttin, A., and Brussow, H. (2003). Prophage genomics. Microbiology and Molecular Biology Review 67, 238-‐276.

37
Casjens, S.R., and Thuman-‐Commike, P.A. (2011). Evolution of mosaically-‐related tailed bacteriophage genomes seen through the lens of phage P22 virion assembly. Virology 411, 393-‐415. Chantranupong, L., and Heineman, R.H. (2012). A common, non-‐optimal phenotypic endpoint in experimental adaptations of bacteriophage lysis time. BMC Evolutionary Biology 12. Chao, L., Levin, B.R., and Stewart, F.M. (1977). Complex community in a simple habitat -‐ experimental studies with bacteria and phage. Ecology 58, 369-‐378. Chao, L., Tran, T.R., and Matthews, C. (1992). Muller's ratchet and the advantage of sex in the RNA virus φ6. Evolution 46, 289-‐299. Chen, J., and Novick, R.P. (2009). Phage-‐mediated intergeneric transfer of toxin genes. Science 323, 139-‐141. Chibani-‐Chennoufi, S., Bruttin, A., Dillmann, M.L., and Brussow, H. (2004). Phage-‐host interaction: an ecological perspective. Journal of Bacteriology 186, 3677-‐3686. Cochran, P.K., Kellogg, C.A., and Paul, J.H. (1998). Prophage induction of indigenous marine lysogenic bacteria by environmental pollutants. Marine Science Faculty Publications 64, 125-‐133. Colomer-‐Lluch, M., Jofre, J., and Muniesa, M. (2011). Antibiotic resistance genes in the bacteriophage DNA fraction of environmental samples. PLoS ONE 6, e17549. Colson, P., Pagnier, I., Yoosuf, N., Fournous, G., La Scola, B., and Raoult, D. (2013). "Marseilleviridae", a new family of giant viruses infecting amoebae. Archives of Virology 158, 915-‐920. Culley, A.I., Lang, A.S., and Suttle, C.A. (2006). Metagenomic analysis of coastal RNA virus communities. Science 312, 1795-‐1798. Cumby, N., Edwards, A.M., Davidson, A.R., and Maxwell, K.L. (2012). The bacteriophage HK97 gp15 moron element encodes a novel superinfection exclusion protein. Journal of Bacteriology 194, 5012-‐5019. Danovaro, R., Corinaldesi, C., Dell'Anno, A., Fuhrman, J.A., Middelburg, J.J., Noble, R.T., and Suttle, C.A. (2011). Marine viruses and global climate change. FEMS Microbiology Reviews 35, 993-‐1034. Danovaro, R., Dell'Anno, A., Corinaldesi, C., Magagnini, M., Noble, R., Tamburini, C., and Weinbauer, M. (2008). Major viral impact on the functioning of benthic deep-‐sea ecosystems. Nature 454, 1084-‐U1027.

38
Darwin, C. (1859). On The Origin of Species by Means of Natural Selection, or the Preservation fo Favoured Races in the Struggle for Life (London: John Murray). Darwin, C. (1862). On the Various Contrivances by Which British and Foreign Orchids Are Fertilised by Insects (London: John Murray). De Paepe, M., and Taddei, F. (2006). Viruses' life history: towards a mechanistic basis of a trade-‐off between survival and reproduction among phages. PLoS Biology 4, e193. Deho, G., and Ghisotti, D. (2006). The satellite phage P4. In The Bacteriophages 2/e. R.L. Calendar, and S.T. Abedon, eds. (Oxford: Oxford University Press), pp. 391–408. Delwart, E.L. (2007). Viral metagenomics. Reviews in Medical Virology 17, 115-‐131. Dennehy, J.J. (2012). What can phages tell us about host-‐pathogen coevolution? International Journal of Evolutionary Biology, 396165-‐396165. Dennehy, J.J., Duffy, S., O'Keefe, K.J., Edwards, S., and Turner, P.E. (2013). Frequent coinfection reduces RNA virus population genetic diversity. Journal of Heredity 104, 704-‐712. Dennehy, J.J., Friedenberg, N.A., Holt, R.D., and Turner, P.E. (2006). Viral ecology and the maintenance of novel host use. American Naturalist 167, 429-‐439. Desnues, C., B. La Scola, N. Yutin, G. Fournous, C. Robert et al. (2012). Provirophages and transpovirons as the diverse mobilome of giant viruses. Proceedings of the National Academy of Sciences of the United States of America 109, 18078-‐18083. Desnues, C., Rodriguez-‐Brito, B., Rayhawk, S., Kelley, S., Tran, T., Haynes, M., Liu, H., Furlan, M., Wegley, L., Chau, B., et al. (2008). Biodiversity and biogeography of phages in modern stromatolites and thrombolites. Nature 452, 340-‐U345. Duffy, S., Shackelton, L.A., and Holmes, E.C. (2008). Rates of evolutionary change in viruses: patterns and determinants. Nature Reviews Genetics 9, 267-‐276. Ebert, D., Zschokke-‐Rohringer, C.D., and Carius, H.J. (2000). Dose effects and density-‐dependent regulation of two microparasites of Daphnia magna. Oecologia 122, 200-‐209. Edwards, R.A., and Rohwer, F. (2005). Viral metagenomics. Nature Reviews Microbiology 3, 504-‐510. Figueroa-‐Bossi, N., and Bossi, L. (1999). Inducible prophages contribute to Salmonella virulence in mice. Molecular Microbiology 33, 167-‐176.

39
Filée, J., Forterre, P., Sen-‐Lin, T., and Laurent, J. (2002). Evolution of DNA polymerase families: Evidences for multiple gene exchange between cellular and viral proteins. Journal of Molecular Evolution 54, 763-‐773. Filée, J., Tétart, F., Suttle, C.A., and Krisch, H.M. (2005). Marine T4-‐type bacteriophages, a ubiquitous component of the dark matter of the biosphere. Proceedings of the National Academy of Sciences 102, 12471-‐12476. Fischer, M.G., and Suttle, C.A. (2011). A virophage at the origin of large DNA transposons. Science 332, 231-‐234. Fisher, R.A. (1930). The Genetical Theory of Natural Selection (Oxford: Clarendon Press). Forterre, P., and Prangishvili, D. (2013). The major role of viruses in cellular evolution: facts and hypotheses. Currunt Opinion in Virology 3, 558-‐65. Freeman, V.J. (1951). Studies on the virulence of bacteriophage-‐infected strains of Corynebacterium diphtheriae. Journal of Bacteriology 61, 675–688. Fuhrman, J.A. (1999). Marine viruses and their biogeochemical and ecological effects. Nature 399, 541-‐548. Gallet, R., Lenormand, T., and Wang, I.-‐N. (2012). Phenotypic stochasticity protects lytic bacteriophage populations from extinction during the bacterial stationary phase. Evolution 66, 3485–3494. Gallet, R., Kannoly, S., and Wang, I.-‐N. (2011). Effects of bacteriophage traits on plaque formation. BMC Microbiology 11. Gallet, R., Shao, Y.P., and Wang, I.-‐N. (2009). High adsorption rate is detrimental to bacteriophage fitness in a biofilm-‐like environment. BMC Evolutionary Biology 9. García-‐Villada, L., and Drake, J.W. (2013). Experimental selection reveals a trade-‐off between fecundity and lifespan in the coliphage Qß. Open Biology 3. Gause, G.F. (1934). The Struggle for Existence (New York: Hafner). Gerla, D.J., Gsell, A.S., Kooi, B.W., Ibelings, B.W., Van Donk, E., and Mooij, W.M. (2013). Alternative states and population crashes in a resource-‐susceptible-‐infected model for planktonic parasites and hosts. Freshwater Biology 58, 538-‐551. Göker, M., Scheuner, C., Klenk, H.-‐P., Stielow, J.B., and Menzel, W. (2011). Codivergence of mycoviruses with their hosts. PLoS One 6.

40
Hacker, J., and Kaper, J.B. (2000). Pathogenicity islands and the evolution of microbes. Annual Review of Microbiology 54, 641-‐679. Hambly, E., and Suttle, C.A. (2005). The viriosphere, diversity, and genetic exchange within phage communities. Current Opinion in Microbiology 8, 444-‐450. Hatfull, G.F., and Hendrix, R.W. (2011). Bacteriophages and their Genomes. Current Opinion in Virology 1, 298-‐303 Heineman, R.H., and Bull, J.J. (2007). Testing optimality with experimental evolution: lysis time in a bacteriophage. Evolution 61, 1695-‐1709. Held, N.L., and Whitaker, R.J. (2009). Viral biogeography revealed by signatures in Sulfolobus islandicus genomes. Environmental Microbiology 11, 457-‐466. Hendrix, R.W., Lawrence, J.G., Hatfull, G.F., and Casjens, S. (2000). The origins and ongoing evolution of viruses. Trends in Microbiology 8, 504-‐508. Hensel, M., and Schmidt, H., eds. (2008). Horizontal gene transfer in the evolution of pathogenesis (Cambridge: Cambridge University Press). Herskowitz, I., and Hagen, D. (1980). The lysis-‐lysogeny decision of phage λ: explicit programming and responsiveness. Annual Review of Genetics, 399-‐445. Horiuchi, K. (1983). Co-‐evolution of a filamentous bacteriophage and its defective interfering particles. Journal of Molecular Biology 169, 389-‐407. Hotopp, J.C.D. (2011). Horizontal gene transfer between bacteria and animals. Trends in Genetics 27, 157-‐163. Huang, A.S. (1973). Defective interfering viruses. Annual Review of Microbiology 27, 101-‐117. Hurwitz, B.L., and Sullivan, M.B. (2013). The Pacific Ocean virome (POV): a marine viral metagenomic dataset and associated protein clusters for quantitative viral ecology. PLoS One 8. Hyman, P., and Abedon, S.T. (2010). Bacteriophage host range and bacterial resistance. Advances in Applied Microbiology 70, 217-‐248. Imamovic, L., Jofre, J., Schmidt, H., Serra-‐Moreno, R., and Muniesa, M. (2009). Phage-‐mediated Shiga toxin 2 gene transfer in food and water. Applied and Environmental Microbiology 75, 1764-‐1768. Iranzo, J., and Manrubia, S.C. (2012). Evolutionary dynamics of genome segmentation in multipartite viruses. Proceedings of the Royal Society B-‐Biological Sciences 279, 3812-‐3819.

41
Joseph, S.B., Hanley, K.A., Chao, L., and Burch, C.L. (2009). Coinfection rates in φ6 bacteriophage are enhanced by virus-‐induced changes in host cells. Evolutionary Applications 2, 24-‐31. Juhala, R.J., Ford, M.E., Duda, R.L., Youlton, A., Hatfull, G.F., and Hendrix, R.W. (2000). Genomic sequences of bacteriophages HK97 and HK022: Pervasive genetic mosaicism in the lambdoid bacteriophages. Journal of Molecular Biology 299, 27-‐51. Kawecki, T. J. and Ebert, D. (2004), Conceptual issues in local adaptation. Ecology Letters, 7: 1225–1241. Kerr, B., West, J., and Bohannan, B.J.M. (2008). Bacteriophages: models for exploring basic principles of ecology. In Bacteriophage Ecology, S.T. Abedon, ed. (Cambridge: Cambridge University Press), pp. 31-‐63. Kerr, P.J. (2012). Myxomatosis in Australia and Europe: a model for emerging infectious diseases. Antiviral Research 93, 387-‐415. Klumpp, J., Fouts, D.E., and Sozhamannan, S. (2013). Bacteriophage functional genomics and its role in bacterial pathogen detection. Briefings in Functional Genomics 12, 354-‐365. Klumpp, J., and Fuchs, T.M. (2007). Identification of novel genes in genornic islands that contribute to Salmonella typhimurium replication in macrophages. Microbiology-‐SGM 153, 1207-‐1220. Koskella, B., and Meaden, S. (2013). Understanding bacteriophage specificity in natural microbial communities. Viruses-‐Basel 5, 806-‐823. Kristensen, D.M., Mushegian, A.R., Dolja, V.V., and Koonin, E.V. (2010). New dimensions of the virus world discovered through metagenomics. Trends in Microbiology 18, 11-‐19. Krupovic, M., Prangishvili, D., Hendrix, R.W., and Bamford, D.H. (2011). Genomics of bacterial and archaeal viruses: dynamics within the prokaryotic virosphere. Microbiology and Molecular Biology Reviews 75, 610. Kunin, V., He, S., Warnecke, F., Peterson, S.B., Martin, H.G., Haynes, M., Ivanova, N., Blackall, L.L., Breitbart, M., Rohwer, F., et al. (2008). A bacterial metapopulation adapts locally to phage predation despite global dispersal. Genome Research 18, 293-‐297. La Scola, B., Audic, S., Robert, C., Jungang, L., de Lamballerie, X., Drancourt, M., Birtles, R., Claverie, J.M., and Raoult, D. (2003). A giant virus in amoebae. Science 299, 2033-‐2033.

42
La Scola, B., Desnues, C., Pagnier, I., Robert, C., Barrassi, L., Fournous, G., Merchat, M., Suzan-‐Monti, M., Forterre, P., Koonin, E., et al. (2008). The virophage as a unique parasite of the giant mimivirus. Nature 455, 100-‐U165. Lawrence, J.E., and Suttle, C.A. (2004). Effect of viral infection on sinking rates of Heterosigma akashiwo and its implications for bloom termination. Aquatic Microbial Ecology, 1-‐7. Lee, K.L., Hubbard, L.C., Hern, S., Yildiz, I., Gratzl, M., and Steinmetz, N.F. (2013). Shape matters: the diffusion rates of TMV rods and CPMV icosahedrons in a spheroid model of extracellular matrix are distinct. Biomaterials Science 1, 581-‐588. Lenski, R.E. (1984). Coevolution of bacteria and phage -‐ are there endless cycles of bacterial defenses and phage counterdefenses? Journal of Theoretical Biology 108, 319-‐325. Lenski, R.E. (1988). Dynamics of interactions between bacteria and virulent bacteriophage. Advances in Microbial Ecology 10, 1-‐44. Lenski, R.E., and Levin, B.R. (1985). Constraints on the coevolution of bacteria and virulent phage -‐ a model, some experiments, and predictions for natural communities American Naturalist 125, 585-‐602. Levin, B.R., Stewart, F.M., and Chao, L. (1977). Resource limited growth, competition, and predation -‐ A model and experimental studies with bacteria and bacteriophage American Naturalist 111, 3-‐24. Little, T.J., and Ebert, D. (2000). The cause of parasitic infection in natural populations of Daphnia (Crustacea : Cladocera): the role of host genetics. Proceedings of the Royal Society B-‐Biological Sciences 267, 2037-‐2042. Lotka, A.J. (1925). Elements of physical biology (Baltimore: Williams and Wilkins). Loverdo, C., and Lloyd-‐Smith, J.O. (2013). Intergenerational phenotypic mixing in viral evolution. Evolution 67, 1815-‐1822. Ludwig, D., and Rowe, L. (1990). Life history strategies for energy gain and predator avoidance under time constraints. American Naturalist 135, 686-‐707. Mari, X., Rassoulzadegan, F., Brussaard, C.P.D., and Wassmann, P. (2005). Dynamics of transparent exopolymeric particles (TEP) production by Phaeocystis globosa under N-‐ or P-‐limitation: a controlling factor of the retention/export balance. Harmful Algae 4, 895-‐914. Marshall, N., Priyamvada, L., Ende, Z., Steel, J., and Lowen, A.C. (2013). Influenza virus reassortment occurs with high frequency in the absence of segment mismatch. PLoS Pathogens 9, e1003421.

43
Marston, M.F., Pierciey, F.J., Jr., Shepard, A., Gearin, G., Qi, J., Yandava, C., Schuster, S.C., Henn, M.R., and Martiny, J.B.H. (2012). Rapid diversification of coevolving marine Synechococcus and a virus. Proceedings of the National Academy of Sciences of the United States of America 109, 4544-‐4549. McDaniel, L.D. (2012). High frequency of horizontal gene transfer in the oceans. Science 337, 911-‐911. McLean, A.R., and Bostock, C.J. (2000). Scrapie infections initiated at varying doses: an analysis of 117 titration experiments. Philosophical Transactions of the Royal Society of London Series B-‐Biological Sciences 355, 1043-‐1050. Mochizuki, T., Krupovic, M., Pehau-‐Arnaudet, G., Sako, Y., Forterre, P., Prangishvili, D. (2012). Archaeal virus with exceptional virion architecture and the largest single-‐stranded DNA genome. Proceedings of the National Academy of Sciences of the United States of America 109, 13386–91. Mokili, J.L., Rohwer, F., and Dutilh, B.E. (2012). Metagenomics and future perspectives in virus discovery. Current Opinion in Virology 2, 63-‐77. Monier, A., Claverie, J.-‐M., and Ogata, H. (2008). Taxonomic distribution of large DNA viruses in the sea. Genome Biology 9. Morgan, A.D., Maclean, R.C., and Buckling, A. (2009). Effects of antagonistic coevolution on parasite-‐mediated host coexistence. Journal of Evolutionary Biology 22, 287-‐292. O'Keefe, K.J., Silander, O.K., McCreery, H., Weinreich, D.M., Wright, K.M., Chao, L., Edwards, S.V., Remold, S.K., and Turner, P.E. (2010). Geographic differences in sexual reassortment in RNA phage. Evolution 64, 3010-‐3023. Ojosnegros, S., Garcia-‐Arriaza, J., Escarmis, C., Manrubia, S.C., Perales, C., Arias, A., Garcia Mateu, M., and Domingo, E. (2011). Viral genome segmentation can result from a trade-‐off between genetic content and particle stability. PLoS Genetics 7. Oppenheim, A.B., Kobiler, O., Stavans, J., Court, D.L., and Adhya, S. (2005 ). Switches in bacteriophage λ development. Annual Review of Genetics, 409-‐429. Paul, J.H. (2008). Prophages in marine bacteria: dangerous molecular time bombs or the key to survival in the seas? The ISME Journal 2, 579-‐589. Pearl, R. (1927). The growth of populations. Quarterly Review of Biology 2, 532-‐548.

44
Pearl, R., and Reed, L.J. (1920). On the rate of growth of the population of the united states since 1790 and its mathematical representation. Proceedings of the National Academy of Sciences of the United States of America 6, 275-‐288. Pepin, K.M., Samuel, M.A., and Wichman, H.A. (2006). Variable pleiotropic effects from mutations at the same locus hamper prediction of fitness from a fitness component. Genetics 172, 2047-‐2056. Philippe, N., Legendre, M., Doutre, G., Coute, Y., Poirot, O., Lescot, M., Arslan, D., Seltzer, V., Bertaux, L., Bruley, C., et al. (2013). Pandoraviruses: amoeba viruses with genomes up to 2.5 Mb reaching that of parasitic eukaryotes. Science 341, 281-‐286. Pianka (1976). Natural selection of optimal reproductive tactics. American Zoologist 16, 775-‐784. Poorvin, L., Rinta-‐Kanto, J.M., Hutchins, D.A., and Wilhelm, S.W. (2004). Viral release of iron and its bioavailability to marine plankton. Limnology and Oceanography 49, 1734-‐1741. Pradel, R. (1996). Utilization of capture-‐mark-‐recapture for the study of recruitment and population growth rate. Biometrics 52, 703-‐709. Prangishvili, D. (2013). The wonderful world of archaeal viruses. Annual Review of Microbiology 67, 565–85. Preisig, O., Wingfield, B.D., and Wingfield, M.J. (1998). Coinfection of a fungal pathogen by two distinct double-‐stranded RNA viruses. Virology 252, 399-‐406. Pressing, J., and Reanney, D.C. (1984). Divided genomes and intrinsic noise. Journal of Molecular Evolution 20, 135-‐146. Rapp, F. (1969). Defective DNA animal viruses. Annual Review of Microbiology 23, 293-‐316. Regoes, R.R., Nowak, M.A., and Bonhoeffer, S. (2000). Evolution of virulence in a heterogeneous host population. Evolution 54, 64-‐71. Roux, L., Simon, A.E., and Holland, J.J. (1991). Effects of defective interfering viruses on virus-‐replication and pathogenesis in vitro and in vivo. Advances in Virus Research 40, 181-‐211. Rohwer, F., and Edwards, R. (2002). The phage proteomic tree: a genome-‐based taxonomy for phage. Journal of Bacteriology 184, 4529-‐4535. Rohwer, F., and Thurber, R.V. (2009). Viruses manipulate the marine environment. Nature 459, 207-‐212.

