ADENOCARCINOMA OF THE COLON AND RECTUM · gastro adenocarcinoma of the colon and rectum — 3 In...
Transcript of ADENOCARCINOMA OF THE COLON AND RECTUM · gastro adenocarcinoma of the colon and rectum — 3 In...

Scientifi c American SurgeryDOI 10.2310/7800.2073
09/14
© 2014 Decker Intellectual Properties Inc
gastrointestinal tract and abdomen
A D E N O C A R C I N O M A O F T H E C O L O N A N D R E C T U M
Martin R. Weiser, MD, and Leonard B. Saltz, MD*
Colorectal cancer (CRC) is one of the most dynamic fi elds in oncology. The molecular events associated with cellular transformation were reported over 20 years ago, and intense study of the mechanisms of carcinogenesis and tumor progression continues. Molecularly based therapies now in use may be harbingers of more elegant, tumor-specifi c CRC therapy. Clinically, CRC is a diverse disease, requiring individually tailored treatment strategies. This topic review discusses the most current data on the epidemiology, screen-ing, diagnosis, staging, and multimodal treatment of CRC.
Incidence and Epidemiologic Associations
Worldwide, over 1 million people are diagnosed with CRC annually, and there are more than 500,000 associated deaths.1 The highest rates of colorectal carcinoma are found in industrialized countries. The rates are signifi cantly lower in eastern Europe, Asia, Africa, and South America.2 However, studies of Japanese migration to the United States, Asiatic Jewish migration to Israel, and eastern European migration to Australia show that migrants acquire the high rates of CRCs prevalent in their adopted countries. There is little question that environmental factors, most likely dietary, account for this.
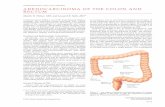
Colon cancer is three times more common than rectal cancer. Interestingly, epidemiologic studies indicate a rising proportion of right-sided colonic lesions. The proximal migration of colon cancer may be associated with changing environmental factors; however, there is no doubt that increased screening successfully detects early lesions in an aging population [see Figure 1].3
CRC ranks as the third most common malignancy in the United States (behind prostate and lung cancer in men and breast and lung cancer in women) and the second leading cause of cancer-related mortality. Approximately 143,000 patients are diagnosed with CRC in the United States each year, and 51,000 die of disease.4,5 The probability of CRC developing during an individual’s lifetime is about 6%. In contrast to the three previous decades, however, the overall incidence and mortality of CRC have declined for both men and women. Age-adjusted incidence and mortality are associated with race and ethnicity; however, the relation-ships are complex, infl uenced by social and economic confounding factors more than tumor biology.6
Genetic Pathways to CRC
Cancer cells are characterized by an ability to avoid normal aging and death. Cumulative sequential mutations
in oncogenes, and an associated mutational deactivation of tumor suppressor genes over time, result in the production of abnormal cells that grow uncontrollably, invading local tissues and metastasizing to distant organs. This model of tumorigenesis, the adenoma-carcinoma cascade in CRC, was fi rst described by Fearon and Vogelstein in 1990.7
Genomic instability is key to tumor development, and several genetic pathways, each characterized by specifi c mutations, lead to the development of CRC. The chromo-somal instability (CIN) pathway is typifi ed by an accumula-tion of deactivated tumor suppressor genes and abnormally active proto-oncogenes. Tumors developing along this path-way demonstrate characteristic mutations of the APC, TP53, and K-ras genes, allelic loss of 18q, and aneuploidy. The APC gene is crucial in tumor development: virtually all (100%) patients with familial adenomatous polyposis (FAP), who carry this mutation, develop CRC in the absence of preven-tive surgery. This progression was recently reevaluated using large-scale genetic “comparative lesion” sequencing of multiple lesions from a single patient.8 The investigators
* The authors and editors gratefully acknowledge the contribu-tions of the previous authors, Bruce M. Brenner, MD, FACS, and David M. Ota, MD, FACS, to the development and writing of this topic review.
Cecum17% (15%)
Rectum18% (21%)
Ascending Colon12% (8%)
DescendingColon4% (6%)
Sigmoid Colon23% (25%)
Rectosigmoid Junction10% (10%)
Transverse Colon13% (13%)
Figure 1 The relative frequencies of colorectal cancer for various anatomic subsites of the colon in 1996. For comparative purposes, fi gures for 1976 are provided in parentheses.

Scientifi c American Surgery
09/14
gastro adenocarcinoma of the colon and rectum — 2
estimated that it takes approximately 17 years for a large adenoma to progress to invasive malignancy but less than 2 additional years to develop the capacity to metastasize. Nearly 80% of tumors develop along the CIN pathway.
The microsatellite instability (MSI) pathway is also impli-cated in the development of CRC. It is typifi ed by defi cient DNA mismatch repair (MMR), a near-diploid karyotype; lower levels of p53, SMAD4, and K-ras mutations; and higher levels of BAX, TGF-BIIR, and BRAF mutations. These tumors, which usually develop proximal to the splenic fl exure, have a better prognosis than tumors arising along the CIN pathway. Patients with Lynch syndrome develop tumors along the MSI pathway, with mutation in DNA-MMR genes. The MSI pathway (also referred to as the replication error [RER] pathway), is responsible for approx-imately 20% of carcinomas. Tumors characterized by MSI appear less responsive to 5-fl uorouracil (5-FU)-based chemotherapy.9,10 Therefore, it is important to identify these tumors correctly so that patients can receive optimal treatment.
Malignancies characterized by excessive gene methylation (CpG island methylator phenotype, or CIMP) are distinct from other colon cancers.11 However, the existence of CIMP as a discrete entity is controversial. CIMP may simply mark one end of a tumor continuum typifi ed by genetic hyper-methylation, or it may comprise a subgroup of CRCs that have a unique molecular etiology. Many precursor lesions associated with CIMP tumors are serrated polyps demon-strating extreme hypermethylation and V600E BRAF muta-tions. This suggests that CIMP colorectal malignancies arise from serrated polyps, which may originate from a stemlike cell different from that of CRCs associated with tubular adenomas. DNA hypermethylation in CRC is currently a prominent topic for investigation.
Some tumors do not fall into any currently known categor y, suggesting that other genetic pathways exist.12 Additional investigations will give us a better understand-ing of the various stages involved in carcinogenesis, offering opportunities for more specifi c molecular staging and, ulti-mately, tumor-specifi c therapy. Genetic pathways of CRC and the potential therapeutic role of chemopreventive agents are demonstrated here [see Figure 2].
CRC Risk Factors
Clearly, CRC is associated with genetic and environmen-tal infl uences. Overt risk factors include a personal or family
history of CRC or colorectal adenoma(s), a personal history of colorectal polyps, infl ammatory bowel disease (IBD), and age greater than 50. Age is the most common risk factor. The risk of CRC increases after the fourth decade of life.13 Most individuals present with disease after the age of 60, and only 10% of CRCs are diagnosed in individuals younger than 40.
Nonhereditary CRCs are referred to as “sporadic” and comprise 75 to 80% of all CRCs. Genetic etiology may be identifi ed in the remaining 20 to 25% of patients, including family history (15 to 20%), Lynch syndrome (5%), and FAP (< 1%). Cancer can arise within a polyp or at another site in the colon or rectum. The likelihood of invasive disease aris-ing in a colorectal polyp is associated with the morphology, histology, and size of the lesion. Polyps can be classifi ed as tubular, villous, or tubulovillous. Large villous polyps are most suspicious for malignancy; about 50% of villous lesions larger than 2 cm harbor cancer. Approximately 40% of patients present with multiple adenomatous polyps and are at high risk for having or developing CRC.14 Patients previ-ously diagnosed with colon or rectal cancer are at risk for metachronous disease; approximately 40% of those treated for sporadic CRC develop metachronous polyps, and about 6% develop a second CRC while under surveillance.15,16
Patients with IBD are at high risk for developing CRC, proportional to the extent and duration of disease. In indi-viduals with ulcerative colitis, the risk of cancer seems to begin after 8 to 10 years, increasing at a rate of 0.5 to 1.0% each year. Some studies report the absolute risk as 2 to 5% at 10 years, 8 to 10% at 20 years, and 20 to 30% at 30 years.17 Patients with disease extending proximal to the splenic fl exure (pancolitis), patients with IBD diagnosed at a young age,18 and those with colitis-associated sclerosing cholangitis are at highest risk.
In patients with ulcerative colitis, cancers may develop in any portion of the large bowel. These tumors are usually diagnosed in the fourth decade of life and apparently carry the same prognosis as colon cancer in general.19 However, accurate diagnostic endoscopy in the setting of active colitis is diffi cult, and for this reason, patients with IBD often pres-ent with late-stage disease. Because all of the screening tests commonly used (including repetitive biopsies) are problem-atic,20 most patients with long-standing colitis will eventu-ally benefi t from prophylactic surgery (proctocolectomy). The risk of CRC is also greater in individuals with Crohn colitis. The cancer risk in patients with Crohn colitis appears
NormalEpithelium
APC/β-catenin K-ras/BRAF DCC/SMAD4/SMAD2 p53 Other Changes?
EarlyAdenoma
IntermediateAdenoma
LateAdenoma
GeneticInstability
Carcinoma Metastasis
Figure 2 Genetic model of colorectal tumorigenesis.186

