Antigen processing and Presentation
description
Transcript of Antigen processing and Presentation

Antigen processing and Presentation
Dr. Sheeba Murad Mall19th March 2012

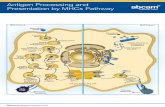
Antigen processing and Presentation• T cell requires that peptides derived from the antigen be displayed
within the cleft of an MHC molecule on the membrane of a cell• The formation of these peptide-MHC complexes requires that a
protein antigen be degraded into peptides by a sequence of events called antigen processing
• The degraded peptides then associate with MHC molecules within the cell interior, and the peptide- MHC complexes are transported to the membrane, where they are displayed- antigen presentation
• Class I and class II MHC molecules associate with peptides that have been processed in different intracellular compartments i.e. cytoplasmic or endocytic
• a third pathway for the presentation of nonpeptide antigens derived from bacterial pathogens is through non-classical MHC molecules

Self-MHC restriction
• A very important trait of antigen recognition in context to MHC molecules is
self- restriction • i.e, Both CD4 and CD8 T cells can recognize
antigen only when it is presented by a self-MHC molecule, an attribute called self-MHC
restriction

Display antigen through MHC class I molecule
• Class I MHC molecules bind peptides derived from endogenous antigens that have been processed within the cytoplasm of the cell– e.g., normal cellular proteins, – tumor proteins, – or viral – and bacterial proteins produced within infected
cells)

• In eukaryotic cells, protein levels are carefully regulated• Every protein is subject to continuous turnover and is
degraded at a rate that is generally expressed in terms of its half life– Some proteins (e.g., transcription factors, cyclins, and key
metabolic enzymes) have very short half-lives– denatured, miss folded, or otherwise abnormal proteins
also are degraded rapidly• Through MHC class I pathway
– normal turnover of intracellular proteins– endogenous antigens are degraded for presentation

Evidence for Antigen processing before presentation to T cells
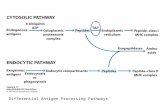
Overview of cytosolic and endocytic pathways fo processing antige TAP (transporter of antigenic peptides)
It should benoted that the ultimate fate of most peptides in the cell is neitherof these pathways, but rather to be degraded completely intoamino acids.

Endogenous Antigens:Degradation
• Intracellular proteins are degraded into short peptides by a cytosolic proteolytic system present in all cells
• Those proteins targeted for proteolysis have a small protein, called ubiquitin
• Proteolytic inhibitors inhibiting the activity of the proteasome- inhibit antigen presentation by MHC class I molecules
Cytosolic proteolytic system for degradation of intracellular
proteins

TAP1 and TAP2 defective cells
• A mutant cell line, called RMA-S, expresses about 5% of the normal levels of class I MHC molecules on its membrane although the despite of the synthesis of normal levels of class I chains and 2-microglobulin
• The DNA of the mutant cells showed that the genes affected encoded members of the ATP-binding cassette (ABC) family of proteins and that these proteins were absent or nonfunctional in the mutant cells
• ABC proteins mediate the ATP-dependent transport of ions, sugars, amino acids, and peptides across membranes
• The two ABC proteins missing from the mutant cells are normally associated with the endoplasmic reticulum membrane and are called transporters associated with antigen processing-I and -2 (TAPI and TAP2)
• The genes TAPl and TAP2 map within the MHC (see Section 6-ll) and are inducible by interferons, which are produced in response to viral infection; indeed, viral infection increases the delivery of cytosolic peptides into the endoplasmic reticulum
• It prefers peptides of between 8 and 10 amino acids in length, with hydrophobic or basic residues at the carboxy terminus-the precise features of peptides that bind MHC class I molecules

Peptides Assemble with Class I MHC Aided
by Chaperone Molecules
• TAP is a membrane spanning heterodimeric protein
• Belong to ATP binding cassette proteins
Generation of antigenic peptide–class I MHC complexes in the cytosolic pathway

Peptide editing before antigen loading• DRiPs (defective ribosomal products) are tagged by ubiquitin for rapid degradation by the
proteasome– 30%-of peptides and proteins known as (DRiPs) including peptides translated from introns in improperly spliced
mRNAs, translations of frameshifts, and improperly folded proteins
• The proteasome excision-splicing mechanism, can increase the pool of peptides through which an internal segment of a protein is removed and the surrounding noncontiguous polypeptide segments are joined and used as the peptide presented by MHC class I
• Certain cellular chaperones such as the TCP-1 ring complex (TRiC) protect some peptides from complete degradation
• Peptides too long to bind MHC class I molecules can still be transported into the endoplasmic reticulum with the help of ERAAP
• amino termini can be trimmed by an aminopeptidase called the endoplasmic reticulum aminopeptidase associated with antigen processing (ERAAP)
• The aminopeptidase ERAAP trims the peptides at their amino termini, allowing peptides that are too long to bind, increasing the repertoire of potential peptides for presentation
• ERAAP is up regulated by IFN-y• ERAAP is an important editor of the normal peptide: MHC repertoire as many unstable and
immunogenic peptides are found in complexes with MHC molecules on the cell surface of ERAAP deficient mice

Peptide loading on MHC class I molecules in the endoplasmic reticulum

• Calnexin: Newly synthesized MHC class I a chains assemble in the endoplasmic reticulum (ER) with the membrane-bound protein calnexin
• Calnexin also associates with partly folded T-cell receptors, immunoglobulins, and MHC class II molecules, and so has a central role in the assembly of many immunological proteins
• Peptide loading complex (PLC): – MHC class I a:B2m dimer dissociates from calnexin– Calreticulin– ERp57 (thiol oxido reductase)– Tapasin– TAP
• This whole complex ensures– Maintaining the receptive state of MHC I– To ensure peptide editing- exchange the low affinity peptides with high affinity
peptides• In vitro binding studies suggest that the ERp57:tapasin heterodimer functions in editing
peptides binding to MHC class I• Cells defective in either calreticulin or tapasin show defects in the assembly of MHC class
I molecules, and those molecules that reach the cell surface are bound to suboptimal, low-affinity peptides

• Most of the peptides transported by TAP are transported back into the cytosol by an ATP-dependent transport complex distinct from TAP, known as the Sec61 complex and are rapidly cleared out of the endoplasmic
reticulum

IMMUNOEVASINS
Immunoevasins produced byviruses interfere with the processingof antigens that bind to MHC class Imolecules