45
Rosario, K., and Breitbart, M. (2011). Exploring the viral world through metagenomics. Current Opinion in Virology 1, 289-‐297. Rosario, K., Nilsson, C., Lim, Y.W., Ruan, Y., and Breitbart, M. (2009). Metagenomic analysis of viruses in reclaimed water. Environmental Microbiology 11, 2806-‐2820. Sanjuán, R., Nebot, M.R., Chirico, N., Mansky, L.M., and Belshaw, R. (2010). Viral mutation rates. Journal of Virology 84, 9733-‐9748. Sano, E., Carlson, S., Wegley, L., and Rohwer, F. (2004). Movement of viruses between biomes. Applied and Environmental Microbiology 70, 5842-‐5846. Saunders, J.R., Allison, H., James, C.E., McCarthy, A.J., and Sharp, R. (2001). Phage-‐mediated transfer of virulence genes. Journal of Chemical Technology and Biotechnology 76, 662-‐666. Schrag, S.J., and Mittler, J.E. (1996). Host-‐parasite coexistence: The role of spatial refuges in stabilizing bacteria-‐phage interactions. American Naturalist 148, 348-‐377. Shao, Y.P., and Wang, I.N. (2008). Bacteriophage adsorption rate and optimal lysis time. Genetics 180, 471-‐482. Short, C.M., and Suttle, C.A. (2005). Nearly identical bacteriophage structural gene sequences are widely distributed in both marine and freshwater environments. Applied and Environmental Microbiology 71, 480-‐486. Short, S.M. (2012). The ecology of viruses that infect eukaryotic algae. Environmental Microbiology 14, 2253-‐2271. Six, E.W., and Klug, C.A. (1973). Bacteriophage P4: a satellite virus depending on a helper such as prophage P2. Virology 51, 327-‐344. Stopar, D., and Abedon, S.T. (2008). Modeling bacteriophage population growth. In Bacteriophage Ecology, S.T. Abedon, ed. (Cambridge: Cambridge University Press). pp. 389-‐414. Suttle, C.A. (1994). The significance of viruses to mortality in aquatic microbial communities. Microbial Ecology 28, 237-‐243. Suttle, C.A. (2007). Marine viruses -‐ major players in the global ecosystem. Nature Reviews Microbiology 5, 801-‐812. Symons, R.H. (1991). The intriguing viroids and virusoids -‐ what is their information content and how did they evolve? . Molecular Plant-‐Microbe Interactions 4, 111-‐121.

46
Derek J. Taylor, D.J., Ballinger, M.J., Bowman, S.M., and Bruenn, J.A. (2013). Virus-‐host co-‐evolution under a modified nuclear genetic code. PeerJ. 1: e50. Thingstad, T.F. (2000). Elements of a theory for the mechanisms controlling abundance, diversity, and biogeochemical role of lytic bacterial viruses in aquatic systems. Limnology and Oceanography 45, 1320-‐1328. Thingstad, T.F., Bratbak, G., and Heldal, M. (2008). Aquatic phage ecology. In Bacteriophage Ecology, S.T. Abedon, ed. (Cambridge, UK: Cambridge University Press), pp. 451-‐480. Thompson, J.N. (1994). The Coevolutionary Process (Chicago: University of Chicago Press). Thompson, J.N. (2012). The role of coevolution. Science 335, 410-‐411. Thurber, R.V., Haynes, M., Breitbart, M., Wegley, L., and Rohwer, F. (2009). Laboratory procedures to generate viral metagenomes. Nature Protocols 4, 470-‐483. Turner, P.E. (2005). Parasitism between co-‐infecting bacteriophages. Advances in Ecological Research, 37, 309-‐332. Uc-‐Mass, A., Loeza, E.J., de la Garza, M., Guarneros, G., Hernandez-‐Sanchez, J., and Kameyama, L. (2004). An orthologue of the cor gene is involved in the exclusion of temperate lambdoid phages. Evidence that Cor inactivates FhuA receptor functions. Virology 329, 425-‐433. Vainio, E.J., Hakanpää, J., Dai, Y.-‐C., Hansen, E., Korhonen, K. & Hantula, J. 2011. Species of Heterobasidion host a diverse pool of partitiviruses with global distribution and interspecies transmission. Fungal Biology 115, 1234-‐1243. Van Etten, J.L., Lane, L.C., and Meints, R.H. (1991). Viruses and virus-‐like particles of eukaryotic algae. Microbiological Reviews 55, 586-‐620. van Wolferen, M., Ajon, M., Driessen, A.J.M., and Albers, S.V. (2013). How hyperthermophiles adapt to change their lives: DNA exchange in extreme conditions. Extremophiles 17, 545-‐563. Volterra, V. (1926). Fluctuations in the abundance of a species considered mathematically. Nature 118, 558-‐560. Wang, I.-‐N., Dykhuizen, D.E., and Slobodkin, L.B. (1996). The evolution of phage lysis timing. Evolutionary Ecology, 545-‐558 Weinbauer, M.G., Bonilla-‐Findji, O., Chan, A.M., Dolan, J.R., Short, S.M., Simek, K., Wilhelm, S.W., and Suttle, C.A. (2011). Synechococcus growth in the ocean may depend on the lysis of heterotrophic bacteria. Journal of Plankton Research 33, 1465-‐1476.

47
Waldor, M.K., and Mekalanos, J.J. (1996). Lysogenic conversion by a filamentous phage encoding cholera toxin. Science 272, 1910-‐1914. Weinbauer, M.G., and Rassoulzadegan, F. (2004). Are viruses driving microbial diversification and diversity? Environmental Microbiology 6, 1-‐11. Weinbauer, M.G., and Suttle, C.A. (1999). Lysogeny and prophage induction in coastal and offshore bacterial communities. Aquatic Microbial Ecology, 217-‐225. Weinbauer, M.G., and Thingstad, T.F. (2010). Trade-‐offs between competition and defense specialists among unicellular planktonic organisms: the "killing the winner" hypothesis revisited. Microbiology and Molecular Biology Reviews 74, 42-‐57.Weitz, J.S., and Dushoff, J. (2008). Alternative stable states in host-‐phage dynamics. Theoretical Ecology 1, 13-‐19. Weitz, J.S., Mileyko, Y., Joh, R.I., and Voit, E.O. (2008). Collective decision making in bacterial viruses. Biophysical Journal 95, 2673-‐2680. Whon, T. W., Kim, M.-‐S., Roh, S. W., Shin, N.-‐R., Lee, H.-‐W., et al. (2012). Metagenomic characterization of airborne viral DNA diversity in the near-‐surface atmosphere. Journal of Virology 86, 8221-‐8231. Williams, H.T.P. (2013). Phage-‐induced diversification improves host evolvability. BMC Evolutionary Biology 13, 17. Wilhelm, S.W., and Suttle, C.A. (1999). Viruses and nutrient cycles in the sea -‐ viruses play critical roles in the structure and function of aquatic food webs. Bioscience 49, 781-‐788. Winget, D.M., Helton, R.R., Williamson, K.E., Bench, S.R., Williamson, S.J., and Wommack,K.E. (2011). Repeating patterns of virioplankton production within an estuarine ecosystem. Proceedings of the National Academy of Sciences of the United States of America 108, 11506-‐11511. Winter, C., Bouvier, T. Winter, C., Bouvier, T., Weinbauer, M.G., Thingstad, T.F. (2010). Trade-‐offs between competition and defense specialists among unicellular planktonic organisms: the "killing the winner" hypothesis revisited. Microbiology and Molecular Biology Review 2010 74, 42-‐57. Wommack, K.E., and Colwell, R.R. (2000). Virioplankton: viruses in aquatic ecosystems. Microbiology Molecular Biology Review 64, 69 -‐ 114. Yau, S., Lauro, F.M., DeMaere, M.Z., Brown, M.V., Thomas, T., Raftery, M.J., Andrews-‐Pfannkoch, C., Lewis, M., Hoffman, J.M., Gibson, J.A., et al. (2011). Virophage control of

48
antarctic algal host-‐virus dynamics. Proceedings of the National Academy of Sciences of the United States of America 108, 6163-‐6168.

49
Chapter 2: Frequency and Fitness Consequences of Bacteriophage Φ6 Host Range Mutations
Brian E. Ford1,2,3, Bruce Sun1,2,4, James Carpino1,3, Elizabeth S. Chapler1, Jane Ching1,5, Yoon
Choi1,6, Kevin Jhun1,7, Jung D. Kim1,8, Gregory G. Lallos1, Rachelle Morgenstern1,9, Shalini Singh1, Sai Theja1, and John J. Dennehy1,3,10
1Biology Department, Queens College of the City University of New York, Queens, New York, 11367 2Co-‐first authors 3The Graduate Center of the City University of New York, New York, NY, 10016 4Current affiliation: Department of Surgery, Columbia University Medical Center, New York, NY 10032 5Current affiliation: University of Maryland School of Pharmacy, Baltimore, Maryland 21201 6Current affiliation: Smilow Research Center, New York University Medical Center, 522 First Avenue, New York, NY 10016 7Current affiliation: Icahn School of Medicine at Mount Sinai, New York, NY 10029 8Current affiliation: Epidemiology and Public Health, CUNY School of Public Health, New York, NY 10065 9Current affiliation: Mailman School of Public Health, Columbia University, New York, NY 10032 10Corresponding author: [email protected] Running Head: Phage Host Range Mutation Frequency Key Words: emerging infectious disease, virus emergence, host range expansion, antagonistic pleiotropy, host attachment protein Research Article publish in PLoS ONE

50
Abstract
Viruses readily mutate and gain the ability infect novel hosts, but few data are available
regarding the number of possible host range-‐expanding mutations allowing infection of any
given novel host, and the fitness consequences of these mutations on original and novel hosts.
To gain insight into the process of host range expansion, we isolated and sequenced 69
independent mutants of the dsRNA bacteriophage φ6 able to infect the novel host,
Pseudomonas pseudoalcaligenes. In total, we found at least 17 unique suites of mutations
among these 69 mutants. We assayed fitness for 13 of 17 mutant genotypes on P.
pseudoalcaligenes and the standard laboratory host, P. phaseolicola. Mutants exhibited
significantly lower fitnesses on P. pseudoalcaligenes compared to P. phaseolicola. Furthermore,
12 of the 13 assayed mutants showed reduced fitness on P. phaseolicola compared to wildtype
φ6, confirming the prevalence of antagonistic pleiotropy during host range expansion. Further
experiments revealed that the mechanistic basis of these fitness differences was likely variation
in host attachment ability. In addition, using computational protein modeling, we show that
host-‐range expanding mutations occurred in hotspots on the surface of the phage's host
attachment protein opposite a putative hydrophobic anchoring domain.

51
Introduction
After a long period of steady decline, mortality due to infectious disease increased over
the past several decades, largely because of the emergence of new infectious diseases including
HIV [1,2]. Of these new diseases, a disproportionate number have been viruses [3,4]. Because
of their high mutation rates and vast population sizes, viruses have higher probabilities of
acquiring the requisite mutation(s) allowing infection of novel hosts than do other types of
pathogens [5]. A common fear is that a highly transmissible and virulent virus will spread
pandemically among humans, causing widespread mortality and economic damage. Thus, there
is a strong motivation to understand and predict virus emergence.
Virus emergence is a two-‐step process. A virus first mutates to gain the ability to infect a
new host, and then fully emerges by achieving positive population growth on that host via
adaptation [6]. Theoretical modeling has shown that emergence probabilities are highly
sensitive towards the type of mutation(s) required to productively infect a novel host [7].
Emergence events requiring single nucleotide substitutions are far more likely to occur than
those that require several simultaneous point mutations or recombination [8]. While mutations
altering virus host specificity can involve large-‐scale genomic rearrangements, most virus host
shifts likely entail the modification of a small number of virus receptor amino acid residues [9].
In fact, single nucleotide substitutions are often sufficient to expand a virus’s host range [10]. If
this mechanism of host range expansion were common, the number of host range expanding
mutations and their frequency of appearance would be important parameters governing the
probability of emergence of a potential human pathogen.

52
Few studies have systematically determined the type, number, frequency and fitness
consequences of host range expanding mutations for any particular virus-‐host combination
[11]. Such data can aid the parameterization of evolutionary ecological model of virus
emergence. Factoring in other parameters, such as transmission rates and population densities,
may allow quantitative predictions of the likelihood a particular virus is able to emerge on a
new host. This type of prioritization is critical before allocating resources to interdict potential
pathogenic viruses before they emerge.
Here we use an experimental model system, the bacteriophage (phage) φ6, to
determine number, frequency, fitness and structural consequences of mutations allowing
infection of a novel host. Phage φ6 (family Cystoviridae) is a dsRNA virus with a tripartite
genome divided into Small (2,948 bp), Medium (4,061 bp) and Large (6,374 bp) segments [12-‐
14]. Mutations allowing φ6 to infect novel hosts have been localized to the gene encoding the
P3 protein on the Medium segment [11,15].
Two previous studies have systematically examined φ6 host range expansion [11,15].
Duffy et al. isolated 10 φ6 host range mutants on each of three different Pseudomonas host
strains including Pseudomonas pseudoalcaligenes [15]. Genetic sequencing revealed that all
mutations occurred in the P3 attachment protein. Moreover, the authors reported that 7 of the
9 host range mutations imposed a fitness cost on the canonical host. Ferris et al. isolated 40 φ6
host range mutants, of which 16 contained novel mutations [11]. The authors used a statistical
approach to predict the existence of a further 39 mutations that were missed by their screen. In
addition, they observed broad fitness costs on the canonical host in agreement with Duffy et al.
[11,15].

53
Our study builds on each of these earlier studies in order to present a more complete
picture of host range expansion in φ6. We isolated 69 independent φ6 mutants able to infect
the novel host, P. pseudoalcaligenes, and sequenced the entire P3 gene for each of them in
order to determine the number, location and frequency of host range expanding genotypes. For
a subset of unique mutant genotype, we quantified plaque size on P. pseudoalcaligenes, and
reproductive capacity (fitness) and host attachment rate on canonical host P. phaseolicola and
P. pseudoalcaligenes. Finally, we used protein structural modeling to predict the effects of host
range mutations on P3 attachment protein structure.
Methods and Materials
Study Organisms and Culture Conditions
Cystovirus φ6 used in these experiments is a descendant of the strain originally isolated
from bean straw in 1973 [16]. φ6’s host of isolation was the Gram-‐negative bacterium, P.
syringae pathovar phaseolicola (ATCC # 21781; hereafter PP) [16]. In our study, we used a
nonpermissive host, P. pseudoalcaligenes East River isolate A (hereafter ERA), to isolate φ6 host
range mutants (HRMs). The ERA receptor to which φ6 binds has not been determined, but is
likely the ERA pili. Two other nonpermissive hosts, P. syringae pv. tomato (hereafter TOM) and
P. syringae pv. atrofaciens (hereafter ATRO), were used in some assays. All bacteria and virus
stocks were obtained from Paul Turner, Yale University, New Haven, CT.
All phages and bacteria were propagated in lysogeny broth (LB: 10 g NaCl, 10 g Bacto™
tryptone, and 5 g Bacto™ yeast extract per liter of water) at pH 7. Bacterial cultures were
initiated by transferring a single colony from a streak plate into 10 mL LB in a sterile 50 mL flask

54
capped with a 20 mL beaker. Culture flasks were incubated with shaking (120 rpm) at 25 °C for
18 hours, allowing bacteria to attain stationary-‐phase density (~6 x 109 cells mL-‐1).
Virus Stock Preparation
High-‐titer phage lysates were prepared by adding 1 μL stock lysate and bacteria (200 μL
of PP/ATRO/TOM or 20 μL ERA) to 3 mL top agar (LB with 0.7% Bacto™ agar; stored as liquid at
45 °C, solidifies at 25 °C), and pouring onto 35 mL bottom agar (LB with 1.5% Bacto™ agar) in a
sterile Petri dish. After 24 hours at 25 °C, the resulting plaques were harvested and
resuspended in 4 mL of LB, followed by 10 min centrifugation at RCF = 1400 x g to pellet agar
and bacterial debris. Bacteria-‐free lysates were obtained by filtering the supernatant through a
0.22 μm filter (Durapore®; Millipore, Bedford, MA). Phage particles per mL in the lysates were
quantified via serial dilution and titering. Plaques were counted on plates where 30 -‐ 500
plaques were visible. The number of plaque forming units per mL (pfu mL-‐1) in the original
lysate was obtained by multiplying the number of plaques times the dilution factor. Lysates
were mixed 1:1 (v/v) with 80% glycerol and were stored at -‐20 °C.
Host Range Mutant Frequency
The frequency of HRMs in a phage population was estimated by plating a known
number of wildtype φ6 on a lawn of a nonpermissive host and counting the resulting number of
plaques. Each plaque represents the descendants of a single HRM in the parent population. To
perform this assay, a single plaque was picked off a lawn of PP and placed in 1 mL LB. This
mixture was serially diluted and plated on PP to estimate phage pfu mL-‐1. Subsequently, 107 or
(108 for TOM) phages were plated on lawns of the nonpermissive hosts, ERA, TOM and ATRO.
Typically, a φ6 plaque contains ~ 5 x 108 pfu/mL so sufficient phage for plating were easily

55
obtained [17]. Following 48 hrs growth, plaques were counted to estimate the number of
spontaneous HRMs among the descendants of a single phage. This assay was repeated at least
twenty times per nonpermissive host strain. Mutant frequency was calculated by dividing the
number of plaques observed by the initial inocula. The resulting data were analyzed using a
one-‐way analysis of variance (ANOVA) model. A Tukey-‐Kramer honest significant difference test
was applied post-‐hoc to ascertain significant differences among mean mutant frequencies on
each host type.
Host Range Mutant Isolation
Each HRM was isolated independently to minimize bias due a “jackpot effect” where
multiple descendants of the same mutational event appear in a population [18]. A φ6 lysate
was serially diluted and plated on a PP lawn such that only a few widely spaced plaques
appeared on the bacterial lawn. A single plaque was picked at random and placed in 1 mL LB.
After vortexing, 100 µL of the plaque suspension was added to 20 µL ERA and 3 mL top agar and
plated. Following 48 hrs growth, a single HRM plaque was picked from the ERA lawn and
suspended in 500 µL LB. 10 µL from this solution was plated on an ERA lawn to obtain phage
lysate for RNA extraction. 500 µL 80% glycerol was added to the remainder which was then
stored at -‐20 °C. This protocol was repeated 69 times to obtain 69 independent HRM isolates.
RNA Extraction and Sequencing
To sequence the region of the Medium segment encoding the P3 protein, 3 mL phage
lysate from each mutant was concentrated by centrifuging at RCF = 100,000 x g for 3 hrs at 4 °C
using a Beckman TL-‐100 ultracentrifuge. The supernatant was discarded and the pellet was
resuspended in 150 µL nuclease-‐free water. RNA was extracted using a QIAamp® Viral RNA Mini

56
Kit (QIAGEN, Valencia, CA). Phage RNA was reverse transcribed using random hexamer primers
and Superscript® III reverse transcriptase (Life Technologies, Grand Island, NY), and the resulting
cDNA was used as template for PCR. Three sets of oligonucleotide primers corresponding to
bases 1298–2142, 2042–3052, and 2877–3873 of φ6’s Medium segment were used for the PCR
amplification of the region encoding the P3 host attachment protein. PCR product was purified
for sequencing using ExoSAP-‐It® (Affymetrix, Santa Clara, CA). PCR product was sequenced in
both directions with a minimum of 3-‐fold replication (6-‐fold coverage). Sequencing was
performed at the DNA Analysis Facility on Science Hill at Yale University. Sequence data were
analyzed using Geneious Pro Ver. 5.4 [19] and MEGA Ver. 5.05 [20]. Chromatograms were
verified via MacVector Ver. 12.5.1 bioinformatics software.
Mutant Characterization
We phenotypically characterized HRM genotypes by determining plaque size on ERA,
and by assaying reproductive capacity (fitness) and attachment rates on ERA and PP. Of our
unique mutant genotypes, we did not assay the three mutants whose mutations were not
identified (see Table 1). Furthermore, we only assayed one mutant in situations where
differences between genotypes were attributable to synonymous amino acid substitutions.
Finally, for one mutant, stored frozen lysate degraded due to a freezer failure following
sequencing, and viable phage could not be recovered for phenotypic characterization. In sum,
we phenotypically characterized 13 of our 17 unique HRM genotypes.
Plaque Size Estimates
Plaque sizes for 13 HRM genotypes were estimated from digital photographs of plaques
formed on ERA. All LB plates used for plaque assays were poured at the same time and were

57
weighed to maintain consistency. Each mutant’s lysate was diluted and plated such that
between 20 to 100 plaques formed on the ERA lawn after 48 hrs growth. Digital photographs
were taken using a Kodak Gel Logic® 440 digital imaging system. ImageJ software (NIH,
Bethesda, MD; http://rsb.info.nih.gov/ij/) was used to estimate the total area of the plaque. For
each genotype, at least 35 plaque size estimates were made across 3 plates.
Mutant Fitness on Native and Novel Hosts
We assayed absolute fitness for 13 of our φ6 mutants and the wildtype on native and
novel hosts using traditional plating methods. Here 105 phages were added to 3 x 108 host cells
in 10 mL LB and incubated at 25 °C with rotary shaking (120 rpm) for 24 hrs. All assays were
replicated 5x. Bacteria-‐free lysates were obtained by centrifuging 3 mL culture at RCF = 2.75 x g
for 10 min to pellet bacterial debris, then passing the supernatant through a 0.22 µm filter.
Phage particles per mL in the lysates were quantified via serial dilution and titering on host
lawns. For each assayed mutant genotype, we estimated absolute fitness using the
equation,𝑊𝑊 = 𝑙𝑙𝑙𝑙 "8"9
, where Ni is the starting number of phage and Nt is the total number of
progeny phage produced during the infection period.
Attachment Rate Assays
The rate of attachment to native and novel hosts of 13 mutant HRM genotypes was
measured using a centrifugation method. This method relies on the fact that, following
centrifugation, attached phages are pelleted with host cells, while unattached phages remain in
the supernatant. The decline of unattached phage over time is quantified to give the rate of
phage attachment to host cells. In this assay, 103 phages, which were titered on the same host
as the assay host, were mixed with 5 × 109 exponentially growing host cells in 10 mL LB with 3-‐