Scientifi c American Surgery
09/14
gastro adenocarcinoma of the colon and rectum — 3
In addition to colonic polyposis, FAP patients often develop other tumors: intra-abdominal desmoids, gastric fundic polyps, and periampullary cancers. Following cura-tive surgery for their cCRC, FAP patients most frequently succumb to periampullary tumors.26 Gardner syndrome is a type of FAP characterized by the development of colorectal adenomas as well as extraintestinal tumors and abnormali-ties: supernumerary teeth, osteomas (usually of the skull and mandible), mesenteric fi bromatosis, soft tissue tumors (i.e., fi bromas, lipomas, epidermoid and sebaceous cysts), desmoid tumors, and congenital hypertrophy of retinal pigmentation epithelium (CHRPE). Another variant of FAP is Turcot syndrome, in which colorectal adenomas are associated with brain tumors. Attenuated FAP syndrome is characterized by the development of fewer polyps at a later age, usually in the right colon.27 Attenuated FAP is clinically diffi cult to distinguish from Lynch syndrome. The variety of FAP phenotypes appears to be associated with the specifi c location of mutation on the APC gene.27 In attentuated FAP, for example, the location of the mutation is usually more proximal or distal on the APC gene than is found in classic FAP syndrome.
Lynch syndrome is a familial disorder characterized by a high incidence of colon cancer but without the high degree of polyposis found in classic FAP. Approximately 5 to 6% of CRCs are attributable to Lynch syndrome.28,29 The pheno-typical characteristics are a relatively early onset of CRC (mean age 46 years), a predominance of right-sided tumors,
to be equivalent to that in patients with ulcerative colitis depending on similar duration and extent of disease.21
Familial and hereditary factors are implicated in 25% of CRCs. Individuals with a fi rst-degree relative affected by CRC are at twice the risk for developing disease. For those with two or more affected fi rst-degree relatives, this risk is nearly threefold. A family history of CRC is associated with younger age at the time of diagnosis, suggesting a genetic predisposition.22 The patients at highest risk are those who carry the genetic mutations typical of FAP and Lynch syndrome [see Figure 3]. Nearly 100% of individuals with FAP and 80% with Lynch syndrome develop CRC in their lifetime.
FAP syndromes account for only 1% of all CRCs, affecting approximately one in every 8,000 to 10,000 people. These hereditary syndromes are characterized by early onset of hundreds to thousands of adenomatous polyps throughout the colon, usually presenting early in the second decade of life. Without prophylactic colectomy, cancer inevitably develops by the fourth or fi fth decade. However, 10 to 20% of cases represent de novo mutations with no apparent family history.23,24 The disease is inherited as an autosomal dominant trait; therefore, 50% of offspring from an affected individual will develop polyposis coli. The adenomatous polyposis coli (APC) gene, which causes FAP, is located on chromosome 5 (5q21). The most common genetic abnor-mality results in the generation of a premature stop codon, resulting in a truncated and nonfunctional protein.25
Figure 3 Colorectal cancer risk.
Cum
ulat
ive
perc
ent i
ncid
ence
of c
olor
ecta
l can
cer
Age (yr)
General population
Family history positive population
Hereditary nonpolyposis colon cancer population
Familial adenomatous polyposis cancer population
100
80
60
40
20
20 30 40 50 60 700