58
fold replication. The mixture was incubated with orbital shaking for 40 min. Immediately after
mixing and every 10 min thereafter, a 1 mL sample from the mixture was centrifuged (RCF =
1,700 x g) for 1 min to pellet the cells then 100 µL from the supernatant was plated on a PP
lawn. The attachment rate constant (k) is calculated as
where Ni is the total number of phage added, Nf is the number of unattached phage, C is the
concentration of bacteria, and t is the incubation time in minutes.
Attachment Protein P3 3D Structure Prediction
Three-‐dimensional structures of canonical bacteriophage φ6 ancestral strain P3
attachment protein was predicted by homologue modeling based on nucleotide sequences
submitted to the online I-‐TASSER server [21,22]. I-‐TASSER generates three-‐dimensional (3D)
atomic models from multiple threading alignments and iterative structural assembly
simulations.
Results
Mutation Frequency
φ6 HRMs were readily isolated by plating wildtype phages on lawns of the
nonpermissive hosts, ERA, TOM and ATRO. The frequency in which ERA-‐infective HRMs
appeared in populations of φ6 phages was 1.15 x 10-‐6 (n = 21, SD ± 5.219 x 10-‐7). This value is
only slightly lower than φ6’s spontaneous mutation rate, 2.7 x 10-‐6 [23]. Rates on TOM and
were 1.39 x 10-‐7 (n =21, SD ± 1.489 x 10-‐8) and 4.45 x 10-‐7 (n =21, SD± 1.001 x 10-‐7) respectively.
We conducted a one-‐way ANOVA on log10 mutant frequency with bacterial host strain as a
CtNiNf
k)ln(
=

59
factor, and found significant differences in mutant frequency across different host strains (F =
198.54, DF = 2, P < 0.0001). A Tukey-‐Kramer post hoc test with α = 0.05 revealed that all
compared means were significantly different from each other. Interestingly, HRMs appeared
more readily on the phylogenetic outgroup P. pseudoalcaligenes ERA than they do on other
conspecific P. syringae pathovars such as P. syringae atrofaciens and P. syringae tomato [24].
We found at least 17 unique genotypes among the 69 ERA HRMs isolated and partially
sequenced (Table 1). Three HRMs had no mutations in the sequenced region of the genome,
thus we count them as, at a minimum, one unique genotype. Out of a combined 78 identified
mutations from three studies, the majority resulted in nonsynonymous substitutions in the P3
amino acid sequence. Only 2 synonymous substitutions were identified (Table 1). This result
conforms to Duffy et al.’s report of only 1 synonymous substitution among 31 mutations [15].
Synonymous substitution frequencies were similar among the two studies (2.5% vs. 3.2%).
Mutation Substitution Frequency
Among all nucleotide substitutions identified by our screen, the estimated
transition/transversion bias R was 1.90. At the 8th residue, at least 5 possible substitutions
(G22A, A23G, A23C, G24T and G24C) allow infection of ERA. However, of all substitutions
observed, the majority were transitions (51 vs. 9). These results suggest that host range
mutations allowing infection of ERA are heavily biased towards transitional substitutions. The
significance of this finding is not clear, and may simply be a consequence of spontaneous
deamination.
87% (60 of 69) of all mutants possessed a mutation at the 8th amino acid residue in the
P3 protein. Only 9 mutants (4 single, 1 double, 1 triple and 3 unknowns) did not show a

60
mutation at the 8th residue. This imbalance is higher than was observed in Duffy et al.’s study,
where only 14 of 30 mutants isolated on ERA possessed mutations at the 8th residue [15].
However, we note that Duffy et al.’s study did not control for the “jackpot effect” and 20 of 30
mutants were isolated from hosts other than ERA. The dominance of a single residue is not
unprecedented. Ferris et al. reported that 12 of 40 mutants isolated on P. glycinea showed a
mutation at the 554th residue [11]. Across all 3 studies and 5 different hosts, there seems to be
3 “hotspots” for host range mutations in φ6. 85.4% of all amino acid substitutions occurred
close to the 8th (54.7%), 138th (16.7%) and 544th (14%) residues (Fig. 1A; Table 2).
Phenotypic Change Analysis
Changes in mass, electrical charge and hydrophobicity presumably can alter host
receptor binding by changing the protein’s tertiary structure and altering protein-‐protein
interactions. In Table 3, we compiled the phenotypic characteristics of all amino acid
substitutions allowing infection of ERA observed in this study and in Duffy et al. [15]. Using this
data, we performed a paired t-‐test on amino acid mass for each mutation with strain type
(wildtype or mutant) as a factor. Both factors had significant effects on amino acid mass.
Substituted amino acids in mutants had significantly less mass than the original amino acids in
the wildtype strain (t = 6.73, DF = 77, P < 0.0001). This effect was most pronounced in mutation
hotspots (F = 7.25, DF = 71, P < 0.0001). Perhaps lower mass substitutions permit greater
flexibility at the host binding site.
Electrostatic interactions between host and phage proteins are most likely the basis of
phage attachment. If so, we expect that charge changes incurred by host range mutations
should be consistently in the same direction. A Χ2 test was used to determine whether chemical

61
properties of substituted amino acids differed significantly from the random expectation based
on the amino acid composition of the P3: 9.16% acidic, 8.69% basic, 24.53% hydrophilic, and
57.45% hydrophobic. We found that mutant amino acids were significantly more likely to be
basic or hydrophilic than expected by chance (Χ2 = 110.008, DF = 3, P < 0.0001). Furthermore,
the frequency of mutations occurring at acidic residues was disproportionately high (81/106 or
76%). Ferris et al. also observed a greater than expected number of loss of charge mutations
[11]. We speculate that these chemical changes make the P3 protein's host-‐binding site more
permissive for binding host receptors.
P3 3D Structure Prediction
Little is known regarding how host range expanding amino acid substitutions affect
phage attachment protein structure. We used I-‐TASSER
(http://zhanglab.ccmb.med.umich.edu/I-‐TASSER) [21,22] and DAS modeling software
(http://www.sbc.su.se/~miklos/DAS/maindas.html) [25] to predict structural features of the P3
protein. I-‐TASSER generates three-‐dimensional atomic models from multiple threading
alignments and iterative structural assembly simulations based on homology to solved
structures (Fig. 1B). The predicted model’s confidence score (C-‐score) was -‐2.12, which is
intermediate confidence where scores range from high (2) to low (-‐5) confidence. When
predicting known structures, and using a C-‐score cutoff > -‐1.5 for the models of correct
topology, both false positive and false negative rates are below 0.1 [21]. While our C-‐score did
not meet this threshold, we are confident that the probability of an incorrect structure is still
low. Our view is supported by the ability of the predicted structure to provide a biologically
plausible interpretation of the mechanistic basis of host range expansion.

62
DAS modeling software predicts transmembrane protein segments based on low-‐
stringency dot-‐plots of query sequences against a collection of non-‐homologous membrane
proteins using a previously derived, scoring matrix. Although P3 is soluble [26], DAS predicted a
21 amino acid hydrophobic membrane-‐interactive domain at residues 271 to 291. Based on the
fact that, on the predicted structure, this domain extends out from the P3 core (Fig. 1B), we
venture that domain likely anchors the P3 protein to the integral membrane protein P6 [27],
thus we will refer to it as the hydrophobic anchoring domain, or HAD. All host range mutations
occurred on the face opposite the HAD, suggesting that the opposite surface binds the host
receptor, and that mutations in this region allow infection of novel hosts. However, this
hypothesis assumes that amino acid substitutions do not substantially alter the protein shape,
and that residues on this face in the ancestor would remain on this face in the mutant. Figure
1C suggests our conjecture is valid as the E8G mutant’s predicted structure does not show
major structural rearrangements compared to the wildtype. Interestingly, the most common
host range mutations found in our study alter the surface charge at this location from negative
to neutral, hinting at a proximate mechanism for host range expansion (Fig. 1C).
Plaque Size
We isolated HRMs by visually identifying and picking plaques off lawns of the
nonpermissive host, ERA. Our results showed that host range mutations were heavily biased
towards the 8th residue. One possible criticism of our mutant isolation process is that it may
have been biased towards certain mutations simply because these mutants formed larger
plaques that were more likely to be spotted by the sampler. To test this hypothesis, we
determined from digital photographs the average plaque size for 13 of 17 of our identified

63
HRMs. Mean plaque size for our mutants ranged from 3.5 to 10.3 mm2 (Table 4). We performed
an ANOVA of mean plaque size with mutant frequency as a factor, and the results confirmed
that plaque size did not predict mutant frequency. While we did find significant differences in
plaque size among genotypes, the two most frequent genotypes found by our study (E8K and
E8G) ranked 4th and 9th respectively in mean plaque size. These data imply that mutant
sampling was not biased. Furthermore, we did not observe any correlation between fitness and
plaque size.
Mutant Fitness on Original and Novel Hosts
The fitness consequences of host range expanding mutations will play a large role in the ability
of these mutants to persist in host populations [28]. With this in mind, we estimated the
absolute fitness of 13 of our mutant genotypes on the canonical host, PP, and the novel host,
ERA (Table 4). A one-‐way ANOVA of absolute fitness with strain as a factor revealed significant
differences among strain fitness on both ERA (Fig. 2A; F = 40.64, DF = 12, P < 0.0001 ) and PP
(Fig. 2B; F = 3.515, DF = 12, P = 0.0008), but mean fitness on ERA was not correlated with mean
fitness on PP nor was fitness on ERA correlated with the number of mutations a mutant
possessed. In fact, genotypes containing multiple mutations tended to be less fit than those
with single mutations, although this trend was not significant. Matching previous results, fitness
on PP was, in all but once case, less than that of the ancestor [11,15]. These results are
indicative of antagonistic pleiotropy, implying a tradeoff in fitness between infection of PP and
ERA. In addition, the coefficient of variation (i.e. standard deviation/mean; CV) in mutant
fitness was considerably greater on ERA as opposed to PP (CV: 0.402 versus 0.015). This

64
suggests that mutations expanding the host range have a much wider range of fitness effects
on the novel host.
Attachment Rate
Bacteriophages initiate infections of host cells by binding to receptors on the surface of
the bacterial outer membrane. As such, the host attachment rate is a critical factor in the
ecological success of a phage. We measured the rate of phage attachment to the original and
novel hosts for 13 mutant genotypes (Table 4). A one-‐way ANOVA of the rate of attachment to
ERA with mutant genotype as a factor revealed significant differences among the strains (F =
10.17, DF = 12, P < 0.0001). In addition, we regressed attachment rate against mutant fitness on
ERA to determine if the two were correlated. Since our HRMs most likely differ only by
mutations in the P3 host attachment protein, we expected that improved attachment would
lead to increased fitness. Indeed, for 13 mutant strains whose fitnesses and attachment rates
were estimated, fitnesses on ERA were correlated with ERA attachment rates (Fig. 3; F = 11.91,
DF = 1, P = 0.0062). However, the linear regression model accounted for roughly half of the
variance in attachment rate (R2 = 0.54). These results are not surprising given the difficulty of
precisely estimating the φ6 attachment rate. Nevertheless, the results conform to our
expectation of a positive correlation between fitness and host binding ability. By contrast,
attachment rates of the various mutants to PP were not significantly different, nor were they
correlated with mutant fitnesses on this host. These results might be expected given the
relatively narrow range of fitness differences on PP (Fig. 2; Table 4).
The rates of attachment to PP were significantly greater than attachment rates to ERA
(One-‐way ANOVA: F = 216.7, DF = 1, P <0.0001). This latter result matches expectations since

65
mutant fitness on PP is approximately an order of magnitude greater than that on ERA [17].
Presumably, the switching of receptor types and the lack of adaptation to an ERA receptor may
account for the significant differences in mutant fitness on the different host types. However,
attachment rates to PP and ERA were not correlated, implying that mutations that increase
binding to ERA do not necessarily increase or decrease binding to PP.
Data Availability
All genetic sequences were deposited with Genbank (Accession numbers KF027227 -‐
KF027297). Data files are available via datadryad.org .
Discussion
Φ6 Host Range Mutation Frequency
Understanding the genetic basis of virus host range expansion is critical to predicting the
emergence of potentially dangerous viruses. The genetic distance a virus must cross to gain the
ability to infect a novel host may be a dominant factor determining the probability of
emergence. Not all viruses readily infect novel hosts [29]. For example, many
mycobacteriophages isolated on Mycobacterium smegmatis are unable to infect M.
tuberculosis, even when large numbers of phage are plated [30]. Presumably infection of M.
tuberculosis requires several simultaneous mutations or even the recombination of whole
genes or gene systems. By contrast, many viruses are able to infect novel hosts via single
nucleotide substitutions [10,31-‐36]. This minimal genetic distance can easily be traversed
because viral population sizes and mutation rates allow them to search available sequence
space rapidly. The phage φ6 is an excellent model to study virus emergence via single

66
nucleotide substitutions because such HRMs are easily isolated, sequenced, and characterized
in the laboratory [11,15].
In this study, we found that φ6 HRMs appear on ERA at a rate (1.17 x 10-‐6) slightly lower
the estimated φ6 mutation rate of 2.7 x 10-‐6 per nucleotide per generation. Thus our figure
seems somewhat low given that there are multiple possible mutations allowing host range
expansion in the φ6 genome (Table 1). However, Chao et al.’s estimate was derived from the
frequency of revertants from an amber mutation (sus297), and it was assumed that there was
only one way to revert [23]. If there are multiple ways to revert from Chao’s et al.’s amber
mutation, then theirs is an overestimate of the mutation rate. Moreover, Chao et al. estimated
the mutation rate at a single locus, but the mutation rate may vary across the genome [37,38].
At any rate, it is clear that, given their potentially enormous population sizes, φ6 HRMs can be
isolated relatively easily.
Our results indicate that there is considerable variation in the ability of φ6 to mutate to
infect nonpermissive host strains. While there are certainly strong coarse-‐grained trends in
infectivity, e.g., φ6 seems mainly restricted to the pseudomonads [39], infectivity within this
group is currently unpredictable. Phage φ6 is better able to mutate to infect P.
pseudoalcaligenes ERA, a distant relative of P. syringae pv phaseolicola [24], than two
pathovars from the same species, P. syringae pv tomato and P. syringae pv atrofaciens [40].
Duffy et al. and Cuppels et al. found many examples of other P.syringae pathovars
nonpermissive for φ6 even at high plating densities [15,39]. For example, Duffy et al. were
unable to isolate HRMs on at least 8 P. syringae pathovars despite plating over 1010 φ6 phages
on each pathovar [15]. Similar results were obtained by Cuppels et al. [39]. It would appear

67
that phylogeny is a poor predictor of infectivity at least at the fine scale level within the
pseudomonads. φ6’s ability to expand its host range appears to be somewhat idiosyncratic,
which is to be expected given myriad possible outcomes for parasite-‐host coevolution [41]. It
may be that the P. syringae strains have experience recent coevolution with φ6 or its close
relatives, and thus have acquired resistance to infection to these phages.
The frequency of mutants lacking mutations in the P3 (4.3%) was similar to that found in
Ferris et al.’s study (2.5%) [11]. These results provide strong evidence that the P3 sequence is
the primary, but not exclusive, determinant of host range among phage φ6 [42]. While it is
tempting to speculate that additional host range mutations might be found in membrane fusion
protein P6, Duffy et al. sequenced the P6 for 30 φ6 HRMs and found no mutations [15]. As of
publication, no other candidate genes for host range expansion on ERA have been explicitly
identified in φ6; however one study has reported that a mutation allowing infection of ERA was
localized to the large segment [43]. This segment contains a gene encoding an RNA-‐dependent
RNA polymerase and genes associated with RNA packaging and procapsid assembly [12].
The number of ways a virus can mutate to infect a novel host is an important parameter
in predicting its potential for emergence [44]. Using a method based on the coupon collector’s
problem of statistical theory, Ferris et al. estimated the total number of possible mutations that
allow φ6 to infect a novel host, P. glycinea [11]. The coupon collector’s problem can be
informally stated as: Given n coupons, how many coupons will need to be sampled before each
coupon is observed at least once [45]? One assumption of the coupon collector’s problem is
that all coupons are equally likely. This assumption does not hold for genetic mutations as some
types are more likely than others are. Ferris et al. accommodate this simplification by adjusting

68
the equation to account for differences in the probabilities of transitions and transversions.
Since they found 19 distinct genotypes among their 40 independent samples, they estimated
that further sampling would uncover an additional 36 mutations [11]. If Ferris et al.’s estimates
are correct, it would mean that 1.3% of all possible nonsynonymous substitutions in P3 confer
the ability to infect ERA (i.e., 55 of 4,380 potential nonsynonymous changes expand host
range).
Although their HRMs were isolated on a different host, P. glycinea, both their study and
ours found similar frequencies of transitions among all mutations (90% in Ferris et al., 84% in
our study). However, out of 69 HRMs, we found only 17 distinct genotypes. Ferris et al. isolated
almost the same number of distinct genotypes in half as many samples [11], which may be a
consequence of the different hosts of isolation. Since Duffy et al. observed 10 unique genotypes
out of 30 isolates (33% unique) [15] and we observed at least 17 unique genotypes out of 69
(26% unique), the implication is that more unique genotypes would be found with further
sampling. However, a closer inspection of our data suggests otherwise. 8 of 17 of our unique
genotypes were only unique because of second-‐ or third-‐site mutations. If we consider only
those mutations that are sensu stricto necessary for infection of ERA, we only find a combined
13/99 (13%) unique genotypes among our and Duffy et al.’s study [15]. In fact, we only found 3
unique sensu stricto substitutions not found by Duffy et al. study and they found 6 not
identified in ours.
If 1.3% of all possible nonsynonymous substitutions allowed φ6 to infect ERA, we would
expect to see more unique genotypes among our isolates. Our results also indicate that some
mutations occur far more frequently than expected by chance even if differences in transitions

69
and transversions are accounted for. One possibility is that low fitness HRMs are eliminated by
within plaque selection and consequently are not represented in the mutant collection
sampled. We have no means to ascertain the validity of this hypothesis at this point, but it
could be an interesting question to approach by deep sequencing of single HRM plaques.
However, at the same time, it seems likely that additional factors that are not currently well
understood, such as RNA structure, codon bias and variation in the mutation rate across the
genome, influence the probability of mutation at any particular locus. Nonetheless, Ferris et
al.’s method is a valuable step forward towards the estimation of an important parameter
relevant to virus emergence.
Mutation Hotspots
We found that mutations expanding the host range of phage φ6 were more likely to
appear in certain regions of the P3 gene than others. Such mutation hotspots are have been
observed among virus drug resistance [46-‐48], host range [49,50], hemagglutinin [51], capsid
[52], and core antigen genes [53] among others. Mutation hotspots are evidence of strong
positive selection for substitutions that provide an adaptive advantage in a particular
environment [54,55]. Growth on a novel host should impose strong positive selection for
nonsynonymous substitutions at loci associated with host range expansion. Thus, we can use
the frequency of mutations found in our survey to identify regions of the P3 protein that are
important in attachment to a host receptor. 85.4% of all mutations identified by our study and
by Duffy et al. [15] were found in just three regions (near 8th, 133rd and 554th residues) of the P3
gene (Fig. 1A; Table 2). We venture that these hotspots on the P3 protein are important in host
range determination among φ6 phages.

70
Structural Speculations
We used the structural modeling software I-‐TASSER [21,22] to predict the structure of
the P3 protein from its amino acid sequence. The resulting structure showed homology to
bacterial alcohol dehydrogenase quinoproteins [56-‐58]. Interestingly, in the best-‐fit model, our
putative mutation hotspots were located close together on one face of the ~60 Å diameter P3
protein (Fig. 1B). Residues 8 and 130 were located at the surface 18 Å from each other, and
residue 554 was located subsurface about 15 Å from residue 8 and 23 Å from residue 130.
Other less frequently observed mutations also occur near this region (Fig. 1B). We propose that
this region of the P3 protein is a host-‐binding domain and directly interacts with host receptors.
This supposition is supported by the fact that the host binding domain is diametrically opposite
the hydrophobic anchoring domain (residues 271-‐291) predicted by DAS (Fig. 1B). The most
parsimonious explanation is that this domain serves to anchor the P3 to the integral membrane
protein P6 [27], which leaves the putative host binding domain exposed to the environment.
Mutations allowing infection of ERA may not significantly alter the tertiary structure of
the P3 protein. I-‐TASSER structural modeling did not show any major structural rearrangements
in predicted structures for mutant strains. Rather mutations may alter the host-‐binding
domain’s electrical charge from negative to positive or neutral (Fig. 1C). This difference in
electrical charge may allow mutant φ6 to bind the ERA host receptor. The presumptive ERA
receptor is its pilus, but this has not been definitively determined. If the ERA receptor were
indeed the pilus, it would be interesting to know if its electrical properties are appreciably
different than those of the pilus of PP. Moreover, it is plausible that neutral or positive electric
charges and smaller mass amino acids confer more flexibility to the binding region, allowing a

71
greater variety of structures to be bound [59] It would be interesting to determine if host range
expanding mutations more frequently result in the substitution of small for large amino acids or
alter the charge of the binding site.
Fitness on Native and Novel Hosts
Fitness on native and novel hosts was assessed using standard flask productivity assays.
Phage φ6 HRMs showed a broad range of fitness values on ERA, some of which were
significantly different from the others (Fig. 2A). Mutant fitnesses on the native host, PP, were
much greater than those on ERA (Fig. 2B). Since φ6 is presumably well adapted to native but
not novel hosts, these results meet our expectations. Supporting these results, we found that
the coefficient of variation (CV) of mutant fitnesses on PP was much lower than CV of mutant
fitnesses on ERA. These results conform to theoretical expectations that there should be less
variation in fitness values close to a fitness peak on an adaptive landscape [60]. Directional
selection should erode the variation in fitness as a population increases in fitness in a particular
environment. Thus, a virus that is adapted to a particular host should have lower variation in
fitness on that host as opposed to a host to which it is not well adapted.
We found that, in concert with previous studies [11,15], mutations expanding the φ6
host range usually reduced fitness on the original host, PP. On average, HRM fitness on PP was
reduced about 2.5% compared to the wildtype. Negative genetic associations between host
types is an example of antagonistic pleiotropy [61,62]. The adage that “a jack of all trades is a
master of none” is well supported, at least among φ6 host infections. However, the ultimate
cause of host specialism or generalism remains opaque. Intuitively one would imagine that a
broader host range would produce greater returns than a narrow one as long as the reduction

72
in productivity on a single host was offset by an increase in overall productivity [63]. With
regard to the present system, it seems unlikely that the relatively minor cost in fitness on the
original host imposed by host range expansion should outweigh the benefits of an expanded
host range. Moreoever, we isolated one mutant (S28) whose fitness on the canonical host
actually increased following the acquisition of a mutation permitting infection of ERA. Why then
are broad host range phages relatively rare? The rarity of generalism may be a result of the
interaction of widespread habitat patchiness, reduced dispersal and the ubiquity of local
adaptation [64]. If these general trends hold, competition within a patch should favor the
evolution of specialism. This hypothesis should be amenable to testing via experimental
evolution studies.
As a general rule, we might expect that novel hosts will present a greater challenge to
virus reproduction than native hosts, a conclusion that is supported by many examples in the
literature [65-‐67]. Novel hosts may represent ecological sinks, defined as habitats where the
basic reproductive rate is < 1. Our fitness results support this conjecture, and suggest that φ6
probably experiences a broader range of sink conditions on ERA than it does on PP.
Consequently, φ6 population extinction is more likely in a habitat populated by ERA than one
populated by PP [17]. Given the many HRM genotypes over a broad range of fitness values, φ6
should be a valuable system to test hypotheses regarding virus emergence [44].
Attachment to Native and Novel Hosts
With the exception of the three non-‐P3 mutants, the mutant strains are most likely
isogenic outside the host attachment protein region. The differences in fitness are expected to
result mainly from differences in binding efficiency to the host receptor. Our results indicate

73
that different suites of mutations had highly divergent attachment rates and fitnesses on the
novel host (Fig. 2 and 3). Nonetheless, a regression of phage fitness on ERA against attachment
rate to ERA revealed a significant positive correlation. Ferris et al. reported a similar result for
φ6 infecting P. glycinea [11]. These results make intuitive sense as mutants that are better able
to bind to the host are expected to reproduce at a higher rate. Moreover, attachment to ERA
was significantly lower than to PP, which is also reflected in the large differences in fitness.
Implications for Disease Emergence
This study and other recent studies of φ6 host range expansion suggest several
generalizations. First, phylogeny may only allow relatively coarse-‐grained predictions of virus
host range. Phage φ6’s ability to mutate to infect close relatives was frequently worse than its
ability to infect distant relatives. Second, nonsynonymous substitutions allowing host range
expansion may occur at hotspots in the host attachment protein. This prediction makes
intuitive sense as host attachment relies on binding affinity between host and virus proteins. In
addition, many host range-‐expanding mutations may not result in large structural
rearrangements in host attachment proteins. Rather, amino acid substitutions may result in
more subtle changes in protein surface charges, allowing binding to different host proteins.
Furthermore, the number of nonsynonymous substitutions allowing host range expansion is
probably relatively small considering the number of possible substitutions. Nonetheless, the
relatively high virus mutation rate allows viruses to rapidly acquire host range expanding
mutations despite their relative rarity. Finally, initial fitness on a novel host is usually much less
than that on the original host, and antagonistic pleiotropy among host range mutations is

74
common. This generalization conforms to our expectations since evolutionary tradeoffs in
different habitats are anticipated to be ubiquitous.
Acknowledgements
We thank Paul Turner for providing phage and bacterial strains and Tim Short for technical
advice. Constructive criticism from Paul Gottlieb and three anonymous reviewers was much
appreciated. This work was completed in part using equipment in the Core Facility for Imaging,
Cellular and Molecular Biology at Queens College.

75
Figure Legends
Fig. 1 – Spatial models of φ6 P3 protein mutants
Panel A: Three host range mutation hotspots (accounting for 86% of all mutations) are
highlighted in this linear representation of the 648 amino acid sequence of the φ6 P3 gene. The
remaining 14% of mutations are not shown. Panel B: Space-‐filling representations of the φ6 P3
protein are shown as predicted by I-‐TASSER. Colored regions correspond to the mutation
hotspots depicted in Panel A. A putative hydrophobic anchoring domain (HAD) is shown in
orange. In our model, the hydrophobic anchoring domain penetrates φ6’s outer lipid
membrane to bind inner membrane protein P6. Panel C: Surface electrical charges of E8G
mutant contrasted with ancestor. Space-‐filling representations showing predicted surface
electrical charges for the φ6 E8G host range mutant and its ancestor were estimated using I-‐
TASSER. Positively-‐ and negatively-‐charged regions are depicted in blue and red respectively.
Arrows indicate the predicted location of the mutated 8th residue. The most prominent
difference between the mutant and the ancestor is the greater surface positive charge at the
presumed host binding domain.
Fig. 2 – Mutant absolute fitness on canonical and novel hosts
Panel A: Absolute fitness of 13 φ6 host range mutants on the novel host, ERA. Each point is the
mean of 5 replicate measurements of fitness. Bars are ±1SE. Panel B: Absolute fitness of 13 φ6
host range mutants on the canonical host, PP. Each point is the mean of 5 replicate

76
measurements of fitness. Fitness of wildtype φ6 is shown by the dotted line for comparison.
Bars are ±1SE.
Fig. 3 – Mean ERA attachment rate (k) is plotted against phage φ6 fitness on ERA
Attachment to ERA was correlated with fitness on ERA for φ6 host range mutants. Each point is
the mean of 3 replicate measurements. Dotted lines show 95% confidence intervals.

77
Figures
Fig. 1 – Spatial models of φ6 P3 protein mutants

78
Fig. 2 – Mutant absolute fitness on canonical and novel hosts

79
Fig. 3 – Mean ERA attachment rate (k) is plotted against phage φ6 fitness on ERA

80
Table 1. Phage φ6 Mutations Allowing Infection of Novel Host ERA In this table, the columns show the nucleotide and amino acid substitutions found it all 69 ERA host range mutants. Rows show unique genotypes. N is the number of mutants with a particular genotype. The penultimate row shows number of times a given mutation appears among all mutants. The last row shows the frequency in which one amino acid residue is replaced by others in amino-‐acid sequences of the same protein from different organisms [59]. Mutations at the same amino acid residue are shaded together. Transitions are indicated by closed squares, transversions with open squares, synonymous substitutions with open circles and unknown changes with a filled triangle. Nucleic Acid
Amino Acid
N
G22A
E8
K
A23G
E8
G
A23C
E8
A
G24T
E8D
G24C
E8
D
T137C
F46S
T138A
F46L
T177C
D59D
A3
89G
Q13
0R
T481C
S161
P
C896G
S299
W
C1588T
L530
L
A1661G
D5
54G
A1661C
D5
54A
C1663T
L555
F
Non
P3
Single (n=56)
20 n 24 n 4 ☐ 3 ☐ 1 ☐ 1 n 2 n 1 ☐
Double (n=8)
1 n n 1 n n 1 n n 1 ☐ ☐ 3 n n
1 n ☐ Triple (n=2)
1 n n ¢ 1 ¢ n n
Unknown 3 ▲ Total #
Observed 69 23 28 5 3 1 1 1 1 7 1 1 1 3 1 1 3
Petsko Frequency — 10 11 27 83 83 4 17 — 12 27 2 — 16 15 17 —

81
Table 2. Host Range Substitution Hotspots in φ6 P3 Proteina Amino acid substitutions close together in the primary sequence are grouped together. We combine data from this study with two other studies of φ6 host range expansion. N is total number of times a substitution was observed across all studies. Frequency is percentage of total substitutions a particular substitution was observed. Combined frequency is percentage of total substitutions constituted by substitutions in a particular region of the primary sequence. Others category includes substitutions found outside substitution hotspots.
Substitution This Study Duffy Ferris N Frequencyb Combined
Frequencyb G5S 2 2 1.3%
54.7% E8K 23 4 1 28 18.7% E8G 28 9 5 42 28% E8D 4 4 2.7% E8A 5 1 6 4%
Q130R 7 7 4.7%
16.7% A133V 9 9 6.0% D145G 3 3 2.0% N146S 6 6 3.9% D533A 1 1 0.7%
14.0%
D535N 1 1 0.7% D554G 3 1 8 12 8.0% D554A 1 1 2 1.3% D554V 1 1 0.7% D554N 2 2 1.3% L555F 1 1 2 1.3% Others 9 6 7 22 14.7% 14.7% Total 81 30 39 150 100% 100%
aData compiled from this study, Duffy et al. 2006 and Ferris et al. 2007 [11,15]. bSome frequencies rounded off to nearest tenth percent.

82
Table 3. Amino Acid Substitutions Associated with φ6 Host Range Expansion on ERA Amino acid substitutions found to allow φ6 infection of Pseudomonas pseudoalcaligenes ERA from data obtained by two separate studies. N is number of times substitution was observed across the two studies. Average mass is residue weight of the original and substituted amino acid [68]. Hydrophobicity index was obtained from Kyte and Doolittle [69]. Negative numbers are more hydrophilic; positive numbers are more hydrophobic.
Amino Acid Substitution TSa Dub N
Average Mass Electrochemical Properties
Hydrophobicity Index
Wildtype Mutant Wildtype Mutant Wildtype Mutant Glutamic Acid to
Lysine (E8K) 23 4 27 129.1 128.2 Acidic (-‐)
Polar Basic (+) Polar -‐3.5 -‐3.9
Glutamic Acid to Glycine (E8G) 28 9
37 129.1 57.1 Acidic (-‐)
Polar Neutral Nonpolar -‐3.5 -‐0.4
Glutamic Acid to Aspartic Acid (E8D) 4 4 129.1 115.1 Acidic (-‐)
Polar Acidic (-‐) Polar -‐3.5 -‐3.5
Glutamic Acid to Alanine (E8A) 5 1 6 129.1 71.1 Acidic (-‐)
Polar Neutral Nonpolar -‐3.5 1.8
Aspartic Acid to Alanine (D35A) 2 2 115.1 71.1 Acidic (-‐)
Polar Neutral Nonpolar -‐3.5 1.8
Phenylalanine to Serine (F46S) 1 1 147.2 87.1 Neutral
Nonpolar Neutral Polar 2.8 -‐0.8
Phenylalanine to Leucine (F46L) 1 1 147.2 113.2 Neutral
Nonpolar Neutral Nonpolar 2.8 3.8
Glutamine to Arginine (Q130R) 7 7 128.1 156.2 Neutral
Polar Basic (+) Polar -‐3.5 -‐4.5
Alanine to Valine (A133V) 9 9 71.1 99.1 Neutral
Nonpolar Neutral Nonpolar 1.8 4.2
Serine to Proline (S161P) 1 1 87.1 97.1 Neutral
Polar Neutral Nonpolar -‐0.8 -‐1.6
Serine to Threonine (S246T) 2 2 87.1 101.0 Neutral
Polar Neutral Polar -‐0.8 -‐0.7
Serine to Tryptophan (S299W)
1 1 87.1 186.2 Neutral Polar
Neutral Slightly Polar
-‐0.8 -‐0.9
Lysine to Threonine(K311T) 1 1 128.2 101.0 Basic (+)
Polar Neutral Polar -‐3.9 -‐0.7
Glycine to Serine (G515S) 1 1 57.1 87.1 Neutral
Nonpolar Neutral Polar -‐0.4 -‐0.8
Aspartic Acid to Glycine (D554G) 3 1 4 115.1 57.1 Acidic (-‐)
Polar Neutral Nonpolar -‐3.5 -‐0.4
Aspartic Acid to Alanine (D554A) 1 1 115.1 71.1 Acidic (-‐)
Polar Neutral Nonpolar -‐3.5 1.8
Leucine to Phenylalanine
(L555F) 1 1 113.1 147.2 Neutral
Nonpolar Neutral nonpolar 3.8 2.8
a = This study; b = Duffy et al. 2006 [15].

83
Table 4 . Phenotypic Characteristics of Bacteriophage φ Mutants. Strain Mutation Frequency
of Mutant Fitness ERA
Fitness PP
Plaque Size ERA
Attachment ERA
Attachment PP
S8 D554G 2 3.31 14.73 6.89 2.58 x 10-‐11 4.79 x 10-‐11 S68 E8D 4 1.97 14.61 5.69 2.22 x 10-‐11 4.72 x 10-‐11 S53 E8A 4 3.97 14.57 7.8 4.80 x 10-‐11 4.68 x 10-‐11 S46 Q130R/S299W 1 0.98 14.80 9.26 1.68 x 10-‐11 5.05 x 10-‐11 S42 S161P/L555F 1 2.20 14.93 9.17 2.40 x 10-‐11 5.31 x 10-‐11 S4 Q130R 1 1.27 14.74 6.28 2.05 x 10-‐11 5.41 x 10-‐11 S30 E8K/Q130R 1 2.51 14.62 3.54 2.49 x 10-‐11 5.71 x 10-‐11 S28 E8A/F46L 1 2.47 15.21 5.94 2.53 x 10-‐11 5.63 x 10-‐11 S26 D554A 1 4.47 14.77 7.85 2.39 x 10-‐11 5.63 x 10-‐11 S154 E8G 24 4.12 14.92 6.29 2.59 x 10-‐11 5.33 x 10-‐11 S14 E8K/D554G 1 2.08 14.79 6.56 2.09 x 10-‐11 4.50 x 10-‐11 S13 E8K/F46S 1 3.16 14.85 10.25 2.24 x 10-‐11 4.60 x 10-‐11 S117 E8K 20 3.91 14.66 8.93 2.51 x 10-‐11 4.39 x 10-‐11 φ6 WT n/a n/a n/a 15.07 n/a n/a 4.91 x 10-‐11

84
References
1. Christensen KLY, Holman RC, Steiner CA, Sejvar JJ, Stoll BJ, et al. (2009) Infectious Disease Hospitalizations in the United States. Clinical Infectious Diseases 49: 1025-‐1035.
2. Armstrong GL, Conn LA, Pinner RW (1999) Trends in infectious disease mortality in the United States during the 20th century. Jama-‐Journal of the American Medical Association 281: 61-‐66.
3. Woolhouse M, Gaunt E (2007) Ecological origins of novel human pathogens. Critical Reviews in Microbiology 33: 231-‐242.
4. Cleaveland S, Laurenson MK, Taylor LH (2001) Diseases of humans and their domestic mammals: pathogen characteristics, host range and the risk of emergence. Philosophical Transactions of the Royal Society of London Series B-‐Biological Sciences 356: 991-‐999.
5. Woolhouse MEJ, Haydon DT, Antia R (2005) Emerging pathogens: the epidemiology and evolution of species jumps. Trends in Ecology & Evolution 20: 238-‐244.
6. Antia R, Regoes RR, Koella JC, Bergstrom CT (2003) The role of evolution in the emergence of infectious diseases. Nature 426: 658-‐661.
7. Gandon S, Hochberg ME, Holt RD, Day T (2012) What limits the evolutionary emergence of pathogens? Philosophical Transactions of the Royal Society of London Series B-‐Biological Sciences 368.
8. Alexander HK, Day T (2010) Risk factors for the evolutionary emergence of pathogens. Journal of the Royal Society Interface 7: 1455-‐1474.
9. Holmes EC, Drummond AJ (2007) The evolutionary genetics of viral emergence. Wildlife and Emerging Zoonotic Diseases: the Biology, Circumstances and Consequences of Cross-‐Species Transmission 315: 51-‐66.
10. Baranowski E, Ruiz-‐Jarabo CM, Pariente N, Verdaguer N, Domingo E (2003) Evolution of cell recognition by viruses: A source of biological novelty with medical implications. Advances in Virus Research, Vol 62 62: 19-‐111.
11. Ferris MT, Joyce P, Burch CL (2007) High frequency of mutations that expand the host range of an RNA virus. Genetics 176: 1013-‐1022.
12. Mindich L, Nemhauser I, Gottlieb P, Romantschuk M, Carton J, et al. (1988) Nucleotide sequence of the large double stranded RNA segment of bacteriophage phi6 -‐ genes specifying the viral replicase and transcriptase. Journal of Virology 62: 1180-‐1185.
13. Mindich L, Nemhauser I, Gottlieb P, Romantschuk M, Carton J, et al. (1988) Nucleotide sequence of the large dsRNA segment of bacteriophage phi6 -‐ genes specifying the viral replicase and transcriptase. Journal of Virology 62: 1180-‐1185.
14. McGraw T, Mindich L, Frangione B (1986) Nucleotide sequence of the small double stranded RNA segment of bacteriophage phi6 -‐ novel mechanism of natural translational control. Journal of Virology 58: 142-‐151.
15. Duffy S, Turner PE, Burch CL (2006) Pleiotropic costs of niche expansion in the RNA bacteriophage Phi 6. Genetics 172: 751-‐757.
16. Vidaver AK, Koski RK, Vanetten JL (1973) Bacteriophage phi6 -‐ lipid containing virus of Pseudomonas phaseolicola. Journal of Virology 11: 799-‐805.
17. Dennehy JJ, Friedenberg NA, Holt RD, Turner PE (2006) Viral ecology and the maintenance of novel host use. American Naturalist 167: 429-‐439.

85
18. Luria SE, Delbruck M (1943) Mutations of bacteria from virus sensitivity to virus resistance. Genetics 28: 491-‐511.
19. Kearse M, Moir R, Wilson A, Stones-‐Havas S, Cheung M, et al. (2012) Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28: 1647-‐1649.
20. Tamura K, Peterson D, Peterson N, Stecher G, Nei M, et al. (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular Biology and Evolution 28: 2731-‐2739.
21. Zhang Y (2008) I-‐TASSER server for protein 3D structure prediction. BMC Bioinformatics 9: 40.
22. Roy A, Kucukural A, Zhang Y (2010) I-‐TASSER: a unified platform for automated protein structure and function prediction. Nat Protoc 5: 725-‐738.
23. Chao L, Rang CU, Wong LE (2002) Distribution of spontaneous mutants and inferences about the replication mode of the RNA bacteriophage phi 6. Journal of Virology 76: 3276-‐3281.
24. Mulet M, Lalucat J, Garcia-‐Valdes E (2010) DNA sequence-‐based analysis of the Pseudomonas species. Environmental Microbiology 12: 1513-‐1530.
25. Cserzo M, Wallin E, Simon I, vonHeijne G, Elofsson A (1997) Prediction of transmembrane alpha-‐helices in prokaryotic membrane proteins: the dense alignment surface method. Protein Engineering 10: 673-‐676.
26. Stitt BL, Mindich L (1983) Morphogenesis of bacteriophage Phi6 -‐ a presumptive viral membrane precursor. Virology 127: 446-‐458.
27. Kenney JM, Hantula J, Fuller SD, Mindich L, Ojala PM, et al. (1992) Bacteriophage phi 6 envelope elucidated by chemical cross-‐linking, immunodetection, and cryoelectron microscopy. Virology 190: 635-‐644.
28. Dennehy J (2009) Bacteriophages as model organisms for virus emergence research. Trends in Microbiology 17: 450-‐457.
29. Ayora-‐Talavera G, Shelton H, Scull MA, Ren JY, Jones IM, et al. (2009) Mutations in H5N1 Influenza virus hemagglutinin that confer binding to human tracheal airway epithelium. Plos One 4.
30. Sampson T, Broussard GW, Marinelli LJ, Jacobs-‐Sera D, Ray M, et al. (2009) Mycobacteriophages BPs, Angel and Halo: comparative genomics reveals a novel class of ultra-‐small mobile genetic elements. Microbiology-‐Sgm 155: 2962-‐2977.
31. Cox J, Putonti C (2010) Mechanisms responsible for a Phi X174 mutant's ability to infect Escherichia coli by phosphorylation. Journal of Virology 84: 4860-‐4863.
32. Baranowski E, Ruiz-‐Jarabo CM, Domingo E (2001) Evolution of cell recognition by viruses. Science 292: 1102-‐1105.
33. Li ZJ, Chen HL, Jiao PR, Deng GH, Tian GB, et al. (2005) Molecular basis of replication of duck H5N1 influenza viruses in a mammalian mouse model. Journal of Virology 79: 12058-‐12064.
34. Subbarao EK, London W, Murphy BR (1993) A single amino acid in the PB2 gene of influenza A virus is a determinant of host range. Journal of Virology 67: 1761-‐1764.
35. Jonah G, Rainey A, Natonson A, Maxfield LF, Coffin JM (2003) Mechanisms of avian retroviral host range extension. Journal of Virology 77: 6709-‐6719.