Scientifi c American Surgery
09/14
gastro adenocarcinoma of the colon and rectum — 4
in patients with Lynch syndrome,29 and this should be taken into account when planning the follow-up regimen.
Other genetic syndromes, including Peutz-Jeghers and familial juvenile polyposis, are associated with increased risk of CRC. These autosomal dominant syndromes are characterized by hamartomatous polyposis. The histology comprises an overgrowth of cells or tissues in the area where they normally arise.36 The molecular mechanisms of these syndromes are being studied, and some specifi c mutations have been identifi ed.37
Peutz-Jeghers syndrome is characterized by multiple gastrointestinal hamartomatous polyps associated with mucocutaneous melanin pigmentation. Patients may pres-ent with bowel obstruction, anemia from gastrointestinal blood loss, or polyp intussusception. The polyps are characterized by a branching muscular framework and are generally nonmalignant, but they can contain carcinoma. Individuals with Peutz-Jeghers are at higher risk for devel-oping extraintestinal malignancies as well (i.e., pancreatic, breast, ovarian, uterine, and testicular carcinomas).38 Unlike patients with FAP, prophylactic colectomy is generally not indicated. Polyps can usually be managed endoscopically; radical surgery is reserved for lesions that are large, are symptomatic, or appear to be neoplastic.
Familial juvenile polyposis syndrome is characterized by multiple (50 to 200) juvenile polyps throughout the gastro-intestinal tract. Patients with this syndrome often have other congenital abnormalities, including cardiac and genitouri-nary anomalies. Patients may present in childhood with anemia caused by chronic gastrointestinal blood loss, abdominal pain caused by intussusception, a protein-losing enteropathy, or frank rectal bleeding. Individuals with this syndrome are at an increased risk for upper and lower gastrointestinal cancers.39 The polyps are usually treated endoscopically; total abdominal colectomy with ileal pouch-anal anastomosis is reserved for patients who develop large or numerous polyps or invasive cancers.40 (Patients presentin g with a self-limited solitary juvenile polyp are not included in this defi nition.)
Additional genetic factors associated with CRC have been identifi ed. Studies from Israel show that eastern European (Ashkenazi) Jews with a polymorphism in the APC gene have the highest CRC incidence of any ethnic group in that country. Six percent of unselected Ashkenazi Jews and 28% of those with a family history of CRC carry an APC missense mutation (I 1307 K). These patients do not demonstrate the phenotype typically associated with FAP. Rather, the polymorphism generates a hypermutable region on the APC gene, creating a predisposition to CRC41 and a high inci-dence of synchronous cancers in individuals with polyps: 13% of those with this polymorphism and identifi ed adeno-matous polyps have invasive cancer.42,43 Mutation in the exon-excision-repair gene MYH has also been described. Patients with this mutation may present with either the FAP or the Lynch syndrome phenotype.44
Finally, it has been shown that hyperplastic polyposis—defi ned as more than 20 hyperplastic polyps measuring at least 1 cm in size and located in areas of the colon and rec-tum other than rectosigmoid—is associated with colorectal adenomas and carcinomas.45 These cancers arise in associa-tion with methylation silencing of MMR genes and the HPP1
and, in 35% of cases, synchronous or metachronous CRCs.30 Patients with Lynch syndrome may also have early-onset ovarian, pancreatic, breast, bile duct, endometrial, stomach, genitourinary tract, and small bowel adenocarcinomas.31 Lynch I syndrome is characterized by CRC only; Lynch II syndrome refers to patients with CRC and other associated adenocarcinomas. Another variant, Muir-Torre syndrome, is associated with sebaceous gland adenomas and carcinoma.32
The molecular genetic marker for Lynch syndrome is MSI, which is caused by mutations in DNA MMR genes (hMSH2, hMLH1, hPMS1, hPMS2). The criteria for diagnosis of Lynch syndrome were established in 1991, at a consensus confer-ence in Amsterdam, to help identify and categorize patients with a familial history of CRC.30 These criteria, referred to as Amsterdam Criteria I, require the following: (1) three relatives of the patient with CRC (one being a fi rst-degree relative of the other two); (2) two or more successive gen-erations with CRC; and (3) one family member diagnosed with CRC before the age of 50. However, the Amsterdam Criteria I underestimated the presence of Lynch syndrome in some family pedigrees.28 This led to development of the Amsterdam Criteria II, which included extracolonic tumors [see Table 1]. Typical cancers associated with Lynch syn-drome are colorectal, endometrial, small bowel, ureteral, or renal pelvic cancers. The term familial colorectal cancer type X is used when the Amsterdam criteria are met but no DNA MMR defect is identifi ed. These families have a lower inci-dence of cancer overall, and a lower risk of non-CRCs, than those with documented DNA MMR defi ciency.33 About 35% of patients meeting the Amsterdam criteria do not have a DNA-MMR gene mutation.34 In diagnosing Lynch syn-drome, the clinician should fi rst exclude FAP, and all tumors must be confi rmed pathologically.
Some tumors with MMR defi ciency do not demonstrate familial inheritance or genetic mutation. These sporadic MMR tumors are associated with gene promoter hyper-methylation. Both sporadic and hereditary MMR-defi cient tumors have a characteristic appearance histologically. Adenomas often show a villous component, with more dys-plasia than is usually seen in sporadic tumors. These tumors are often poorly differentiated, with infl ammatory cell infi ltrate (tumor infi ltrating lymphocytes) and signet-ring histology.28,35 Despite this aggressive histologic appearance, however, the stage-for-stage survival in CRC patients with MMR defi ciency is better than in patients with normal MMR.31 From the clinical standpoint, it is important to note that colorectal malignancies appear to develop more rapidly
Table 1 Amsterdam Criteria IIAt least three relatives with an HNPCC-associated cancer
(colorectal, endometrium, small bowel, ureter, or renal pelvis). One affected relative should be a first-degree relative of the other two.
At least two successive generations should be affected.At least one relative should have been diagnosed before age
50 years.Familial adenomatous polyposis should be excluded.Tumors should be verified by pathologic examination.
HNPCC = hereditary nonpolyposis colon cancer.

Scientifi c American Surgery
09/14
gastro adenocarcinoma of the colon and rectum — 5
gene. Eventually, the identifi cation and thorough character-ization of all the various molecular pathways in colorectal carcinogenesis may lead to tumor-specifi c therapy.
Numerous lifestyle and dietary factors have been pro-posed as potentially increasing the risk of CRC [see Table 2]. Lower levels of physical activity and increased body mass are associated with an increased risk of CRC in both men and women.46 The Western-style diet, which is high in calo-ries and fat and low in fi ber, is associated with high rates of CRC. There is evidence that increased dietary intake of calcium may confer some protection against the develop-ment of CRC and adenomatous polyps. The Calcium Polyp Prevention Study, a large randomized trial done in the United States, reported a small but statistically signifi cant reduction in the incidence of recurrent colorectal adenomas with dietary calcium supplementation.47 To date, the evi-dence from randomized trials has not shown dietary fi ber supplementation to have a similar effect. In Japan, where the incidence of CRC has traditionally been low, CRC has become considerably more common in the past few decades.48 This is believed to be the result of post–World War II lifestyle changes (e.g., increased consumption of animal fat and decreased expenditure of energy) that mirror Western habits.
CRC Screening
The aim of CRC screening in asymptomatic patients is to identify and remove premalignant adenomatous polyps and diagnose early malignancies. When an adenomatous polyp is detected, the entire large bowel should be visualized endoscopically because synchronous lesions are found 35 to 40% of the time. Sessile polyps, villous polyps, and large polyps (> 1.5 cm) are more likely to contain invasive cancers than pedunculated, tubular, or small polyps. In the National Polyp Study, patients who underwent endoscopic removal of adenomas were found to have a lower probability of developing CRC compared with (1) a reference group that did not have polyps removed and (2) individuals in a population-based registry (Surveillance, Epidemiology, and End Results [SEER]), most of whom did not have polyps.49 In essence, this study confi rmed that colonoscopic polypectomy reduce s colon cancer mortality; it validated the colorectal adenoma-to-adenocarcinoma sequence and reinforced the importance of screening. The details of a CRC screening regimen are based on an understanding of the individual patient’s risk [see Table 3, Table 4 and Table 5]. In general, average-risk, asymptomatic individuals are candidates for routine screen-ing, whereas those at higher risk should be followed more often and more closely.15
Average-risk men and women should begin routine CRC screening at age 50 [see Table 3]. Several surveillance options
exist. The fi rst includes stool occult blood testing annually and fl exible sigmoidoscopy every 5 years. In the event of a positive stool blood test, the patient should undergo a complete colonoscopy. On screening sigmoidoscopy, single small lesions should be biopsied, and additional treatment should be based on histology. If the lesion is an adenoma-tous polyp, colonoscopy (to complete the polypectomy and assess the proximal colon for synchronous lesions) should be done. In the case of a benign hyperplastic polyp, no additional testing is needed. However, if a screening sigmoidoscopy reveals a large polyp or multiple polyps, the initial biopsy can be omitted in favor of complete colonos-copy with biopsy. The second option for screening of the average-risk individual is complete colonoscopy, repeated at 7- to 10-year intervals if negative for neoplasia. (This is the preferred screening method.) The third option (which is done least frequently) includes double-contrast barium enema with fl exible sigmoidoscopy every 5 to 10 years. A positive test should be followed up by complete colonoscopy.
High-risk individuals are those with a personal history of adenomas or cancers, a family history of colorectal cancers, genetic syndromes, or predisposing medical conditions such as infl ammatory bowel disease [see Table 5]. Patients with a history of colorectal adenomas should undergo increased surveillance for metachronous polyps or small synchronous polyps, which are found in 15% of cases.50 A shorter follow-up interval is necessary after excision of multiple adenomas or of an adenoma with invasive cancer; incomplete or piece-meal removal of a large sessile adenoma and suboptimal examination due to poor preparation also require closer follow-up. The National Polyp Study recommended that a repeat examination be done 3 years following polypectomy.49 If the 3-year follow-up colonoscopy is clear, the surveillance interval can be increased to once every 5 years.51
Patients with a personal history of CRC require more rigorous surveillance for metachronous disease. The fi rst surveillance colonoscopy is usually performed 1 year after resection of a CRC. If the colon was not fully evaluated before surgery, the fi rst colonoscopy should be performed sooner than 1 year from surgery. If this fi rst postoperative colonoscopy is normal, the interval can be lengthened to once every 3 years. If additional abnormalities or disease is noted, however, more frequent examinations are necessary.
Patients with a family history of CRC or adenomas, including affected fi rst-degree relatives, should also under-go more rigorous surveillance. This includes screening colonoscopy starting at age 40 or earlier (when the patient is 10 years younger than the affected family members were at the initial diagnosis).
Patients with long-standing IBD are at increased risk for developing CRC and should undergo routine surveillance. The cancer risk in chronic Crohn disease and ulcerative
Table 2 Dietary and Lifestyle Risk Factors for Colon and Rectal CancerLikelihood of Association Decreased Risk Increased Risk
Probable Physical activity, foliate, vegetables Obesity, smoking, red meat
Possible Fruit, calcium, vitamin D, methionine Alcohol, processed meat, heavily cooked meat, iron
Unknown Fiber supplement —