86
36. Yamada S, Suzuki Y, Suzuki T, Le MQ, Nidom CA, et al. (2006) Haemagglutinin mutations responsible for the binding of H5N1 influenza A viruses to human-‐type receptors. Nature 444: 378-‐382.
37. Baer CF, Miyamoto MM, Denver DR (2007) Mutation rate variation in multicellular eukaryotes: causes and consequences. Nature Reviews Genetics 8: 619-‐631.
38. Ellegren H, Smith NGC, Webster MT (2003) Mutation rate variation in the mammalian genome. Current Opinion in Genetics & Development 13: 562-‐568.
39. Cuppels DA, Vanetten JL, Lambrecht P, Vidaver AK (1981) Survey of phytopathogenic pseudomonads for a restriction and modification system active on the double-‐stranded ribonucleic acid phage Phi6. Current Microbiology 5: 247-‐249.
40. Bono LM, Gensel CL, Pfennig DW, Burch CL (2013) Competition and the origins of novelty: experimental evolution of niche-‐width expansion in a virus. Biology Letters 9.
41. Dennehy JJ (2012) What Can Phages Tell Us about Host-‐Pathogen Coevolution? Int J Evol Biol 2012: 396165.
42. Gottlieb P, Metzger S, Romantschuk M, Carton J, Strassman J, et al. (1988) Nucleotide sequence of the middle dsRNA segment of bacteriophage phi6 -‐ placement of the genes of membrane associated proteins. Virology 163: 183-‐190.
43. Mindich L, Mackenzie G, Strassman J, McGraw T, Metzger S, et al. (1985) cDNA cloning of portions of the bacteriophage phi6 genome Journal of Bacteriology 162: 992-‐999.
44. Dennehy JJ (2009) Bacteriophages as model organisms for virus emergence research. Trends in Microbiology 17: 450-‐457.
45. Dawkins B (1991) Siobhan's Problem: The Coupon Collector Revisited. The American Statistician 45: 76-‐82.
46. Yamada S, Matsumoto Y, Takashima Y, Otsuka H (2005) Mutation hot spots in the canine herpesvirus thymidine kinase gene. Virus Genes 31: 107-‐111.
47. Sasadeusz JJ, Tufaro F, Safrin S, Schubert K, Hubinette MM, et al. (1997) Homopolymer mutational hot spots mediate herpes simplex virus resistance to acyclovir. Journal of Virology 71: 3872-‐3878.
48. Ng TI, Shi Y, Huffaker HJ, Kati W, Liu Y, et al. (2001) Selection and characterization of varicella-‐zoster virus variants resistant to (R)-‐9-‐[4-‐Hydroxy-‐2-‐(hydroxymethy)butyl] guanine. Antimicrobial Agents and Chemotherapy 45: 1629-‐1636.
49. Wu K, Peng G, Wilken M, Geraghty RJ, Li F (2012) Mechanisms of Host Receptor Adaptation by Severe Acute Respiratory Syndrome Coronavirus. Journal of Biological Chemistry 287: 8904-‐8911.
50. Hall AR, Scanlan PD, Buckling A (2011) Bacteria-‐Phage Coevolution and the Emergence of Generalist Pathogens. American Naturalist 177: 44-‐53.
51. Hoeper D, Kalthoff D, Hoffmann B, Beer M (2012) Highly pathogenic avian influenza virus subtype H5N1 escaping neutralization: more than HA variation. Journal of Virology 86: 1394-‐1404.
52. Tapparel C, Cordey S, Junier T, Farinelli L, Van Belle S, et al. (2011) Rhinovirus genome variation during chronic upper and lower respiratory tract infections. Plos One 6.
53. Yuang TTT, Shih C (2000) A frequent, naturally occurring mutation (P130T) of human hepatitis B virus core antigen is compensatory for immature secretion phenotype of another frequent variant (I97L). Journal of Virology 74: 4929-‐4932.

87
54. Chattopadhyay S, Weissman SJ, Minin VN, Russo TA, Dykhuizen DE, et al. (2009) High frequency of hotspot mutations in core genes of Escherichia coli due to short-‐term positive selection. Proceedings of the National Academy of Sciences of the United States of America 106: 12412-‐12417.
55. Hughes AL, Nei M (1988) Pattern of nucleotide substitution at major histocompatibility complex class I loci reveals overdominant selection. Nature 335: 167-‐170.
56. Keitel T, Diehl A, Knaute T, Stezowski JJ, Höhne W, et al. (2000) X-‐ray structure of the quinoprotein ethanol dehydrogenase from Pseudomonas aeruginosa: basis of substrate specificity. J Mol Biol 297: 961-‐974.
57. Ghosh M, Anthony C, Harlos K, Goodwin MG, Blake C (1995) The refined structure of the quinoprotein methanol dehydrogenase from Methylobacterium extorquens at 1.94 A. Structure 3: 177-‐187.
58. Oubrie A, Rozeboom HJ, Kalk KH, Huizinga EG, Dijkstra BW (2002) Crystal structure of quinohemoprotein alcohol dehydrogenase from Comamonas testosteroni: structural basis for substrate oxidation and electron transfer. J Biol Chem 277: 3727-‐3732.
59. Petsko GA, Ringe D (2004) Protein Structure and Function. Sunderland, MA: New Science Press.
60. Fisher RA (1930) The Genetical Theory of Natural Selection. Oxford, UK: Clarendon Press. 61. Rose MR (1982) Antagonistic pleiotropy, dominance and genetic variation. Heredity 48: 63-‐
78. 62. Cooper VS, Lenski RE (2000) The population genetics of ecological specialization in evolving
Escherichia coli populations. Nature 407: 736-‐739. 63. Kassen R (2002) The experimental evolution of specialists, generalists, and the maintenance
of diversity. Journal of Evolutionary Biology 15: 173-‐190. 64. Dennehy JJ (2014) What Ecologists Can Tell Virologists. Annu Rev Microbiol. 65. Sokurenko EV, Gomulkiewicz R, Dykhuizen DE (2006) Opinion -‐ Source-‐sink dynamics of
virulence evolution. Nature Reviews Microbiology 4: 548-‐555. 66. Chattopadhyay S, Feldgarden M, Weissman SJ, Dykhuizen DE, van Belle G, et al. (2007)
Haplotype diversity in "source-‐sink" dynamics of Escherichia coli urovirulence. Journal of Molecular Evolution 64: 204-‐214.
67. Williams PD (2010) Darwinian interventions: taming pathogens through evolutionary ecology. Trends in Parasitology 26: 83-‐92.
68. Lide DR (1991) Handbook of Chemistry and Physics. Boca Raton, FL: CRC Press. 69. Kyte J, Doolittle RF (1982) A simple method for displaying the hydropathic character of a
protein. Journal of Molecular Biology 157: 105-‐132.

88
Chapter 3: Host-‐Acquired Factor Impacts Fitness on Subsequent Hosts in an RNA Virus Brian E. Ford1,2, Kevin Mu1,3 and John J. Dennehy*,1,2 1 Department of Biology, Queens College of the City University of New York, Queens, NY 2 Molecular, Cellular and Developmental Biology Program, The Graduate Center of the City University of New York, New York, NY 3 Macaulay Honors College of the City University of New York, New York, NY *Corresponding author: [email protected] Correspondence: Prof. John J. Dennehy Department of Biology, Queens College 65-‐30 Kissena Blvd. Queens, NY 11367-‐1597 Keywords: host legacy effect, bacteriophage, Cystoviridae, Φ6, Φ9, Φ13, Φ14, enveloped virus, RNA virus, maternal effect, epigenetics, phenotype, Pseudomonas syringae Research Article to be submitted to Axios Review (http://axiosreview.org). Target journals:
1. eLife 2. PLoS Biology 3. BMC Biology 4. American Naturalist

89
Abstract
Many viruses are able to infect multiple hosts, often across species and genus barriers. The
ability to predict how well a virus will fare on a new host would be invaluable to
epidemiologists. Here we describe our findings that a virus’ lipid envelope—notable for being
both host derived and of non genetic origin—plays a significant role in virus fitness in
subsequent infections. We demonstrate significant fitness tradeoffs for host shifts between
numerous bacteriophage with different host ranges and across different mechanisms of
infection. Our results demonstrate that for some viruses, passage through a novel host may
hinder that virus’ ability to reinfect the original native host. However, in other cases, passage
through a novel host actually improves reproductive fitness on both native and novel hosts,
suggesting a host range expansion. This expansion is likely facilitated by a host-‐derived factor.
Our study demonstrates that other factors besides evolutionary adaptation may influence how
successfully viruses reproduce on their hosts.

90
Introduction
Most viruses are able to infect multiple host types even when host cell physiologies are
different [1]. Although permissive for infection, hosts may vary in intrinsic factors, such as
receptor structure and number, codon usage patterns, circuitry of genetic interactions,
metabolic capacities and pathways, immune system activity [2], etc. These intrinsic
physiological differences result in antagonistic pleiotropy, epistasis and other tradeoffs across
hosts, giving rise to the common observation of differential virus reproductive output across
different hosts [3-10]. While these intrinsic factors are major causes of fitness differences
across hosts, they are not the sole causes. Here we describe a new class of extrinsic factors that
can affect virus fitness in a specific host, the genotype and phenotype of the previous host.
This effect, which we term the host legacy effect, occurs when the reproductive output of a
virus infecting a particular host differs according to the genotype or phenotype of the previous
host that produced the virus. Host legacy effect was first observed in the bacteriophage Φ6,
which can infect its native host Pseudomonas syringae pv phaseolicola (hereafter PP) or a novel
host Pseudomonas pseudoalcaligenes East River Isolate A (hereafter ERA). Interestingly, it was
shown that Φ6 viruses acquired from infections of PP were more productive on subsequent
hosts than Φ6 viruses acquired from infections of ERA [4,11]. In other words, some factor
acquired from the previous host affects Φ6's ability to infect and reproduce in ERA. The goal of
our study was to experimentally determine whether a host legacy effect is present in other
bacteriophages in the Cystoviridae family, including Φ9, Φ13 and Φ14.

91
Materials and Methods
Bacterial Host Strain Determination and Selection
Members of Cystoviridae family (Φ6, Φ7, Φ8, Φ9, Φ10, Φ11, Φ12, Φ13 and Φ14) were
screened via spot titer against a panel of bacterial strains for viability (supplementary table
ST1). To ensure that fitness assays would be representative for the whole bacteriophage family,
two members of the “close relatives”—to the canonical member Φ6—[12], Φ6 and Φ9, and
two members of the “distant relatives”, Φ13 and Φ14, were selected for testing. All bacterial
host pairings consisted of the canonical bacterial host, Pseudomonas syringae pv phaseolicola
(PP) and a secondary bacterial host. Secondary bacterial hosts were different for each virus
strain-‐ Φ6: LM2489; Φ9: Pseudomonas syringae pv atrofaciens (hereafter Atro); Φ13: LM2509;
Φ14: Pseudomonas syringae pv tomato (hereafter Tomato). LM2489 [13] is a derivative of PP
susceptible to infection of all members of the Cystoviridae (Φ12 inclusive). LM2509 is another
derivative of PP that lacks pili [13]. Secondary bacteria hosts were chosen to be different as
possible, both from each other and for unique sensitivity. Bacteria host sensitivities are
summarized in Table 1.

92
Table 1. Bacterial Strains Bacteria AKA Sensitive to Used with Pseudomonas syringae pv phaseolicola
PP, HB Φ6, Φ9, Φ13, Φ14
Φ6, Φ9, Φ13, Φ14
Pseudomonas syringae pv atrofaciens
Atro Φ9 Φ9
Pseudomonas syringae pv tomato
Tomato Φ14 Φ14
“Common host” LM2489 Φ6, Φ9, Φ13, Φ14
Φ6
“Non-‐piliated” LM2509 Φ13 Φ13 Susceptibility to infection by bacteriophage strains used in this study. Paired Bacteriophage Strain Preparation
Bacteria were propagated in lysogeny broth (LB: 10g NaCl, 10g Bacto tryptone and 5g Bacto
yeast extract per liter of water) at pH 7. Bacterial cultures were initiated from a single colony
from a streak plate in 10 ml of LB in a sterile 50 ml flask capped by a 20 ml beaker. Cultures
were incubated for 18 H at 25°C with shaking at 120 rpm. Viruses were propagated in 3 ml top
agar (0.7% agar) containing diluted virus lysate and bacteria (200 μl PP or 100 μl for all other
hosts). Top agar was poured over LB agar plates (containing 1.5% agar) and incubated overnight
at 25°C. Top agar from four replicate plates was scraped into 4 ml of LB broth and
centrifuged (RCF = 1,400x g) for 40 min. Viral lysates were passed through 0.22 μm filters
to remove bacteria and stored at 4°C for the duration of the experiments. Paired strains
were made in tandem; virus stock propagated on PP was passed through either PP to
create a base strain or its secondary bacterial host to create a differentially lipid-‐‑enveloped
test strain (Figure 1A). Bacteriophage lysates were titered on both PP and the secondary
strain and the highest titer values were used in multiplicity of infection (MOI) calculations.

93
Fitness Assay Conditions
A single colony of both Pseudomonas syringae pv phaseolicola and one of the secondary hosts
(either LM2489, Pseudomonas syringae pv atrofaciens, LM2509 or Pseudomonas syringae pv
tomato) was used to inoculate 10 ml of LB broth each and incubated overnight (18H) at 25°C
with shaking at 110 rpm. 150 μl of this 18H culture was used to inoculate 15 ml LB and grown at
25°C to an optical density (OD) of 0.2. 30 μl of PP and each secondary host strain was
serially diluted and plated to verify equivalent starting bacterial colony forming units (CFU)
for the different strains.
Both bacterial hosts, as well as both of the differentially enveloped paired virus strains,
were measured concurrently in a 96-‐‑well flat-‐‑bottom plate (Figure 1B). Virus
concentration was titered for each strain and chosen to maximize bacterial growth before
complete host lysis. In order to keep the phage infection curves consistent between strains,
an inoculation concentration of 100 plaque forming units (pfu) for Φ6 or 10,000 pfu for Φ9,
Φ13 and Φ14 was used. Appropriate virus concentrations were diluted in a final volume of
100 μl. 100 μl of 0.2 OD bacteria was added to 100 μl of virus dilution (initial MOI of
1:40,000 for Φ6 and 1:400 for the three remaining virus strains) following Figure 1C.
Subsequent optical density (OD) was measured in a Tecan Infinite Pro plate reader at 600
nm. ODs were measured every 5 minutes, 200 sec of orbital shaking, 5 flashes, with the lid
on over the course of 18H at 25°C. Each sample was plated in octuplicate and each
experiment repeated in triplicate (final n=24 for each treatment). Due to lack of change in
the readings from 15H to 18H for all strains, all results are only reported until 15H.

94
Statistical Analysis
Statistical analyses were carried out using GraphPad Prism for Macintosh (6.0c) (GraphPad
Software, La Jolla, CA). Optical density (OD) was plotted over time (15 hours total) for each
paired strain. Column statistics was used to determine the Area Under the Curve (AUC), the
time to maximal infection (x-‐maximum) and the maximal growth (OD-‐max, or y-‐maximum) for
each replicate and experiment (8 replicates per treatment times 3 repeats of each experiment
equals 24 replicates total per treatment). One-‐way analysis of variance (ANOVA) with multiple
comparisons was used to compare differences between groups (bacteria growth alone, bacteria
growth with phage-‐canonical coat and bacterial growth with phage-‐secondary coat) using each
of the three variables (AUC, x-‐max and y-‐max) on each bacterial host (canonical or secondary).
Due to close agreement between the results for the three measures (AUC, x-‐max and y-‐max,
see ST2. and ST3.), only results from the AUC measures were used in the results and discussion.
Results
Φ6
For Φ6 strains passed through either PP or LM2489 (a PP derivative susceptible to infection by
all members of the Cystoviridae), there is no difference in number of progeny produced,
indicating that previous host identity does not change growth dynamics for either of the paired
strains grown on PP (Figure 2A). However, for those same paired strains grown on LM2489,

95
there is a slight but still significant advantage (p = 0.0028, Supplementary Table ST3) to virus
grown previously on PP (Figure 2B).
Φ9
For the paired Φ9 strains assayed on PP, virus that had been grown first on Atro demonstrated
a significantly increased fitness in comparison to virus that had been grown on PP (figure 2C).
This trend of Φ9-‐atro growing better than Φ9-‐PP was also observed for the two strains when
assayed on Atro (Figure 2D). The difference in progeny production between these paired strains
was more pronounced on Atro than PP (Figure 2C,D).
Φ13
Φ13 was passaged through either PP or LM2509 (a non-‐piliated PP derivative) to make paired
strains. Previous host exposure (i.e. those virus strains that had been previously passaged
through and possessed the same lipid envelope) leads to an increased fitness on both hosts and
a significant decrease in fitness for both strains lacking prior exposure (Figure 2E-‐F; p < 0.0001,
supplementary figure ST3).
Φ14
Distant Φ6 relative Φ14 was passaged through either PP or Tomato to make paired strains.
Without pre-‐exposure to Tomato, Φ14 was unable to infect Tomato at the tested MOI (Figure
2H). However, Φ14 pre-‐exposed to Tomato was even better at infecting PP than Φ14 that had
been only exposed to PP (Figure 2G).

96
These results are summarized below in Table 2.
Table 2. Relative Virus Productivity On Mismatched Hosts Strain Previous Host Current Host Productivity Relative Change
Φ6
PP LM2489 Increased
LM2489 PP No change
Φ9
PP Atro Decreased
Atro PP Increased
Φ13
PP LM2509 Decreased
LM2509 PP Decreased
Φ14
PP Tomato Decreased
Tomato PP Increased
Summary of the relative productivity of each virus strain on the bacterial host not matching the previous passage (i.e. unmatched lipid membrane). Outcomes are relative to productivity measured for each strain on the bacterial strain matching the previous passage (i.e. matching lipid membrane). An increased productivity indicates higher progeny production (greater reduction in OD600). Relative change column indicates the difference in growth between paired strains (red = decrease relative to concordant envelope strain; green = increase relative to concordant envelope strain; blue = no significance; more color indicates greater difference).