Scientifi c American Surgery
09/14
gastro adenocarcinoma of the colon and rectum — 6
colitis appears to be the same, and these patients should have similar follow-up. In patients with pancolitis (disease extending proximal to the splenic fl exure), surveillance colon oscopy should begin 8 years after the initial symptoms. Surveillance can begin later in patients with left-sided colitis, generally after 12 to 15 years. Colonoscopy should be performed every 1 to 2 years. Biopsies should routinely be taken at 10 to 12 cm intervals throughout the colon, from normal- and abnormal-appearing mucosa. In the setting of low- or high-grade dysplasia or diffi cult-to-control colitis, or
Table 3 Screening Guidelines for Average-Risk IndividualsTest Internal (beginning at age 50) Comment
FOBT and flexible sigmoidoscopy
FOBT annually and flexiblesigmoidoscopy every 5 yr
Flexible sigmoidoscopy together with FOBT is preferred compared with FOBT or flexible sigmoidoscopy alone; all positive tests should be followed up with colonoscopy
Flexible sigmoidoscopy, FOBTs
Every 5 yr annually All positive tests should be followed up with colonoscopy; the recommended take-home multiple-sample method should be used
Colonoscopy Every 10 yr All positive tests should be followed up with colonoscopy; colonoscopy provides an opportunity to visualize, sample, and/or remove significant lesions
Double-contrast barium enema
Every 5 yr All positive tests should be followed by colonoscopy
FOBT = fecal occult blood test.
Table 4 Screening Guidelines for Increased-Risk IndividualsRisk Category Age to Begin Recommendation Comment
Patient with a single small (< 1 cm) adenoma
3–6 yr after the initial polypectomy
Colonoscopy If examination is normal, they can thereafter be screened as per average-risk guidelines
Patient with a large (> 1 cm) adenoma, multiple adenomas, or adenomas with high-grade dysplasia or villous change
Within 3 yr after the initial polypectomy
Colonoscopy If normal, repeat examination in 3 yr; if normal then, the patient can thereafter be screened as per average-risk guidelines
Personal history of curative-intent resection of colorectal cancer
Within 1 yr after cancer resection
Colonoscopy If normal, repeat examination in 3 yr; if normal then, repeat every 5 yr
Either colorectal cancer or adenomatous polyps, in any first-degree relative before age 60 yr, or in > 2 first-degree relatives at any age (if not a hereditary syndrome)
Age 40 yr or 10 yr before the youngest case in the immediate family
Colonoscopy Every 5–10 yr; colorectal cancer in relatives more distant than first-degree does not increase risk substantially above the average-risk group
Table 5 Screening Guidelines for High-Risk IndividualsRisk Category Age to Begin Recommendation Comment
Family history of familial adenomatous polyposis (FAP)
Puberty Early surveillance with endoscopy and counseling to consider genetic testing
If the genetic test is positive, colectomy is indicated; these patients are best referred to a center with experience in the management of FAP
Family history of hereditary nonpolypo-sis colon cancer (HNPCC)
Age 21 yr Colonoscopy and counseling to consider genetic testing
If the genetic test is positive or if the patient has not had genetic testing, every 1–2 yr until 40 yr of age, then annually; these patients are best referred to a center with experience in the management of HNPCC
Inflammatory bowel disease, chronic ulceralive colitis, Crohn disease
Cancer risks begin to be significant 8 yr after the onset of pancolitis or 12–15 yr after the onset of left-sided colitis
Colonoscopy with biopsies for dysplasia
Every 1–2 yr; these patients are best referred to a center with experience in the surveillance and management of inflammatory bowel disease
in patients who cannot comply with routine surveillance, colectomy is indicated.
Members of FAP families who have not been tested for an APC mutation should begin routine screening at puberty with annual fl exible sigmoidoscopy. If polyps are not identi-fi ed by age 40, the frequency of the examination can be decreased to once every 3 years. However, individuals with the FAP phenotype should have upper endoscopy of the periampullary region. Because stage-specifi c survival of colorectal cancer appears to be the same for polyposis