97
Discussion
Considered the type strain for the Cystoviridae family, Φ6 is the member against which other
canonical members are classified. Close family members are (Φ6) Φ7, Φ9, Φ10 and Φ11, while
distant family members are Φ8, Φ12, Φ13 and Φ14 [13]. This close and distant classification is
based on infection dynamics; for close family members the host bacteria pilus plays a crucial
role in the infection cycle [14] while distant relatives infect via the lipopolysaccharide (LPS) [15]
present on the cell membrane.
It is worth noting that—of the novel bacterial hosts tested—two are naturally occurring strains
(Pseudomonas syringae pathovars atrofaciens and tomato) and two are mutant derivatives of
PP. The two mutant derivatives are notable for their susceptibility dynamics. LM248 is a PP
derivative allowing infection by any of the Cystoviridae, while LM2509 is a PP mutant that does
not produce pili (hence the non-‐piliated designation). As pili are essential components for
producing a productive infection for the Cystoviridae type strain Φ6 and its close relatives (Φ7,
Φ9, Φ10, Φ11) but not its distant relatives (Φ8, Φ12, Φ13, Φ14) we would not expect LM2509
to be susceptible to infection by any of the close Φ6 relatives. This is the exact infection pattern
that we observe with spot titers (Supplementary Table ST1). LM2509 is only susceptible to
infection by Φ12 and Φ13 in our studies. In other words, the two mutant derivatives can be
considered special cases almost opposite in their infection outcomes; LM2489 in an

98
exceptionally permissive host and LM2509 is an exceptionally non permissive host. This
uniqueness is again apparent in their effect on Cystovirus infection patterns.
Passage through LM2489, the extremely permissive host, is the only case where there was no
difference in fitness between the paired strains on the canonical host PP. Stated differently,
jumping to an extremely permissive host carries very little cost in switching back to the original
host (Figure 2A). This is not to imply that there is no cost moving to a new host species as the
data demonstrate that following a jump to the new LM2489 host, subsequent Φ6 infection in
that same host (LM2489) is slightly less than that of unexposed Φ6 (Figure 2B). This seems in
direct opposition to the case of LM2509, the non-‐piliated or extremely non-‐permissive host.
Here, without prior passage through LM2509, Φ13 is unable to infect the host at the tested
MOI (Figure 2F). Once the virus has made the jump to this mutant host, it is extremely impaired
at infecting the original host, PP (Figure 2E). Unlike in the previous case of LM2489 of an easier
jump to a permissive host, in LM2509, the shift to a relatively inhospitable host carries a
significant fitness cost on the original host (Figure 2E). Once the virus successfully replicates on
an exceptionally impervious host, it has higher reproductive fitness on subsequent infections of
the same host, but is also much worse on the original host.
The two naturally occurring strains (Atro and Tomato) demonstrate a slightly different infection
dynamic. Here, in both cases, a jump to the novel host is greatly facilitated by previous
exposure to that host (Figure 2D,H) and passage through the secondary novel host significantly
increases fitness on the canonical host (Figure 2C,G). These results are interesting in that they

99
are consistent across both Φ9 and Φ14 (a close and distant relative of Φ6 respectively). That
these data for these two viruses—although related—are consistent, is noteworthy given that
both viruses display different host ranges and follow different infection dynamics (targeting the
pilus vs the LPS respectively) [16]. These data would suggest that certain host range expansions
could improve a generalist host recognition mechanism or that there are other factors
responsible for bacteria host recognition in addition to recognition sites on either the pilus or
LPS. A prime candidate for the responsible factor is the lipid membrane.
The Cystoviridae are enveloped viruses. They consist of about 10% tripartite dsRNA, 70%
protein and 20% lipids [17]. Their lipid membrane is derived from the host’s cytoplasmic
membrane [18]. In our paired strain experiments, each virus is passaged once though PP or a
novel host. We kept the passage cycles to a minimum to reduce genetic evolution as a
confounding factor. Each paired strain should significantly differ only in their host-‐derived lipid
membrane (Figure 1C). In our test scenario, resultant differences in fitness between each
paired strain should be attributable to the composition of the host-‐derived membrane. Our
data suggest the host-‐derived lipid membrane is a significant factor in subsequent virus fitness.
Further experimentation is required in order to determine if it is the composition of the lipid
membrane itself [19] or that of the host-‐derived proteins embedded in the membrane [20] as
the responsible party for the subsequent fitness tradeoffs.
For organisms—such as viruses—that multiply exponentially, given a finite amount of both time
and resources, initial conditions can play a large role in the final reproductive outcome. The

100
host legacy effect plays a direct role only in the initial infection cycle (Figure 3). In the first
scenario, having a concordant lipid envelope—where the lipid envelope composition of the
virus matches that of the bacteria—virus amplification for each round of infection is relatively
equivalent to each other, differing only in the ratio of virus to bacterial hosts (Figure 3A). In the
second case, having a discordant virus envelope—where the lipid envelope composition
matches a different bacterial host—virus production can either be impaired or facilitated,
leading either to little or great amplification of the virus (Figure 3B). For the bacteriophage Φ6,
one infection cycle takes roughly 3H [21,22]. For the majority of the paired strain results
(Supplementary Figure 1A,B,C,E,F,G), differences in virus amplification are readily apparent
following the second round of infection, at 6H (Supplementary Figure 1, Figure 2), indicating the
importance of the host legacy effect and initial infection conditions.
Viruses have very defined host ranges and niches. Many factors have been identified either
allowing or restricting expansion of these—usually narrow—host ranges [23]. Here we identify
a new factor important for the success or failure of of these segmented dsRNA viruses in
encountering a new host: the host-‐derived lipid membrane. These data are suggestive of a
“host legacy effect”—analogous to the eukaryotic maternal effect concept—of bacterial host
identity impacting virus fitness in subsequent infection. While we do not see a generality in all
tested infection scenarios, we do see that in all cases save one—in an extremely permissive
host where the exception proves the rule—that the lipid membrane plays a significant role in
subsequent virus fitness. Further experimentation will be required to determine both the
identity—whether the effect is a function of differing proteins embedded in the envelope, the

101
lipid composition of the envelope itself, or some other factor—and the mechanism of action
responsible for this effect.
Author Contributions
BEF & JJD designed the study and analyzed the data, BEF & KM conducted the assays, and all
three authors wrote the manuscript.
Conflict of Interest Statement
None of the authors have affiliations with, or involvement in any organization or entity with any
financial interest, or non-‐financial interest in the subject matter or materials discussed in this
manuscript.
Acknowledgements
We would like to thank Leonard Mindich and Paul E. Turner for their kind donations of
bacteriophage and bacteria strains. We appreciate assistance from Marko Baloh and Elizabeth
S. Chapler and other members of the Dennehy Lab. This work was funded by National Science
Foundation Early Career Award to JJD (DEB #1148879). The Macaulay Honors College provided
financial support to KM.

102
Captions
Figure 1. Experimental Design
General experimental overview. (A) Reciprocal fitness assay setup for the four paired strains:
each virus pair is concurrently assayed on both bacteria host strains along with a bacteria
control. Close relatives (Φ6, Φ9) in top row; distant relatives (Φ13, Φ14) on bottom row. (B)
Fitness assay design. Each paired strain is assayed simultaneously on both bacterial hosts in
octuplicate. Each experiment was repeated three times. White: not used; Red: blank; Blue: Φ9-‐
canonical on canonical strain; Purple: canonical alone; Cyan: Φ9-‐secondary on canonical; Dark
green: Φ9-‐canonical on secondary; Bright green: secondary alone; Army green: Φ9-‐secondary
on secondary. (C) Paired strain preparation. Each of the four strains (Φ6, Φ9, Φ13, Φ14) was
passaged through one round of infection on both the canonical host (PP) and a secondary
bacterial host (LM2489, Atro, LM2509 and Tomato) resulting in genetically identical viruses
differing only in the host-‐derived lipid membrane.
Figure 2. Paired Fitness Assays
Growth curves for the four paired-‐strain reciprocal fitness assays. (A) Φ6 on Pseudomonas
syringae pv phaseolicola (PP); (B) Φ6 on LM2489, a bacterial strain determined to be
susceptible to the entire Cystoviridae family. (C) Φ9 on PP; (D) Φ9 on Pseudomonas syringae pv
atrofaciens (Atro). (E) Φ13 on PP; (F) Φ13 on 2509, a non-‐piliated bacteria. (G) Φ14 on PP; (H)
Φ14 on Pseudomonas syringae pv tomato (Tomato). (A,C,E,G) represent growth on the

103
canonical host Pseudomonas syringae pv phaseolicola. (B,D,F,H) bacteriophage fitness on
secondary bacteria hosts; each bacterial host is different for each virus. N=24 for all
experiments. Error bars represent the mean ± standard error of the mean (SEM).
Figure 3. Host Legacy Effect Infection Dynamics
The host legacy effect can change after each round of infection. (A) In a concordant infection—
virus envelope matches bacteria type—infection dynamics and virus amplification proceed as
normal. (B) For discordant infections—virus envelope matches a different bacteria type—the
first round of infection can greatly differ from subsequent rounds of infection, leading to
greater or reduced virus amplification.
Supplementary Figure 1. Paired Fitness Assay Summary at 6H (~2 Infection Cycles)
Paired strain fitness data from Figure 2 reformatted to emphasis differences at 6H, about two
infectious cycles for Φ6.
Supplementary Table ST1. Host range of Cystoviridae on selected strains
Summary of selected bacteria strain susceptibility to members of the Cystoviridae.
Supplementary Table ST2. One-‐way ANOVA tables for the effect of virus lipid membrane
composition on fitness at 15 hours, measured by Area Under Curve (AUC), time to infection
(x-‐max) and maximum bacterial growth (y-‐max).

104
Fitness is measured for each paired strain on both bacterial hosts. Degrees of freedom (df) for
all analyses is 2. Rows with discordant significance values between measures are boxed in red.
AUC, area under curve.
Supplementary Table ST3. ANOVA post-‐hoc Tukey’s multiple comparison test.
Degrees of freedom (df) for all analyses is 23. Control, bacteria alone; AUC, area under curve;
Mean diff., mean difference; Summ., summary; Adj. P, adjusted P; ns, not significant. Rows with
discordant significance values between measures are boxed in red.
Figures
Figure 1. Experimental Design

105
Figure 2. Paired Fitness Assays

106
Figure 3. Host Legacy Effect Infection Dynamics

107
Supplemental Figures
Supplementary Figure 1. Paired Fitness Assay Summary 6H (~Two Infection Cycles)

108
1
Supp
lemen
tary Tab
le ST1. C
ysto
virid
ae H
ost R
ange
on
Sele
ct H
ost S
trai
ns
Nam
e La
b co
de
Desig
natio
n De
scrip
tion
Susc
eptib
le
Curr
ent S
tudy
Re
fere
nce
Eschericha
coli
JJD1
E. c
oli
N
one
Pseudo
mon
as
pseudo
alcaligenes
Ea
st R
iver
Isol
ate
A
JJD33
ER
A N
ovel
Φ6
host
Φ
8, Φ
10, Φ
11,
Φ12
, Φ13
, Φ
14*
Pseudo
mon
as
syrin
gae
pv
phaseolicola
JJD34
HB
, PP
Host
of
isola
tion
for
clos
e re
lativ
es
Φ6,
Φ7,
Φ8,
Φ
9, Φ
10, Φ
11,
Φ13
, Φ14
Φ6,
Φ9,
Φ13
, Φ
14
JJD
50
LM23
33
Perm
issiv
e ho
st
Φ6,
Φ7,
Φ8*
, Φ
9, Φ
10, Φ
11,
Φ12
*, Φ
13*,
Φ
14*
M
indi
ch 1
999
“Non
-‐pili
ated
” JJD
53
LM25
09
Φ
8*, Φ
12, Φ
13 Φ
13
Min
dich
199
9 Pseudo
mon
as
syrin
gae
pv
tomato
JJD98
To
mat
o
Φ8,
Φ10
*,
Φ11
*, Φ
13,
Φ14
*
Φ14
Pseudo
mon
as
syrin
gae
pv
atrofaciens
JJD99
At
ro
Φ
7, Φ
9, Φ
10,
Φ11
Φ
9
JJD
138
S4M
Φ8
M
indi
ch 1
999
“Com
mon
hos
t”
JJD
139
LM24
89
Extr
emel
y pe
rmiss
ive
host
Φ6,
Φ7,
Φ8,
Φ
9, Φ
10, Φ
11,
Φ12
, Φ13
, Φ14
Φ6
Min
dich
199
9
JJD
140
LM25
72
Φ
6, Φ
7, Φ
8,
Φ9,
Φ10
, Φ11
, Φ
12, Φ
13, Φ
14
* in
fect
ivity
onl
y at
hig
h tit
er
2

109
3
Supp
lemen
tary Tab
le ST2. O
ne-‐w
ay A
NO
VA ta
bles
for t
he e
ffect
of v
irus l
ipid
mem
bran
e co
mpo
sitio
n on
fit
ness
at 1
5 ho
urs a
s mea
sure
d by
Are
a Un
der C
urve
(AUC
), tim
e to
infe
ctio
n (x
-‐max
) and
max
imum
bac
teria
l gr
owth
(y-‐m
ax).
Fitn
ess i
s mea
sure
d fo
r eac
h pa
ired
stra
in o
n bo
th b
acte
rial h
osts
. Fa
ctor
AU
C
X-‐M
ax
Y-‐
Max
SS
F
P
SS
F P
SS
F
P Φ6
PP
361.
1 27
18
<0.000
1
671.
0 11
04
<0.000
1
0.97
34
610.
1 <0.000
1
LM
2489
19
1.6
3097
<0.000
1
1093
26
41
<0.000
1
1.10
9 11
96
<0.000
1 Φ9
PP
5531
.7
8621
<0.000
1
1320
43
45
<0.000
1
2.79
6 50
79
<0.000
1
atr
o 17
7.6
974.
4 <0.000
1
792.
8 83
6.7
<0.000
1
1.89
4 71
2.6
<0.000
1 Φ13
P
P 49
8.7
2969
<0.000
1
939.
8 66
9.1
<0.000
1
2.52
5 14
62
<0.000
1
LM
2509
36
2.7
7202
<0.000
1
1374
16
46
<0.000
1
3.10
7 57
33
<0.000
1 Φ14
P
P 65
7.7
1244
3 <0.000
1
1033
14
44
<0.000
1
3.14
7 16
70
<0.000
1
tom
ato
99.1
3 33
5.1
<0.000
1
658.
4 16
11
<0.000
1
1.66
8 50
6.0
<0.000
1 AU
C, a
rea
unde
r cur
ve; M
ean
diff.
, mea
n di
ffere
nce;
Sum
m.,
sum
mar
y; A
dj. P
, adj
uste
d P;
ns,
not s
igni
fican
t. 4
Degr
ees o
f fre
edom
(df)
for a
ll an
alys
es e
qual
s 2.
5
6

110
Supp
lemen
tary Tab
le ST3. A
NO
VA P
ost-‐h
oc T
ukey
’s M
ultip
le C
ompa
rison
Tes
t Fa
ctor
AU
C
X-‐M
ax
Y-‐
Max
M
ean
diff
Sum
m.
Adj.
P
Mea
n di
ff Su
mm
. Ad
j. P
M
ean
diff
Sum
m.
Adj.
P Φ6
PP
Con
trol
vs Φ
6-‐PP
4.
712
****
<0
.000
1
6.43
0 **
**
<0.0
001
0.
2455
**
**
<0.0
001
C
ontr
ol v
s Φ6-‐
LM24
89
4.78
8 **
**
<0.0
001
6.
521
****
<0
.000
1
0.24
78
****
<0
.000
1
Φ6-‐
PP v
s Φ6-‐
LM24
89
0.07
608
ns
0.50
79
0.
0902
5 ns
0.
3633
0.00
2379
ns
0.
9315
LM24
89
Con
trol
vs Φ
6-‐PP
3.
519
****
<0
.000
1
8.37
1 **
**
<0.0
001
0.
2798
**
**
<0.0
001
C
ontr
ol v
s Φ6-‐
LM24
89
3.39
9 **
**
<0.0
001
8.
159
****
<0
.000
1
0.24
29
****
<0
.000
1
Φ6-‐
PP v
s Φ6-‐
LM24
89
-‐0.1
195
**
0.00
28
-‐0
.211
9 **
* 0.
0003
-‐0.0
3688
**
**
<0.0
001
Φ9
PP
Con
trol
vs Φ
9-‐PP
5.
642
****
<0
.000
1
8.76
8 **
**
<0.0
001
0.
3840
**
**
<0.0
001
C
ontr
ol v
s Φ9-‐
atro
5.
880
****
<0
.000
1
9.36
5 **
**
<0.0
001
0.
4453
**
**
<0.0
001
Φ
9-‐PP
vs Φ
9-‐at
ro
0.23
81
***
0.00
04
0.
5979
**
**
<0.0
001
0.
0612
7 **
**
<0.0
001
atro
Con
trol
vs Φ
9-‐PP
1.
473
****
<0
.000
1
5.00
2 **
**
<0.0
001
0.
2090
**
**
<0.0
001
C
ontr
ol v
s Φ9-‐
atro
3.
814
****
<0
.000
1
8.05
0 **
**
<0.0
001
0.
3971
**
**
<0.0
001
Φ
9-‐PP
vs Φ
9-‐at
ro
2.34
1 **
**
<0.0
001
3.
048
****
<0
.000
1
0.18
81
****
<0
.000
1 Φ13
PP
Con
trol
vs Φ
13-‐P
P 5.
696
****
<0
.000
1
7.60
8 **
**
<0.0
001
0.
4083
**
**
<0.0
001
C
ontr
ol v
s Φ13
-‐LM
2509
0.
2335
*
0.01
18
-‐0
.110
4 ns
0.
9097
0.02
309
* 0.
0189
Φ13
-‐PP
vs Φ
13-‐L
M25
09
-‐5.4
62
****
<0
.000
1
-‐7.7
19
****
<0
.000
1
-‐0.3
852
****
<0
.000
1 LM25
09
Con
trol
vs Φ
13-‐P
P 0.
0583
3 ns
0.
4187
0.19
92
ns
0.46
44
0.
0161
8 *
0.02
68
C
ontr
ol v
s Φ13
-‐LM
2509
4.
790
****
<0
.000
1
9.36
5 **
**
<0.0
001
0.
4485
**
**
<0.0
001
Φ
13-‐P
P vs
Φ13
-‐LM
2509
4.
732
****
<0
.000
1
9.16
6 **
**
<0.0
001
0.
4323
**
**
<0.0
001
Φ14
PP
Con
trol
vs Φ
14-‐P
P 6.
140
****
<0
.000
1
7.85
7 **
**
<0.0
001
0.
3985
**
**
<0.0
001
C
ontr
ol v
s Φ14
-‐tom
ato
6.65
3 **
**
<0.0
001
8.
200
****
<0
.000
1
0.47
78
****
<0
.000
1

111
Cont
rol,
bact
eria
alo
ne; A
UC, a
rea
unde
r cur
ve; M
ean
diff.
, mea
n di
ffere
nce;
Sum
m.,
sum
mar
y; A
dj. P
, adj
uste
d P;
ns,
not
7 sig
nific
ant.
8 De
gree
s of f
reed
om (d
f) fo
r all
com
paris
ons e
qual
s 23.
9
Row
s with
disc
orda
nt si
gnifi
canc
e va
lues
bet
wee
n m
easu
res a
re b
oxed
in re
d.
10
11
12
Φ
14-‐P
P vs
Φ14
-‐tom
ato
0.51
26
****
<0
.000
1
0.34
29
****
<0
.000
1
0.07
930
****
<0
.000
1 tom
ato
Con
trol
vs Φ
14-‐P
P 0.
0191
7 **
0.
8708
0.00
4167
ns
0.
8990
0.02
136
**
0.00
55
C
ontr
ol v
s Φ14
-‐tom
ato
2.49
9 **
**
<0.0
001
6.
417
****
<0
.000
1
0.33
30
****
<0
.000
1
Φ14
-‐PP
vs Φ
14-‐to
mat
o 2.
479
****
<0
.000
1
6.41
3 **
**
<0.0
001
0.
3116
**
**
<0.0
001

112
References
1. Parrish CR, Holmes EC, Morens DM, Park EC, Burke DS, Calisher CH, et al. Cross-‐Species Virus Transmission and the Emergence of New Epidemic Diseases. Microbiol Mol Biol Rev. 2008;72: 457–470. doi:10.1128/MMBR.00004-‐08
2. Feng Z, Hensley L, McKnight KL, Hu F, Madden V, Ping L, et al. A pathogenic picornavirus acquires an envelope by hijacking cellular membranes. Nature. Nature Publishing Group; 2013;496: 367–371. doi:10.1038/nature12029
3. Ford BE, Sun B, Carpino J, Chapler ES, Ching J, Choi Y, et al. Frequency and Fitness Consequences of Bacteriophage Φ6 Host Range Mutations. van Raaij MJ, editor. PLoS ONE. Public Library of Science; 2014;9: e113078. doi:10.1371/journal.pone.0113078
4. Dennehy JJ, Friedenberg NA, Holt RD, Turner PE. Viral ecology and the maintenance of novel host use. Am Nat. 2006;167: 429–439. doi:10.1086/499381
5. Crill WD, Wichman HA, Bull JJ. Evolutionary reversals during viral adaptation to alternating hosts. Genetics. 2000;154: 27–37.
6. Woolhouse ME, Taylor LH, Haydon DT. Population biology of multihost pathogens. Science. American Association for the Advancement of Science; 2001;292: 1109–1112. doi:10.1126/science.1059026
7. Longdon B, Hadfield JD, Webster CL, Obbard DJ, Jiggins FM. Host Phylogeny Determines Viral Persistence and Replication in Novel Hosts. Schneider DS, editor. PLoS Pathog. 2011;7: e1002260. doi:10.1371/journal.ppat.1002260
8. Seed KD, Lazinski DW, Calderwood SB, Camilli A. A bacteriophage encodes its own CRISPR/Cas adaptive response to evade host innate immunity. Nature. 2013;494: 489–491. doi:10.1038/nature11927
9. Richter M, Reimann I, Schirrmeier H, Kirkland PD, Beer M. The viral envelope is not sufficient to transfer the unique broad cell tropism of Bungowannah virus to a related pestivirus. Journal of General Virology. Society for General Microbiology; 2014;95: 2216–2222. doi:10.1099/vir.0.065995-‐0
10. Ciota AT, Payne AF, Ngo KA, Kramer LD. Consequences of in vitro host shift for St. Louis encephalitis virus. Journal of General Virology. Society for General Microbiology; 2014;95: 1281–1288. doi:10.1099/vir.0.063545-‐0
11. Duffy S, Turner PE, Burch CL. Pleiotropic Costs of Niche Expansion in the RNA Bacteriophage Φ6. Genetics. Genetics Society of America; 2006;172: 751–757. doi:10.1534/genetics.105.051136
12. Gottlieb P, Potgieter C, Wei H, Toporovsky I. Characterization of phi12, a bacteriophage