Scientifi c American Surgery
09/14
gastro adenocarcinoma of the colon and rectum — 7
patients as for those with sporadic bowel cancers, relatives of FAP kindred or patients with a known genetic mutation should be treated with colectomy as soon as polyps develop.
As patients with Lynch syndrome are predisposed to develop proximal colonic lesions, and because the adenoma-to-carcinoma sequence appears to take place more rapidly in this group, they should undergo full colonoscopy every 1 to 2 years. Screening should begin at 21 years of age for those with known mutations or a family history consistent with the Amsterdam criteria.4,15 Screening for extracolonic disease should be done as well. This includes urinalysis/urine cytol-ogy, pelvic ultrasonography, and periodic endometrial biopsy.
Virtual colonoscopy, which uses high-resolution compute d tomographic (CT) scanning to image the colon, has been evaluated in at least two multicenter trials in the United States, with varying results.52,53 One of the studies reported a sensitivity and a specifi city of 89% and 80%, respectively, for polyps larger than 6 mm and up to 94% and 96%, respec-tively, for polyps larger than 10 mm.52 The sensitivities were equivalent to those of optical colonoscopy in this group of asymptomatic average-risk patients. The second study, however, found that virtual colonoscopy had a sensitivity of only 39% for lesions larger than 6 mm and 55% for lesions larger than 10 mm.53 Given these divergent fi ndings, it appears that issues related to equipment, software, training, and overall sensitivity in the study remain to be addressed before virtual colonoscopy can be recommended as a routine screening modality. Another consideration is that patients with lesions detected by means of virtual colonos-copy must still undergo optical colonoscopy for treatment or tissue diagnosis.
Fecal DNA assays have been developed to test for muta-tions in multiple genes known to be involved in colorectal neoplasia and are currently being evaluated in clinical trials.54 A study comparing fecal DNA testing with a com-mercially available multigene panel with guaiac-based testing in asymptomatic patients showed both to have a poor sensitivity in detecting cancers and advanced adeno-mas detected by screening colonoscopy.55 DNA testing was signifi cantly better but still only detected 52% of cancers and 18% of advanced adenomas.
CRC Staging
clinical staging
Clinical staging is based on the history and physical examination, endoscopic fi ndings, and biopsy results. If colonoscopy cannot be completed, CT colonography or air-contrast barium enema can be considered to evaluate the remainder of the colon. If these procedures are not viable or pose signifi cant risk, short-interval postoperative colonos-copy should be performed. Additional staging information may be obtained by means of imaging studies, which gener-ally includes a CT scan of the chest, abdomen, and pelvis with oral and intravenous (IV) contrast. Additional imaging may be useful, including magnetic resonance imaging (MRI) of the liver or positron emission tomographic (PET) scan-ning to further work up abnormalities seen on CT. PET is a sensitive study, but its routine use in staging primary CRC is not generally recommended. PET may be considered for
high-risk patients in whom the detection of metastases would change initial management.56
In cases of rectal cancer, locoregional staging may signifi -cantly affect therapeutic decision making. Such staging includes determination of the depth of invasion of the rectal wall and the degree of regional node involvement [see Figure 4]. Modalities commonly used include CT, MRI, and endoscopic ultrasonography (EUS). Optimally, either MRI or EUS is used to stage primary rectal cancer.
pathologic staging
The prognosis for patients with CRC is associated with the stage of disease at diagnosis as well as tumor histology (e.g., differentiation, lymphatic invasion, perineural invasion, single-cell infi ltration) and the extent of tumor-free surgical margins. In the future, molecular genetic markers may help identify subsets of patients who are more or less likely to develop tumor recurrence. This would lead to more precise, individualized application of adjuvant multimodality treat-ment.57,58 However, the use of such molecular markers as a basis for tailored treatment remains investigational at this time.
The standard CRC staging system is the tumor, node, and metastasis (TNM) staging system of the American Joint Committee on Cancer (AJCC) and International Union Against Cancer (UICC) [see Table 6 and Table 7 for an outline of the 7th edition of the AJCC staging manual]. The symbols “c” and “p” denote clinical and pathologic staging, respectively; the prescript “y” denotes posttreatment tumor staging (e.g., ypT2N1M0 describes a pathologically staged tumor extend-ing into the muscularis propria, with metastases in one to three regional lymph nodes, in a patient who received preoperative treatment).
In colorectal carcinomas, the staging category pTis (carci-noma in situ) denotes either the presence of malignant cells confi ned by glandular basement membrane (intraepithelial carcinoma) or tumor invading beyond the basement membrane into the mucosal lamina propria (intramucosal carcinoma). The terms high-grade dysplasia and intraepithelial carcinoma are often used synonymously. The defi nition of invasive CRC, pT1, includes tumor cell invasion through the muscularis mucosa into the submucosa, where lymphatics are found in abundance. This is in marked contrast to other gastrointestinal and solid tumors, in which invasion below the lamina propria is considered malignant.
The TNM staging system has several other nuances as well. Extramural tumor deposits are classifi ed as N1c. pT4 refers to tumor penetration to the surface of the visceral peritoneum with or without free perforation into the peritoneal cavity (T4a) or extension into adjacent organs or structures (T4b).
As is true in the setting of most epithelial cancers, the presence of metastases in regional lymph nodes has a sig-nifi cant impact on survival. Proper staging and treatment of advanced CRC require adequate lymphadenectomy. In their study of T3 colorectal tumors, Goldstein demonstrated the association between staging accuracy and lymphadenec-tomy: lymph node metastases were found in 85% of cases when 15 or more nodes were recovered and identifi ed in the specimen but in only 22% when fewer than 15 were identi-fi ed.59 Furthermore, among the patients who did not appear

Scientifi c American Surgery
09/14
gastro adenocarcinoma of the colon and rectum — 8
DistantMetastases
A(T1N0M0)Mucosa
Astler-Coller Stage
Submucosa
Muscularis SerosaLymph Nodes
B1(T2N0M0)
B2(T3–4N0M0)
C1(T2N1M0)
C2(T3–4N1M0)
D(TXNXM1)
LungsLiver
Table 6 American Joint Committee on Cancer–Union Internationale Contre le Cancer Tumor, Node, Metastasis Stage Grouping187
Primary tumor (T)
TX Primary tumor cannot be assessed
T0 No evidence of primary tumor
Tis Carcinoma in situ: lntraepithellal or invasion of lamina propria*
T1 Tumor invades submucosa
T2 Tumor invades muscularis propria
T3 Tumor invades through the muscularis propria into pericolorectal tissues
T4a Tumor penetrates to the surface of the visceral peritoneum†
T4b Tumor directly invades or is adherent to other organs or structures†‡
Regional lymph nodes (N)
NX Regional lymph nodes cannot be assessed
N0 No regional lymph node metastasis
Nl Metastasis in 1–3 regional lymph nodes
Nla Metastasis in 1 regional lymph node
N1b Metastasis in 2–3 regional lymph nodes
N1c Tumor deposit(s) in the subserosa, mesentery, or nonperitonealized pericolic or perirectal tissues without regional nodal metastasis
N2 Metastasis in 4 or more regional lymph nodes
N2a Metastasis in 4–6 regional lymph nodes
N2b Metastasis in 7 or more regional lymph nodes
Distant metastasis (M)
M0 No distant metastasis
M1 Distant metastasis
M1a Metastasis confined to one organ or site (e.g., liver, lung, ovary, nonregional node)
M1b Metastases in more than one organ/site or the peritoneum
*T1s include cancer cells confi ned within the glandular basement membrane (intraepithelial) or mucosal lamina propria (intramucosal), with no extension through the muscularis mucosae into the submucosa.†Direct invasion in T4 includes invasion of other organs or other segments of the colorectum as a result of direct extension through the serosa, as confi rmed on microscopic examination (e.g., invasion of the sigmoid colon by a carcinoma of the cecum) or, for cancer in a retroperitoneal or subperitoneal location, direct invasion of other organs or structures by virtue of extension beyond the muscularis propria, respectively, a tumor on the posterior wall of the descending colon invading the loft kidney or lateral abdominal wall; or a mid- or distal rectal cancer with invasion of the prostate, seminal vesicles, cervix, or vagina.‡Tumor that is adherent to other organs or structures, grossly, is classifi ed cT4b. However, if no tumor is present in the adhesion, microscopically, the classifi cation should be pT1–4a depending on the anatomic depth of wall invasion. The V and L classifi cations should be used to identify the presence or absence of vascular or lymphatic invasion, whereas the PN site-specifi c factor should be used for perineural invasion.
Figure 4 Classifi cation of colorectal cancer takes into account the depth of tumor penetration and involvement of lymph nodes.

Scientifi c American Surgery
09/14
gastro adenocarcinoma of the colon and rectum — 9
to have nodal metastases, survival was greatest in those with high lymph node recovery. Although many factors infl uence the number of nodes examined—including the extent of the resection and the diligence of the pathologist—these data lend credence to the concept that proper onco-logic resection is associated with improved outcome. Based on several studies, it is recommended that at least 12 lymph nodes be examined to accurately stage CRC patients.60
Alternative methods for identifying very small amounts of metastatic disease now include immunohistochemistry and molecular biology–based techniques. Recent studies show that a polymerase chain reaction (PCR)-based assay can differentiate patients with occult nodal disease and a higher risk of recurrence from those with histologically and molecularly negative lymph nodes.61 Identifi cation of occult nodal disease on molecular analysis is currently not part of the 7th edition of the AJCC/UICC TNM staging N1. Biopsy of sentinel nodes for intestinal malignancy62 remains inves-tigational, and the results of various studies have been inconsistent. One cooperative group reported an unaccept-ably high rate of false negatives (negative sentinel lymph nodes and positive nonsentinel lymph nodes), which sug-gests that this may not be a valid practice in CRC.63 These discordant results, and the fact that lymphadenectomy is a standard component of colorectal resection, have curtailed the analysis of sentinel nodes.
The association between pathologic stage and outcome is shown here [see Figure 5].64 Along with bowel wall penetra-tion and lymph node status, there are other pertinent patho-logic features that are predictive of oncologic outcome [see
Table 8 for itemization]. Lymphovascular invasion is associ-ated with nodal and distant disease and is also an indepen-dent prognosticator.65,66 When the data are corrected for nodal involvement and histologic differentiation, the prog-nosis in patients with CRC—unlike that of patients with many other solid tumors—is not infl uenced by the size of the primary lesion.
Treatment of Primary Colon and Rectal Carcinoma
Surgery is the mainstay of therapy for locoregional colon and rectal cancer. In colon cancer, adjuvant chemotherapy is administered to reduce the risk of recurrence, which usually appears as distant failure. In rectal cancer, neoadjuvant com-bined modality therapy (CMT), including chemotherapy and radiation, is used to improve the resectability of the primary lesion, enhance the probability of sphincter preser-vation, and reduce the risk of local and distant recurrence. Adjuvant chemotherapy is primarily used to reduce the risk of distant metastatic disease [see Figure 6 and Figure 7 for treatment algorithms for colon and rectal cancer].
In treating CRC, it is crucial to understand that surgical extirpation of the primary tumor is done when there is a realistic possibility of cure or for patients with symptomatic tumors that cause acute obstruction or clinically signifi cant bleeding. For those who present with synchronous primary tumors and incurable metastatic disease, resection is not routinely indicated. Advances in systemic chemotherapy (outlined below) have signifi cantly increased the probability of managing the tumor medically, and chemotherapy can begin immediately in the setting of an asymptomatic or minimally symptomatic primary. In other words, there is no need to delay initiation of systemic chemotherapy by palliative resection of a primary tumor that is not actively symptomatic. In truth, resection of a primary lesion in the setting of metastatic disease is likely to cause signifi cant morbidity and even mortality. A review of Medicare/SEER data focusing on patients 65 and older reported a 30-day post operative mortality of 10% with resection of a synchronou s primary tumor.67
Furthermore, in a large retrospective series, Poultsides and colleagues reported that 93% of patients presenting with synchronous stage IV disease without overt obstruction never required specifi c intervention on their primary tumor.68
imaging
Prior to defi nitive surgical management, patients require full body imaging with a contrast-enhanced CT scan of the chest, abdomen, and pelvis. For those with contraindications to IV contrast, a noncontrast CT scan of the chest or an MRI of the abdomen and pelvis is an acceptable alternative. Rou-tine preoperative chest CT should be regarded as standard practice, both to rule out the presence of synchronous lung metastases and to establish a baseline for postoperative surveillance, because chest CT is part of the postoperative surveillance algorithm. PET scanning has no role in the pre-operative staging or the postoperative surveillance of CRC and is specifi cally not recommended in National Cancer Care Network (NCCN), American Society of Clinical Oncol-ogy (ASCO), and Cancer Care Ontario (CCO) guidelines. The popular misconception that PET is somehow more “accurate” or “sensitive” than CT scanning is simply not the
Table 7 American Joint Committee on Cancer–Union Internationale Contre le Cancer Tumor, Node, Metastasis Staging of Colon and
Rectal Cancer187
Stage T N M
0 Tis N0 M0
I T1, T2 N0 M0
IIA T3 N0 M0
IIB T4a N0 M0
IIC T4b N0 M0
IIIA T1–T2T1
N1/N1cN2a
M0—
IIIB T3–T4aT2–T3T1–T2
N1/N1cN2aN2b
M0——
IIIC T4aT3–T4aT4b
N2aN2bN1–N2
M0——
IVA Any T Any N M1a
IVB Any T Any N M1b
cTNM is the clinical classifi cation, pTNM is the pathologic classifi cation. The prefi x is used for those cancers that are classifi ed after neoadjuvant pretreatment (e.g., ypTNM). Patients who have a complete pathologic response are ypT0N0cM0, which may be similar to stage group 0 or 1. The r prefi x is to be used for those cancers that have recurred after a disease-free interval (rTNM).