113
related to phi6: nucleotide sequence of the large double-‐stranded RNA. Virology. 2002;295: 266–271. doi:10.1006/viro.2002.1436
13. Mindich L, Qiao X, Qiao J, Onodera S, Romantschuk M, Hoogstraten D. Isolation of additional bacteriophages with genomes of segmented double-‐stranded RNA. J Bacteriol. 1999;181: 4505–4508.
14. Leo-‐Macias A, Katz G, Wei H, Alimova A, Katz A, Rice WJ, et al. Toroidal surface complexes of bacteriophage ϕ12 are responsible for host-‐cell attachment. Virology. Elsevier Inc; 2011;: 1–7. doi:10.1016/j.virol.2011.03.020
15. Joseph SB, Hanley KA, Chao L, Burch CL. ORIGINAL ARTICLE: Coinfection rates in φ6 bacteriophage are enhanced by virus-‐induced changes in host cells. Evolutionary Applications. 2008;2: 24–31. doi:10.1111/j.1752-‐4571.2008.00055.x
16. Daugelavicius R, Cvirkaite V, Gaidelyte A, Bakiene E, Gabrenaite-‐Verkhovskaya R, Bamford D. Penetration of Enveloped Double-‐Stranded RNA Bacteriophages 13 and 6 into Pseudomonas syringae Cells. J Virol. 2005;79: 5017–5026. doi:10.1128/JVI.79.8.5017-‐5026.2005
17. King AM, Lefkowitz E, Adams MJ, Carstens EB, editors. Virus Taxonomy: Ninth Report of the International Committee on Taxonomy of Viruses. 1st ed. Elsevier; 2011.
18. Laurinavičius S, Käkelä R, Somerharju P, Bamford D. Phospholipid molecular species profiles of tectiviruses infecting Gram-‐negative and Gram-‐positive hosts. Virology. 2004;322: 328–336. doi:10.1016/j.virol.2004.02.009
19. Cvirkaite-‐Krupovic V, Poranen MM, Bamford D. Phospholipids act as secondary receptor during the entry of the enveloped, double-‐stranded RNA bacteriophage 6. Journal of General Virology. 2010;91: 2116–2120. doi:10.1099/vir.0.020305-‐0
20. Cantin R, Methot S, Tremblay MJ. Plunder and Stowaways: Incorporation of Cellular Proteins by Enveloped Viruses. J Virol. 2005;79: 6577–6587. doi:10.1128/JVI.79.11.6577-‐6587.2005
21. Dennehy JJ, Turner PE. Reduced fecundity is the cost of cheating in RNA virus 6. Proceedings of the Royal Society B: Biological Sciences. 2004;271: 2275–2282. doi:10.1098/rspb.2004.2833
22. Chao L, Rang CU, Wong LE. Distribution of spontaneous mutants and inferences about the replication mode of the RNA bacteriophage phi6. J Virol. 2002;76: 3276–3281. doi:10.1128/JVI.76.7.3276–3281.2002
23. Longdon B, Brockhurst MA, Russell CA, Welch JJ, Jiggins FM. The Evolution and Genetics of Virus Host Shifts. Hobman TC, editor. PLoS Pathog. Public Library of Science; 2014;10: e1004395. doi:10.1371/journal.ppat.1004395

114
Chapter 4: Benefits of Sticking Together: Fitness Reduced in Virus-‐Challenged Pseudomonas Species Following Shift to Planktonic Phenotype Emily Y. Lin,1,2, David Toubiyan1, Marko Baloh1, Glennon V. Bythrow2,3, Brian E. Ford1,2 and John J. Dennehy1,2,* 1Department of Biology, Queens College of the City University of New York, Queens, NY 2Molecular, Cellular and Developmental Biology Program, The Graduate Center of the City University of New York, New York, NY 3Department of Biology, Brooklyn College of the City University of New York, Brooklyn, NY *Corresponding author: [email protected] Correspondence: Prof. John J. Dennehy Department of Biology, Queens College 65-‐30 Kissena Blvd. Queens, NY 11367-‐1597 Keywords: Clumping, Pseudomonas, ERA, Phenotypic Shift, Planktonic, Sessile, bacteriophage Original Research Article to be submitted to Axios Review (http://axiosreview.org). Target journals:
1. Molecular Ecology 2. American Naturalist 3. BMC Evolutionary Biology 4. Evolution

115
Abstract
Many bacteria can switch between planktonic and sessile states. Single, motile, flagellated cells
characterize the planktonic state, whereas cells in the sessile state are not motile and aggregate
into clumps. Canonical Pseudomonas pseudoalcaligenes (ERA) predominantly exhibits the
sessile phenotype. Here we describe the selection and isolation of a planktonic strain of P.
pseudoalcaligenes through serial passage evolution. The planktonic strain has both longer and
more numerous flagella than its ancestor, and demonstrates a buoyancy that is not movement
dependent. Although the planktonic strain generates smaller colony forming units (CFU) than
the sessile strain, total cell mass was not significantly different, suggesting that cell growth
rates were similar. However, phage growth data suggested that cell aggregation decreases
susceptibility to phage infection. These data imply a scenario where an increase in bacteria
motility—a factor associated with virulence—in response to environmental stress (our
selection), is tempered by an increase in susceptibility to bacteriophage infection. This model
system can provide insights into a possible regulatory mechanism of bacterial phenotype in the
host-‐virus coevolutionary arms race.

116
Introduction
Pseudomonas pseudoalcaligenes East River Isolate A (hereafter ERA) as a model system to
study virus host shifts has a long and well-‐established history [1-6]. While it is a non-‐permissive
host for the Pseudomonas bacteriophage Φ6 (hereafter Φ6). However, several different labs
have isolated Φ6 host range mutants (HRMs) capable of infecting ERA [6-9]. These HRMs are
able to infect both ERA and the canonical host for Φ6, Pseudomonas syringae pathovar
phaseolicola (hereafter PP). Previous work with these HRMs has provided anecdotal evidence
that HRM grown on PP have better fitness on ERA than HRMs grown only on ERA [2,8]. Direct,
simultaneous comparison of fitness of these HRMs on both hosts is made challenging by the
different growth characteristics between the two strains. Here, we describe a set of
experiments designed to extend this model system to study new concepts. In order to be able
to compare fitness of HRMs on their original host, we selected, through serial passage, an ERA
strain with growth dynamics closer to PP. Standard ERA—isolated from the East River in New
York [9]—exhibits a sessile phenotype, grows quickly to stationary phase, and forms clumps.
We selected for a free-‐floating, non-‐clumping, planktonic ERA that reaches stationary phase
much later. Characterization of this new resultant planktonic phenotype of ERA has revealed a
number of properties that allow for an even greater number of new types of assays than
originally planned.

117
Materials and Methods
Bacteria Culture Preparation
All bacteria were propagated in lysogeny broth (LB: 10 g NaCl, 10 g Bacto tryptone, and 5 g
Bacto yeast extract per liter of water) at pH 7. Bacterial cultures were initiated by transferring a
single colony from a streak plate into 10 mL LB. Culture flasks were incubated with shaking (120
rpm) at 25 °C for 18 hours.
Selection for planktonic (non clumping) strain
Figure 1 illustrates our selection scheme. Single colonies of ERA were transferred to liquid
culture to establish two lineages (each in triplicate): the first with selection and the second
without. The first was lineage briefly centrifuged following overnight incubation with only
supernatant (bacterial cells that did not sediment) used to inoculate subsequent passages. The
second lineage—the passage control— was not centrifuged prior to inoculating subsequent
passages. Lineages were tested on days 7 and 14. By passage 14, all three selected lineages had
achieved a planktonic phenotype. For consistency, the starting, unevolved lab ERA strain is
labeled “sessile” in all graphs (in contrast to the evolved planktonic phenotype).
Isolation and Verification of Non Clumping Phenotype
As the selection scheme has resulted in a mixed bacterial population, passage 14 lineages were
streaked out for single colonies and assayed for planktonic phenotype via 18H growth curves on
a Tecan Infinite 200 Plate Reader (Tecan, Morrisville, NC). Single colonies were grown over

118
night (O/N) at 25 °C with shaking at 120 rpm. At 18H, 200 μl of this stationary phase bacteria
were used to inoculate 10 ml LB at 25 °C with shaking at 120 rpm. Liquid cultures were grown
to an optical density of 0.2 at 600 nm (OD600). 200 μl of these exponential bacteria cultures
were then plated in quadruplicate (starting OD600 = 0.02) along with their appropriate controls
(Day 0, corresponding passage control, unevolved sessile ERA).
Heat Shock Buoyancy Test
A single colony from each strain was used to inoculate 30 ml LB and grown O/N at 25 °C with
shaking at 120 rpm. At 24H, 30 ml stationary phase cultures were divided in to three 10 ml
aliquots and moved to three different temperatures: 25 °C, 37 °C and 55 °C and incubated O/N
with shaking at 120 rpm. At 18H, cultures were plated on LB agar for growth. Experiment was
repeated in duplicate.
Determination of Cell Number in Clumps
Six single colonies from each strain were used to inoculate in triplicate either 10 ml LB or 10 ml
LB containing 1%-‐tween-‐80 and grown O/N at 25 °C with shaking at 120 rpm. At 18H, samples
from each treatment were diluted to give approximately 20 – 200 CFUs and plated on LB agar.
Following an O/N incubation at 25 °C, colonies on the plates were counted. Original culture
concentration was determined by dividing the number of counted CFUs by the dilution factor.

119
Colony Size Determination
A single colony from each strain was used to inoculate 10 ml LB at grown O/N at 25 °C with
shaking at 120 rpm. At 18 H, 200 μl of these stationary phase cultures was used to inoculate 10
ml LB broth (= exponential phase culture). Aliquots were taken from each of these three
exponential phase cultures at 1-‐hour intervals up to 6 hours, serially diluted and plated on LB
agar to give 20 – 200 colonies per plate. Plates were incubated O/N at 25 °C. Three photos—
each containing ≥5 colonies—from each plate were taken for each time point (1H – 6H) and
treatment (with or without 1% tween). Colony sizes were measured using the ImageJ (version
1.49) software (NIH, Bethesda, MD). ImageJ workflow: 1) Image: Adjust -‐> Threshold to black
and white (B&W); 2) Process: Binary -‐> Fill in holes; 3) Process: Binary -‐> Convert to masks; 4)
Process: Binary -‐> Watershed; 5) Analyze: Analyze Particles.
TEM Analysis of Cell Morphology
Cell morphology for the three strains was analyzed via Transmission Electron Microscopy (TEM)
using a JEOL 1200 EX Transmission Electron Microscope, (JOEL USA, Columbia, MD). Bacteria
was incubated for 20 min at room temperature (RT) on Carbon Type-‐B, 200 mesh, copper grids
(Ted Pella, Redding, CA), washed twice with deionized water and negatively stained using a 1%
uranyl acetate solution for 2 min at room RT. Multiple micrographs for each strain were taken
at 10k, 15k and 30k x magnifications.

120
Bacteriophage Strain Preparation
Φ6-‐derived host range mutant (HRM) S154 [6] was propagated on Pseudomonas syringae
pathovar phaseolicola (hereafter PP). Lysates were obtained by adding 1 μl of frozen S154 stock
and 200 μl PP to 3 ml top agar (LB with 0.7% Bacto agar, liquid at 45 °C, solid at 25 °C) poured
over 35 ml bottom agar (LB with 1.5% Bacto agar) in a sterile petri dish. Following O/N
incubation at 25 °C, top agar is scraped off and suspended in 3 ml LB and centrifuged at RCF =
1400xg to pellet agar and cellular debris. Cell-‐free lysate is obtained by passing the supernatant
through a 0.22 μm filter (Durapore; Millipore, Bedford, MA). Phage particles per ml were
quantified via serial dilution and titering. Plaques were counted on plates where 30–500
plaques were visible. The number of plaque forming units (PFU) per ml in the original lysate was
obtained by multiplying the number of plaques counted by the dilution factor.
Growth Curve Optical Density to Cell Number Correlation
Because we were using multiple strains—of which at least two are clump-‐forming—the
relationship between OD600 and bacteria cell number (assessed via direct plate count) needed
to be determined. To account for cell clumping, all growth curve and cell plating experiments
were carried out both in the presence and absence of the surfactant Tween-‐80 (1% by v/v). A
single colony from each of the three bacterial strains was used to inoculate 10 ml LB and grown
O/N at 25 °C with shaking at 120 rpm. At 18H, 200 μl from each stationary-‐phase overnight
culture was used to inoculate both 20 ml LB and 20 ml LB with 1%-‐Tween. The relation between
OD and plating number was determined for all three bacteria over the course of 6 hours. At 1H

121
intervals, 1 ml aliquots were taken from each liquid culture, measured in an Implen
Spectrophotometer P 330 (Implen, Westlake Village, CA) at 600 nm and diluted (10-‐3, 10-‐4, 10-‐5
for the two control strains and 10-‐5, 10-‐6, 10-‐7 for the planktonic strain) to give plates with
between 20 – 200 CFU. Each experiment was repeated in triplicate. Results were plotted on
two graphs: 1) OD—in the presence or absence of Tween— over time and 2) number of cells—
with or without Tween—plotted over time. For the OD over time graphs, R-‐square values were
calculated using the non-‐linear curve fitting function of GraphPad Prism Macintosh v6.0f
(GraphPad, La Jolla, CA). For number of cells over time graphs, calculated CFU was plotted over
the course of the 6H experiment. To calculate the original CFU per time point, the total number
of CFUs were divided by the dilution factor. Note that the fitted curves for these experiments
are most accurate during the first four hours of the experiment (Supplementary Figure 1),
which was, fortunately, also the relevant time period for our assays. 1%-‐Tween CFU graphs
were used to determine cell number for all assays.
Flask Fitness Analysis
A single colony of each bacteria strain was used to inoculate 10 ml LB, the incubated O/N at 25
°C with shaking at 120 rpm. Following 18H of incubation, 109 bacteria was withdrawn from the
O/N cultures and added along with 104 bacteriophages to inoculate 10 LB in quintuplicate for a
multiplicity of infection (MOI) of 1:100,000. Bacteria and phage are incubated O/N at 25 °C with
shaking at 120 rpm. After another 18H, bacteria and phage cultures are centrifuged for 10 min
at RCF = 1400xg to pellet bacteria and debris then passed through a 0.22 μm filter to obtain a

122
bacteria-‐free lysate. Lysates are subsequently titered on PP. Experiment was repeated in
triplicate (n = 15).
High-‐Throughput Microplate Fitness Analysis
Three flasks with 10 ml LB were inoculated each from a single colony of the three bacteria
strains and grown O/N at 25 °C with shaking at 120 rpm. At 18H, 200 μl of the stationary
cultures were used to inoculate 20 ml LB and grown at 25 °C with shaking at 120 rpm. OD600
was measured for each culture until they reached an OD600 corresponding to 109 bacteria per
100 μl (Supplemental Figure 1). Once each culture reached the target OD600, those cultures
were incubated at 4 °C on ice until all three cultures reached the desired OD. ODs were verified
before plating on a 96-‐well microplate. Each strain was plated in 12 replicates, either bacteria
alone (109), or bacteria plus 104 phages (HRM S154). OD was measured in a Tecan Infinite Pro
(Tecan; Morrisville, NC) microplate reader at 600 nm. ODs were measure every 5 minutes, 200
sec of orbital shaking, 5 flashes, with the lid on over the course of 15H at 25°C. Each sample was
plated in replicates of 12 and each experiment repeated in triplicate (n=36).
Statistics
All statistical analyses were calculated using GraphPad Prism Macintosh v6.0f (GraphPad, La
Jolla, CA). Bacterial growth with surfactant (Figure 3) and Colony Forming Unit Area Comparison
(Figure 4) were both analyzed via two-‐way analysis of variance (ANOVA) followed by a post-‐hoc
Tukey’s multiple comparison test (column effect = surfactant; row effect = strain). Flask fitness
(Figure 5A) was analyzed by one-‐way ANOVA. The column statistics function was used to

123
calculate the area under the curve (AUC), maximum time to infection (x-‐max) and maximum
bacteria growth (y-‐max) for microplate fitness data, which in turn were analyzed by an unpaired
two-‐tailed t-‐test (Figure 5B) and summarized in Supplementary Table 1. The non-‐linear curve
fitting function was used to determine the formula to correlate OD with time (Supplementary
Figure 1).
DNA Preparation for Next Generation Sequencing
A single colony of each of the three bacterial strains was used to inoculate 10 ml LB and grown
O/N at 25 °C with shaking at 120 rpm. At 24H, cultures were processed using a PowerSoil DNA
Isolation Kit (Mo Bio, Carlsbad, CA) and sent out for Illumina Next Generation Sequencing (NGS)
at the Genomic Sciences Lab at North Carolina State University.
Results
1. Characterization
1.1 Growth
Growth parameters of each strain were characterized by growth parameters, cell morphology,
colony morphology and virus susceptibility.

124
1.1.1 Phenotype Screening
As the original selection protocol resulted in a mixed population (Figure 1), strains were tested
for phenotypic heterogeneity. In order to distinguish between phenotypes, we screened their
growth parameters using a 96-‐well microplate reader. At 15H, colonies with the planktonic
phenotype reached an OD600 of ~0.9 while all control strains only reached ~0.2 (data not
shown). Colonies displaying the planktonic phenotype were mixed 1:1 (v/v) with 80%-‐glycerol
and frozen at -‐20 °C. These selected homogeneous strains were used in all subsequent assays.
1.1.2 Buoyancy
The planktonic phenotype is characterized as an unattached, free-‐floating and motile form of
the bacteria [10]. To test whether the buoyancy demonstrated by our lab-‐evolved strain is
motility dependent, we grew them at a non permissive temperature and observed resultant
buoyancy. Results are summarized below in Table 1.
Table 1. Heat Shock Growth Verification Strain 25 °°C 37 °°C 55 °°C Sessile + + -‐
Passage Control + + -‐ Planktonic + + -‐
Samples from 24H replicate strains were divided and moved to three temperatures: 25°C, 37°C and 55°C for 24H and subsequently assayed for growth. + = growth, -‐ = no growth.
Despite the death of the bacteria grown O/N at 55 °C, no settling was observed for the
planktonic strain (Figure 2C) but observed in the two control strains (Figure 2A-‐B), even after 96
H.

125
1.1.3 Clumping
To test for the presence of clumping, bacteria strains were grown in the presence or absence of
a surfactant (1%-‐Tween-‐80) and subsequently plated. There was a significant difference in
number of observed colonies for the two control strains but not the planktonic strain (Figure 3)
suggesting the planktonic strain does not clump. The surfactant Triton-‐X-‐100 was also tested
but did not give as good a separation of cells as Tween-‐80 (data not shown).
1.2 Morphology
1.2.1 Cell Morphology
ERA, whether the unevolved, sessile phenotype or the planktonic phenotype, is a gram negative
bacillus. Representative images for each strain: Sessile, Passage Control and Planktonic are
presented in Figure 2. The two control strains, Sessile and Passage Control both present similar
morphologies and observed frequencies of pili and flagella (Figure 2D-‐F). The planktonic strain,
by contrast, consistently presented both more numerous and longer pili and flagella (Figure 2F).
1.2.2 Colony Morphology
Colony morphology was measured over the course of 6 hours. Aliquots were taken from
cultures in exponential phase at six time points both in the presence and absence of the
surfactant tween-‐80 (1% by volume). Representative images of colonies (grown in the absence
of tween) for each strain are presented in Figure 2G-‐I. As we did not measure any significant

126
difference between colony sizes based on time point (data not shown), data were aggregated
across time points for comparison of grown with and without surfactant for a final n ≥ 90
colonies per strain (3 photos x ≥5 colonies x 6 time points = ≥90) and summarized in Figure 4.
Results demonstrate that addition of surfactant does not affect CFU area. However, we do see a
significant difference in CFU area size between the planktonic strain and its two control strains
(Figure 4).
1.3 Virus Susceptibility
1.3.1 Flask Fitness
Flask fitness is a standard measure of bacteriophage fitness on a given host. We measured the
fitness of the previously isolated Φ6-‐derived host range mutant (HRM) S154 [6] on the three
strains. S154 was significantly better (1-‐way ANOVA; F = 37.97, DF = 2, p < 0.0001) at infecting
the planktonic strain over the two control strains (Figure 5A). We measured no significant
difference between S154 fitness on the two control strains. The fitness of S154 on the—
canonical bacteriophage Φ6—host PP is again an order of magnitude larger than for the
planktonic phenotype (data not shown).
1.3.2 High Throughput Fitness Assays
HRM S154 fitness was measured on all three strains via a 96-‐well Tecan Infinite 200 microplate
reader. Fitness, for the purpose of our assay, is the number of progeny a phage is able to
produce on a particular host. The higher the number of progeny (meaning the greater the

127
number of new virus) produced, the more the phage is able to infect, leading to a decrease in
measured OD600 (as infection leads to lysis and subsequent clearing of the host cells). Fitness of
Φ6 on its canonical host PP is relatively easy to measure in a high-‐throughput manner. PP’s OD
over 15H increases from 0.02 to over 9 thereby taking advantage of the full resolution of the
spectrophotometer. By contrast, sessile ERA grows to stationary phase much quicker and forms
clumps thereby reducing the reproducibility of of the assay. Unlike sessile ERA phenotype, the
planktonic phenotype OD increases from 0.02 to more than 9 over the course of 15H, thereby
increasing the resolution of this bacteria in this assay and adding sensitivity (as shifts in growth
patterns were be more easily visualized) (Figure 5B). In our assay, the addition of S154
significantly (unpaired t-‐test; p < 0.0001, Supplementary Table 1) alters the growth of the
planktonic phenotype and to a lesser degree the passage control strain (unpaired t-‐test; p =
0.0132) but not the sessile strain (Figure 5B).
1.4 Comparisons
1.4.1 Planktonic vs Passage Control
In a microplate reader setting, growth of the passage control was significantly (Supplementary
Table 1) different from that of the planktonic strain (Figure 5B) indicating that simple serial
passage of sessile ERA is insufficient to induce a planktonic phenotype. Interestingly, both the
passage control strain and the planktonic strain demonstrated a significant susceptibility to
bacteriophage infection (Figure 5B; p = 0.01 and p < 0.0001 respectively), unlike the sessile
strain.