Scientifi c American Surgery
09/14
gastro adenocarcinoma of the colon and rectum — 10
Table 8 Selected Pathologic Prognostic Factors in Colorectal Cancer
Adjacent organ involvement (colon)Radial margin (rectum)Degree of differentiationBlood vessel invasionLymphatic vessel invasionPerineural invasionImmune responseDNA contentProliferative indexAllelic loss of chromosome 18q (DCC)K-ras mutationMMR deficiency
MMR = mismatch repair.
Figure 5 Five-year survival by American Joint Committee on Cancer, fi fth edition, system stages I to IV.
case and has never been supported by data. PET abnormali-ties that lack clear CT correlates have a high propensity to be false positives, and a clinically meaningful proportion of CRCs, especially those with high mucinous components, do not image well on PET. There are rare circumstances in which it may be useful to further evaluate a CT or MRI abnormality with PET; however, more often than not, due to the possibility of both false positives and false negatives in PET scanning, PET will be insuffi ciently defi nitive to meaningfully change management.
surgery
The purpose of curative-intent surgery for CRC is to remove the primary tumor with adequate margins, as well as regional lymph nodes. Planning the extent of lymphade-nectomy is based on a complete understanding of anatomy and the pattern of lymphatic spread in intestinal cancer. This is one of the most challenging aspects of cancer surgery.
The regional lymphatics of the colon have been well described.69 Abundant lymphatic capillaries are located in the submucosa, and efferent vessels proceed peripherally through the circular and longitudinal layers of the muscula-ris propria, communicating with a clearly defi ned subsero-sal plexus. Lymphatic fl ow in the subserosal network is mainly circumferential. Longitudinal intramural lymphatic spread is usually limited to 2 cm, which explains the gen-eral rule of obtaining a 5 cm proximal and distal intestinal margin of resection. Most of the subserosal lymphatics pass into the mesentery, to the paracolic lymph nodes. Normally, lymph fl ow within the colonic mesentery proceeds centrally, in an orderly fashion, from smaller to larger collecting lymphatics and eventually to the root of the mesentery. Lymphatic vessels are closely associated with the vascular pedicles, and the centrally directed fl ow proceeds along the nearest (or most immediately accessible) route to the apex of the mesentery. Therefore, we can describe the pathways of lymphatic fl ow in relation to the appropriate vascular
Surv
ival
dis
trib
utio
n fu
ncti
on
Months
IV
III
II
I
Stage 0 mo
Survival(%)
Survival(%)
N
IIIIIIIV
100100100100
14,50034,36126,94920,802
96.189.272.717.3
8,59119,49212,1821,832
—< .0001< .0001< .0001
—< .0001< .0001< .0001
93.282.559.58.1
4,51510,1055,514
432
N p pSurvival(%)
N
30 mo 60 mo
1.00
0.75
0.50
0.25
0
0 10 20 30 40 50 60

Scientifi c American Surgery
09/14
gastro adenocarcinoma of the colon and rectum — 11
Figure 6 Algorithm outlining treatment of colon cancer. CT = computed tomography; IV = intravenous.
pedicle, including the ileocolic, right colic, and midcolic routes of the superior mesenteric system and the left colic, sigmoidal, and superior rectal routes of the inferior mesenteric system [see Figure 8].
Although there are many variations in the arrangement of lymph nodes along the pathways of fl ow, three roughly separable groups can be identifi ed. First-echelon lymph nodes are paracolic, associated with the marginal vessel of Drummond. These nodes are the most numerous and, in surgical terms, the most important. Second-echelon or intermediate lymph nodes are located in the mesentery, at the level of division of the mainstem blood vessels into peripheral branches. Third-echelon nodes, represented by the central or principal nodes, are closest to the root of the mesentery and associated with takeoff of the major vascular pedicles. Cancer emboli usually follow the most direct route to regional lymph nodes. This constitutes a stepwise progression: centrally from the paracolic nodes adjacent to tumor, to the intermediate nodes along the most contiguous mesenteric vascular pedicle, and fi nally to the main or prin-cipal lymph nodes at the apex of the mesentery. However, variations and “skip metastases” exist. These metastases occur because of retrograde lymphatic fl ow, secondary to tumor blockage of the main efferent lymphatic channels. Commonly seen atypical sites of lymph node metastases include the gastrocolic omentum, associated with transverse colon lesions and paracolic lymph nodes at a distance from the primary tumor. In light of the extensive nature of nodal disease, skip metastases are generally associated with a poor prognosis.69
In the rectum, at about 7 to 8 cm above the anal verge and approximately at the level of the middle valve of Houston, there is a “lymphatic watershed.” In other words, all lymph from the rectum above this point drains upward along the superior hemorrhoidal vessels; below this level, however, there is dual drainage. Although the direction of fl ow is mostly superior, there may be independent or associated drainage laterally along the middle hemorrhoid vessels to the internal iliac chain of lymph nodes and from there through retroperitoneal vessels to the para-aortic nodes. Very distal lesions may drain along the superfi cial perineal lymphatics, with the fl ow directed toward the superfi cial inguinal lymph nodes.
Regardless of location, the purpose of surgery is to remove the primary tumor with adequate margins en bloc with regional lymph nodes. As noted above, longitudinal spread along the colon rarely extends beyond 2 cm, and this is the rationale for resecting 5 cm of normal intestine proxi-mal and distal to the lesion. In practice, however, the length of intestinal resection is determined by devascularization from the lymphadenectomy. Lymph nodes at risk for metas-tases include those along the primary vascular pedicle clos-est to the tumor, as well as adjacent vessels. These secondary routes have been well described [see Figure 8].66 There is a tendency to extrapolate from these studies and perform radical or extended lymph node resection in the hope of improving patient outcome; however, this has not proved effi cacious. For example, “high ligation” of the inferior mes-enteric artery at its takeoff from the aorta does not appear to improve outcome70,71; it is instead associated with increased
Patient has invasive colon cancer
Order investigative studies to stage and assess resectability of tumor:• Complete colonoscopy • CT of chest, abdomen and pelvis with oral and IV contrast
Tumor is resectable
Tumor is stage I orlow-risk stage II
Tumor is unresectable
No metastases are present
Perform segmental colectomy with regional lymphadenectomy.
Tumor is stage III or high-risk stage II
Administer adjuvantchemotherapy.
Tumor remainsunresectable
Administer systemic chemotherapy orsupportive care.
Metastases are present
Perform staged or concurrent resection.Administer systemic chemotherapy.
Patient is asymptomatic
Administer systemic chemotherapyor supportive care.
Patient is symptomatic
Perform palliative resection, stenting, or diversion.Administer systemic chemotherapy.
Follow up according to protocol.
Tumor becomesresectable
Perform staged or concurrent resection ofprimary tumor and metastases.

Scientifi c American Surgery
09/14
gastro adenocarcinoma of the colon and rectum — 12
perioperative morbidity, autonomic nerve injury, and sexual and bladder dysfunction.
The standard extents of resection in various colon cancers are provided here [see Figure 9]. For tumors of the cecum and the ascending colon, a right hemicolectomy that includes the right branch of the middle colic artery at its origin should be performed. For tumors of the hepatic fl exure, an extended right colectomy that includes the entire middle colic artery
is indicated. For tumors of the transverse colon, an extended right or left colectomy or a transverse colectomy may be per-formed. For tumors of the splenic fl exure region, a left hemi-colectomy is performed, and for sigmoid tumors, a sigmoid colectomy is performed.
In patients who have small or fl at tumors or who are undergoing resection after a polypectomy, intraoperative identifi cation of the tumor may be diffi cult. This is especiall y
Figure 7 Algorithm outlining treatment of rectal cancer. APR = abdominoperineal resection; CEA = carcinoembryonic antigen; CT = computed tomography; IV = intravenous; LAR = low anterior resection; MRI = magnetic resonance imaging; TAE = transanal excision; TEM = transanal endoscopic microsurgery.
Order investigative studies to stage and assess resectabilityof tumor:• Complete colonoscopy • CT of chest, abdomen, and pelvis with oral and IV contrast• CEA level. Rectal ultrasonography, MRI, or both
Patient has rectal cancer
LAR or APR with definite pathologic staging.
Follow up according to protocol.
Turmor is resectable
Tumor is early stage (T1–2, N0 on
ultrasonography or MRI)
Perform palliative stenting or diversion if tumor is obstructing/symptomatic.Administer chemotherapy,radiation therapy, or both.
Tumor is unresectable (T4 or M1)
T1, N0T2, N0
Perform TAE or TEM.
Candidate for local excision
Tumor is locally advanced (T3–4 or N1–2 on ultrasonography)
Resect primary tumor and metastases.
Tumor becomes resectable
Administer systemicchemotherapyor supportivecare.
Tumor remains unresectable
T1, negativemargins,
low-risk feature
T1–2,high-risk
features* or T2
T1–2, negativemargins
Adjuvant chemoradiation.
T3–4 or N1–2
Not candidatefor local excision
Perform staged or concurrent resectionof primary tumorand metastases.Administer systemicchemotherapy.
Tumor has metastasized,
but metastases areresectable
Chemoradiationfollowed by LAR or APR.
T3–4 or N1–2
Administer adjuvant chemoradiationtherapy preoperatively.Perform LAR or APR. If patient isunfit for major operation, considerTAE.Consider systemic chemotherapy.