128
1.4.2 Planktonic versus Sessile
Growth of the sessile strain is significantly different from that of the planktonic phenotype
(Figure 5B). In a microplate reader setting, planktonic growth parameters are ideal for the
spectrophotometer's range (0 to 1) with an OD increase of 0.02 to >9 over the course of 15H.
This growth of ~0 to 9 is much different from that for the sessile phenotype, ~0 to ~2, indicating
an increase in sensitivity for assays which perturb ERA growth (e.g. bacteriophage fitness
assays). The planktonic phenotype produces longer and more abundant pili and flagella
compared to the sessile strain.
1.4.3 Planktonic versus PP
Just as for its two control strains, the planktonic phenotype produces longer and more
abundant pili and flagella compared to the sessile strain compared to PP. However, in a plate
reader setting, both the planktonic phenotype and PP demonstrate similar OD increases over
the same amount of time (data not shown). Although S154 fitness on PP is still greater than
that on the planktonic phenotype ERA, the fitness difference between those two is much less
than it is between PP and either the ERA sessile phenotype or the passage control strain. These
data suggest the planktonic ERA as a better candidate to study phage fitness consequences
from shifts back and forth between hosts than the sessile phenotype.

129
1.5 Genotyping
We have received the sequences from the Illumina NGS and coverage for the three strains is
high: sessile (~130 x), passage control (~146 x) and planktonic (~154 x). Preliminary contigs and
raw reads have been deposited to the National Center for Biotechnology Information (NCBI)
Whole Genome Sequencing (WGS) portal under the BioProject number PRJNA287670, with
individual accession numbers LFMA00000000 (sessile phenotype), LFMB00000000 (passage
control) and LFMC00000000 (planktonic phenotype). Sequences have been sent to a
collaborator at Hunter College for assembly.
Discussion
Starting with a sessile phenotype, serial passage emphasizing phenotypes that evade
sedimentation results in a population with an ability to grow to a higher density (Figure 3) and
that does not form clumps (Figure 2C). This increased growth and loss of clumping comes at a
cost: a heightened susceptibility to predation by phage (Figure 5A). Whether the increased
susceptibility to phage is a result of the loss of the ability to clump or due to the increased
production of flagella and pili requires further experimentation. Different sets of pilins have
been shown to be expressed in sessile growth compared to planktonic growth [11,12]
suggesting another possible route for increased predation by phage, keeping in mind that the
first step in a Φ6 infection is adsorption to the pilus [13,14]. We observed a marked increase in

130
both the length and abundance of flagella in the planktonic strain. Flagella have been
demonstrated to be important for adhesion to surfaces [15,16]; perhaps they play a
mechanical role by increasing the chances of phage and pilus encounters. Previous studies have
demonstrated that motility is regulated by the envelope stress response in Pseudomonas [17]
opening the possibility that the observed increase in flagella may not be in response to the
sedimentation per se but rather the stressful conditions of centrifugation. However, the sessile
phenotype has previously been demonstrated to be the precursor for biofilm formation
[18,19], which in turn is a well established defense against predation by phage [20-22]. These
studies would seem to support the theory that the increased predation by phage is a result of
the loss of clumping. It is well established that bacteriophage alter their host’s environment
[23], for example by affecting the bacterial quorum sensing, genome, motility, etc. [24]. The
observation that the addition of low MOI phage affects the growth dynamics of the planktonic
phenotype but not the sessile phenotype (Figure 5B) suggests that at one point in time the
original ERA strain may have been subject to predation.
We have observed this change in phenotype, but as of yet the source of the variation is
unknown. In order to determine the source of the planktonic phenotype—whether it is a
reversible phenotypic switch [25-27], normal phenotype heterogeneity [28], or a genetic
mutation [29,30]—we sent out the three strains for next generation sequencing (NGS) with the
goal of identifying gene candidates for the switch. Although we lack a reference strain, we will
look for homologs of pilus and flagella genes as a starting point.

131
One area where the planktonic phenotype presents a particular advantage is in a high-‐
throughput—or microplate reader—setting. High-‐throughput fitness screening has been
previously validated for PP[31] but not yet ERA. Flask fitness is reagent, space and labor
intensive; being able to assay more strains with less reagents offers both cost and time savings
advantages. It also opens the way to testing more combinations and iterations in a more
reproducible manner, increase replication and thereby increase the power of the assays. Under
our initial assay conditions, a concentration of phage that significantly altered the growth of of
the planktonic phenotype, had no measurable effect on the sessile phenotype (Figure 5B). This
novel assay is of particular interest as we previously isolated 69 host range mutants of Φ6 [6]
and will now be able to simultaneously test combinations of these mutants on ERA and PP
together. Previously it was not possible to compare phage fitness on host strains with
dramatically different growth differences. This assay also opens the door to testing how well
these phages—which have genetically mutated, often with single point mutations but also
double and triple mutations—fare when put back in the context of their original host of
isolation, PP.
Author Contributions
BEF & JJD designed the study and analyzed the data, EYL & GVB isolated the non clumping
strains and performed initial fitness assays, BEF & DT conducted phenotype isolation, fitness
assays, and physical characterization, EYL isolated the DNA for NGS; BEF & EYL assembled the

132
NGS; BEF carried out TEM analysis, MB prepared the reagents, and all authors wrote the
manuscript.
Acknowledgements
This work was funded by the National Science Foundation Early Career Award to JJD (DEB
#1148879).
Captions
Figure 1. Selection
Triplicate overnight colonies of ERA were grown each from a single colony, briefly centrifuged
to sediment the bacteria and supernatant was transferred to fresh media daily for 14 days.
Colony morphology was assayed on days 7 and 14. Planktonic colonies were streaked out for
single colonies, with each colony assayed for desired phenotype.
Icons from thenounproject.com.
Figure 2. Phenotype, Cellular and Colony Morphologies
(A-‐C) Bacterial buoyancy following 96H incubation at 4. (D-‐F) Transmission electron
micrographs (magnification = 15,000 x) of cell morphology. Multiple and longer flagella and pili
are visible on the planktonic form. (G-‐H) Planktonic colony forming units are significantly
smaller than the control strains.

133
Figure 3. Bacterial Growth with Surfactant
Bacteria strains were grown in triplicate (n=3) in the presence or absence of tween-‐20 in flasks.
Error bars represent SEM.
Figure 4. Colony Forming Unit Area Comparison
Strains were grown in the presence or absence of the surfactant Tween-‐80 at 1%. Cell areas (n ≥
90) were measured using built-‐in functions of the ImageJ software. Error bars represent SEM.
Figure 5. Susceptibility to Bacteriophage Infection
(A) Flask fitness. Strains were assayed in quintuplicate and repeated in triplicate (n = 15).
Colored bars indicate the mean. PP fitness is significantly higher than all three treatments (data
not shown). (B) Plate fitness. Each strain had 12 replicates per experiment and each experiment
was repeated in triplicate (n = 36). Error bars representing ±SEM are smaller than icons for all
strains except “Passage Control + S154”. ns = not significant; **** indicates a p < 0.0001.
Supplementary Figure 1. Growth Curve and Plating Number
Determination of relationship between OD600 and cell number. This relationship was somewhat
complicated by the clumping of cells in the control strains. To rectify this discrepancy, each of
the three strains was grown in both the presence and absence of 1%-‐Tween-‐80 allowing
calculation of true cell number for each measured OD. Error bars represent ±SEM. Experiments
were repeated in triplicate.

134
Supplementary Table 1. Microplate Fitness Statistics Summary
Summary of the statistical results of an unpaired two-‐tailed t-‐test (assuming a Gaussian
distribution) using three measures for each bacteria strain: area under curve (AUC), time to
maximum infection (x-‐max) and maximum bacteria growth (y-‐max).
Figures
Figure 1. Planktonic Selection Scheme

135
Figure 2. Phenotype, Cellular and Colony Morphologies

136
Figure 3. Bacterial Growth with Surfactant

137
Figure 4. Colony Forming Unit Area Comparison

138
Figure 5. Susceptibility to Bacteriophage Infection

139
Supplementary Tables and Figures
Supplementary Figure 1. Growth Curve and Plating Number Supplementary Table 1. Microplate Fitness (Bacteria vs Bacteria + Phage) Unpaired T-‐Test Summary AUC x-‐max y-‐max t P Sig. t P Sig. t P Sig. Sessile 1.092 0.2788 ns 1.484 0.1423 ns 1.057 0.2940 ns Passage Control 2.544 0.0132 * 2.609 0.0111 * 3.002 0.0037 **
Planktonic 11.63 <0.0001 **** 10.69 <0.0001 **** 17.46 <0.0001 **** AUC = area under curve; x-‐max = time at highest OD; y-‐max = highest OD; t = t ratio; Sig. = significance; ns = not significant. All reported P values are from two-‐tailed t-‐tests.

140
References
1. Montville R, Froissart R, Remold SK, Tenaillon O, Turner PE. Evolution of Mutational Robustness in an RNA Virus. PLoS Biol. 2005;3: e381. doi:10.1371/journal.pbio.0030381.st002
2. Dennehy JJ, Friedenberg NA, Holt RD, Turner PE. Viral ecology and the maintenance of novel host use. Am Nat. 2006;167: 429–439. doi:10.1086/499381
3. Duffy S, Burch CL, Turner PE. EVOLUTION OF HOST SPECIFICITY DRIVES REPRODUCTIVE ISOLATION AMONG RNA VIRUSES. Evolution. 2007;61: 2614–2622. doi:10.1111/j.1558-‐5646.2007.00226.x
4. Turner PE, Morales NM, Alto BW, Remold SK. ROLE OF EVOLVED HOST BREADTH IN THE INITIAL EMERGENCE OF AN RNA VIRUS. Evolution. 2010;64: 3273–3286. doi:10.1111/j.1558-‐5646.2010.01051.x
5. Bono LM, Gensel CL, Pfennig DW, Burch CL. Competition and the origins of novelty: experimental evolution of niche-‐width expansion in a virus. Biology Letters. 2012;9: 20120616–20120616. doi:10.1098/rsbl.2012.0616
6. Ford BE, Sun B, Carpino J, Chapler ES, Ching J, Choi Y, et al. Frequency and Fitness Consequences of Bacteriophage Φ6 Host Range Mutations. van Raaij MJ, editor. PLoS ONE. Public Library of Science; 2014;9: e113078. doi:10.1371/journal.pone.0113078
7. Turner PE, McBride RC, Duffy S, Montville R, Wang L-‐S, Yang YW, et al. Evolutionary genomics of host-‐use in bifurcating demes of RNA virus phi-‐6. BMC Evol Biol. BMC Evolutionary Biology; 2012;12: 1–1. doi:10.1186/1471-‐2148-‐12-‐153
8. Duffy S, Turner PE, Burch CL. Pleiotropic Costs of Niche Expansion in the RNA Bacteriophage Φ6. Genetics. Genetics Society of America; 2006;172: 751–757. doi:10.1534/genetics.105.051136
9. Mindich L, Cohen J, Weisburd M. Isolation of nonsense suppressor mutants in Pseudomonas. J Bacteriol. 1976;126: 177–182.
10. Abedon S. Bacteriophages & Biofilms: Ecology, Phage Therapy, Plaques. 2011.
11. Pohlschroder M, Esquivel RN. Archaeal type IV pili and their involvement in biofilm formation. Front Microbiol. 2015;06: 1–16. doi:10.3389/fmicb.2015.00190
12. Klausen M, Heydorn A, Ragas P, Lambertsen L, Aaes-‐Jørgensen A, Molin S, et al. Biofilm formation by Pseudomonas aeruginosa wild type, flagella and type IV pili mutants. Mol Microbiol. 2003;48: 1511–1524. doi:10.1046/j.1365-‐2958.2003.03525.x

141
13. Kenney JM, Hantula J, Fuller SD, Mindich L, Ojala PM, Bamford D. Bacteriophage phi 6 envelope elucidated by chemical cross-‐linking, immunodetection, and cryoelectron microscopy. Virology. 1992;190: 635–644.
14. Romantschuk M, Olkkonen VM, Bamford D. The nucleocapsid of bacteriophage phi 6 penetrates the host cytoplasmic membrane. EMBO J. 1988;7: 1821–1829.
15. Bruzaud J, Tarrade J, Coudreuse A, Canette A, Herry J-‐M, de Givenchy ET, et al. Flagella but not type IV pili are involved in the initial adhesion of Pseudomonas aeruginosa PAO1 to hydrophobic or superhydrophobic surfaces. Colloids and surfaces B, Biointerfaces. Elsevier B.V; 2015;131: 1–33. doi:10.1016/j.colsurfb.2015.04.036
16. Pratt LA, Kolter R. Genetic analysis of Escherichia coli biofilm formation: roles of flagella, motility, chemotaxis and type I pili. Mol Microbiol. 1998;30: 285–293.
17. Dougherty K, Smith BA, Moore AF, Maitland S, Fanger C, Murillo R, et al. Multiple Phenotypic Changes Associated with Large-‐Scale Horizontal Gene Transfer. Brown SP, editor. PLoS ONE. 2014;9: e102170. doi:10.1371/journal.pone.0102170
18. Oliveria NM, Martinez-‐Garcia E, Xavier J, Durham WM, Kolter R, Kim W, et al. Biofilm Formation As a Response to Ecological Competition. Laub MT, editor. PLoS Biol. 2015;13: e1002191–23. doi:10.1371/journal.pbio.1002191
19. Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. American Association for the Advancement of Science; 1999;284: 1318–1322. doi:10.1126/science.284.5418.1318
20. Tan D, Dahl A, Middelboe M. Vibriophages Differentially Influence Biofilm Formation by Vibrio anguillarum Strains. Wommack KE, editor. Appl Environ Microbiol. 2015;81: 4489–4497. doi:10.1128/AEM.00518-‐15
21. Tan D, Svenningsen SL, Middelboe M. Quorum Sensing Determines the Choice of Antiphage Defense Strategy in Vibrio anguillarum. MBio. 2015;6: e00627–15–11. doi:10.1128/mBio.00627-‐15
22. Lacqua A, Wanner O, Colangelo T, Martinotti MG, Landini P. Emergence of Biofilm-‐Forming Subpopulations upon Exposure of Escherichia coli to Environmental Bacteriophages. Appl Environ Microbiol. 2006;72: 956–959. doi:10.1128/AEM.72.1.956-‐959.2006
23. Clokie MR. Bacteriophage behavioral ecology: How phages alter their bacterial host's habits. Bacteriophage. 2014;4: e29866. doi:10.4161/bact.29866
24. Boyd EF, Brüssow H. Common themes among bacteriophage-‐encoded virulence factors and diversity among the bacteriophages involved. Trends Microbiol. 2002;10: 521–529.

142
25. van Vliet S, Ackermann M. Bacterial Ventures into Multicellularity: Collectivism through Individuality. PLoS Biol. 2015;13: e1002162–5. doi:10.1371/journal.pbio.1002162
26. McElroy KE, Hui JGK, Woo JKK, Luk AWS, Webb JS, Kjelleberg S, et al. Strain-‐specific parallel evolution drives short-‐term diversification during Pseudomonas aeruginosa biofilm formation. Proc Natl Acad Sci USA. 2014;111: E1419–E1427. doi:10.1073/pnas.1314340111
27. Be'er A, Florin EL, Fisher CR, Swinney HL, Payne SM. Surviving Bacterial Sibling Rivalry: Inducible and Reversible Phenotypic Switching in Paenibacillus dendritiformis. MBio. 2011;2: e00069–11–e00069–11. doi:10.1128/mBio.00069-‐11
28. Norman TM, Lord ND, Paulsson J, Losick R. Memory and modularity in cell-‐fate decision making. Nature. 2013;503: 481–486. doi:10.1038/nature12804
29. Hallet B. Playing Dr Jekyll and Mr Hyde: combined mechanisms of phase variation in bacteria. Current Opinion in Microbiology. 2001;4: 570–581.
30. Dhar N, McKinney JD. Microbial phenotypic heterogeneity and antibiotic tolerance. Current Opinion in Microbiology. 2007;10: 30–38. doi:10.1016/j.mib.2006.12.007
31. Turner PE, Draghi JA, Wilpiszeski R. High-‐throughput analysis of growth differences among phage strains. J Microbiol Methods. Elsevier B.V; 2012;88: 117–121. doi:10.1016/j.mimet.2011.10.020

143
Conclusion
Three regions on the host attachment P3 gene—located on the middle segment—appear to be
important for host range expansion in Φ6 (Chapter 2, Figure 1A). That these three regions all lie
on the same face of the molecule (Chapter 2, Figure 1B), opposite the putative P6 binding
domain, lends credence to the validity of the predicted 3D structure. Though all of the 69 host
range mutants are able to infect the novel host ERA (Figure 1), they are not able to do so
equally (Chapter 2, Figure 2A). In all save one case, the Φ6 host range mutants had reduced
fitness on their canonical host PP in relation to their ancestor (Chapter 2, Figure 2B), an
example of antagonistic pleiotropy (gaining a new host comes with costs on the old host).
Figure 1. Pseudomonas pseudoalcaligenes East River Isolate A (ERA). Electron micrograph of ERA—the novel host of Φ6 mutants—at 20,000 x magnification. Cells were grown overnight at 25 °C and negatively stained with a 1% uranyl acetate (UA) solution.
A non genetic host-‐acquired factor—most likely either the lipid envelope or proteins contained
therein—was demonstrated to have a role in subsequent bacteriophage fitness (Chapter 3,
cba Brian E. Ford, The Dennehy Lab

144
Figure 2). Similar trends were observed across different viruses with differing niches, host
ranges and infection dynamics. These finding suggest that the host factor impacts virus
emergence: they are able to gain an ability from their host that changes how well they are able
to infect subsequent hosts.
Finally, the last study demonstrates how bacteriophage can affect their bacteria host
phenotype. The planktonic strain shows an almost 2-‐fold increase in susceptibility to
bacteriophage infection in relation to sessile form (Chapter 4, Figure 5A). The planktonic strain
displays superior growth dynamics in plate fitness assays in comparison to sessile strain
(Chapter 4, Figure 5B) suggesting this lab-‐evolved strain to be a valuable tool for testing the
fitness of host range mutants switching between hosts. These combined findings also
emphasize a bacteria’s growth options: increased growth (with an increased susceptibility to
infection) or protected growth (improved protection but to a lower density).

145
(Introduction) References
1. Vidaver AK, Koski RK, Van Etten JL. Bacteriophage phi6: a Lipid-‐Containing Virus of
Pseudomonas phaseolicola. J Virol. American Society for Microbiology; 1973;11: 799–805.
2. Mindich L, Qiao X, Qiao J, Onodera S, Romantschuk M, Hoogstraten D. Isolation of additional bacteriophages with genomes of segmented double-‐stranded RNA. J Bacteriol. 1999;181: 4505–4508.
3. King AM, Lefkowitz E, Adams MJ, Carstens EB, editors. Virus Taxonomy: Ninth Report of the International Committee on Taxonomy of Viruses. 1st ed. Elsevier; 2011.
4. Ojosnegros S, García-‐Arriaza J, Escarmís C, Manrubia SC, Perales C, Arias A, et al. Viral Genome Segmentation Can Result from a Trade-‐Off between Genetic Content and Particle Stability. Malik HS, editor. PLoS Genet. 2011;7: e1001344. doi:10.1371/journal.pgen.1001344.t002
5. O’Keefe KJ, Silander OK, McCreery H, Weinreich DM, Wright KM, Chao L, et al. GEOGRAPHIC DIFFERENCES IN SEXUAL REASSORTMENT IN RNA PHAGE. Evolution. 2010;: no–no. doi:10.1111/j.1558-‐5646.2010.01040.x
6. Gottlieb P, Potgieter C, Wei H, Toporovsky I. Characterization of phi12, a bacteriophage related to phi6: nucleotide sequence of the large double-‐stranded RNA. Virology. 2002;295: 266–271. doi:10.1006/viro.2002.1436
7. Leo-‐Macias A, Katz G, Wei H, Alimova A, Katz A, Rice WJ, et al. Toroidal surface complexes of bacteriophage ϕ12 are responsible for host-‐cell attachment. Virology. Elsevier Inc; 2011;: 1–7. doi:10.1016/j.virol.2011.03.020
8. Jäälinoja HT, Huiskonen JT, Butcher SJ. Electron Cryomicroscopy Comparison of the Architectures of the Enveloped Bacteriophages ϕ6 and ϕ8. Structure. 2007;15: 157–167. doi:10.1016/j.str.2006.12.004
9. Turner PE, Morales NM, Alto BW, Remold SK. ROLE OF EVOLVED HOST BREADTH IN THE INITIAL EMERGENCE OF AN RNA VIRUS. Evolution. 2010;64: 3273–3286. doi:10.1111/j.1558-‐5646.2010.01051.x
10. Dennehy JJ. Bacteriophages as model organisms for virus emergence research. Trends Microbiol. 2009;17: 450–457. doi:10.1016/j.tim.2009.07.006
11. Freeland JR. Molecular Ecology. 1st ed. Wiley; 2006.