Scientifi c American Surgery
09/14
gastro adenocarcinoma of the colon and rectum — 13
true with laparoscopic procedures, in which the bowel often cannot be palpated. If the lesion is in the cecum, the ileocecal valve and the appendiceal orifi ce are visualized endoscopi-cally, and localization of the tumor is simple. If the lesion is at another location, endoscopic measurements of the dis-tance from the anus or estimates of the location of the tumor may be inaccurate. Endoscopic tattooing, a process in which an agent is injected into the bowel wall submucosally at or near the site of the lesion, has been employed to facilitate intraoperative identifi cation of the tumor site. India ink is the agent most commonly used for this purpose and generally yields excellent results.72 As an alternative, many institutions use a commercially available sterile suspension of carbon particles, which is also very safe and effective.73 Intraoperative endoscopy is another option for locating these lesions.
Rectal Cancer Surgery
Rectal cancer surgery is challenging due to the limitations of the surrounding bony pelvis and need to preserve critical structures such as the pelvic autonomic nerves. Historically,
rectal cancer surgery had been performed with a blunt technique associated with incomplete mesorectal excision, presacral bleeding due to tearing of the endopelvic fascia, and impotence. In 1982, Heald and colleagues published their landmark article describing an anatomic rectal resec-tion technique, total mesorectal excision (TME).74 They described sharp dissection under direct visualization of the visceral and parietal layers of the endopelvic fascia, result-ing in a specimen with an intact mesorectum and negative tumor margins in the majority of resectable rectal cancers. Heald and colleagues’ fi rst series of 122 curative anterior resections reported an astounding 2.7% rate of local recur-rence at 5 years and 88% overall survival. These excellent results were subsequently matched in a series by Enker and colleagues, which included 246 curable Dukes stage B and C patients.75 Enker further demonstrated excellent mainte-nance of sexual and urinary function with anatomic rectal resection.76
Not surprisingly, studies demonstrate a relationship between the quality of the surgical technique and the out-come. Blunt pelvic dissection is associated with local recur-rence rates as high as 25%. Adequate mesorectal excision (resecting at least 5 cm below a high rectal lesion and TME for middle or low rectal cancers) is associated with local failure rates of 5 to 10%.75,77 Attention must be given to all margins, including the circumferential resection margin.78 Surgeon and hospital volume appear to infl uence the outcome of CRC surgery.79,80
The widespread application of TME has signifi cantly enhanced the effi cacy of rectal cancer surgery. TME paired with sphincter-sparing surgery, including low anterior resection with coloanal anastomosis—particularly in combi-nation with CMT—allows most patients to avoid abdomino-perineal resection and a permanent colostomy. The tech-nique of intersphincteric dissection has been used more and more to gain an adequate distal margin and avoid a perma-nent colostomy.81 In intersphincteric dissection, the internal sphincter (which is a continuation of the rectal muscularis propria) is resected with the rectum, providing an addition-al 1 cm of distal margin [see Figure 10]. Postoperatively, patients are reliant on the external sphincter for continence. These technical advances now mean that sphincter preserva-tion is possible for the majority of rectal cancer patients. Abdominoperineal resection is reserved for patients with poor preoperative function or those with tumors extending into the external sphincter complex.82 Functional outcomes and quality of life associated with ultra-low coloanal anastomosis are now the focus of much research.83
The increasingly common use of these sphincter-saving techniques has spurred renewed interest in defi ning the length of suffi cient distal bowel margin. Although 5 cm was originally thought necessary,84 2 cm is now widely accepted as adequate.85 Recent studies have suggested that even shorter margins may suffi ce, especially if there is signifi cant tumor regression in response to CMT.86 Possibly of more importance than the length of distal intestine removed beyond the tumor is the status of the lateral and circumfer-ential resection margins. Although these have been over-looked in the past, they are as critical as the distal margin in terms of tumor recurrence.78
100
93
20
17
17
12
59
47
100
Figure 8 Lymphatic drainage for colon cancer.

Scientifi c American Surgery
09/14
gastro adenocarcinoma of the colon and rectum — 14
a
Resected Colonand Vessels
Tumor
b
c d
Figure 9 Operative strategies for colorectal cancer: (a) ascending colon; (b) hepatic fl exure; (c) rectosigmoid; (d) splenic fl exure.

Scientifi c American Surgery
09/14
gastro adenocarcinoma of the colon and rectum — 15
IntersphictericDissection
Figure 10 Intersphincteric resection.
Special Circumstances
surgical treatment of hereditary bowel cancer
Surgical resection for patients with hereditary CRC syndromes may be therapeutic or prophylactic. In patients with FAP, the most common procedures include total abdominal colectomy with ileorectal anastomosis or total proctocolectomy with either ileal pouch-anal anastomosis or end ileostomy. Total abdominal colectomy with ileorectal anastomosis is reserved for individuals with minimal rectal disease amenable to endoscopic control. The advantages of ileorectal anastomosis include a simpler operation, relatively normal postoperative bowel function, and preservation of bladder and sexual function. However, the remaining rectum requires frequent surveillance as there is a 10 to 50% risk of the patient developing rectal cancer87; in fact, 40 to 75% of patients eventually require rectal resection. The advantage of total proctocolectomy with ileal pouch-anal anastomosis includes complete elimination of the at-risk colorectal mucosa. However, this is a more complex proce-dure and carries a risk of postoperative bladder and sexual dysfunction, as well as worse (although generally tolerable) bowel function. Total proctocolectomy with end ileostomy is reserved for patients with advanced rectal cancer or those unwilling or unable to undergo an ileal pouch-anal anastomosis.
The surgical management of Lynch syndrome depends on the patient’s initial presentation. Those with cancer or polyps not amenable to endoscopic removal should be considered for total abdominal colectomy with ileorectal anastomosis. Other options include segmental resection with frequent endoscopic follow-up and enrolment in chemopre-vention trials. Women, especially those who have completed childbearing, should be considered for total abdominal
hysterectomy with bilateral salpingo-oophorectomy. Patients with rectal cancer should be considered for total proctocolectomy with end ileostomy or ileal pouch-anal anastomosis. Segmental rectal resection followed by frequent endoscopic surveillance, although generally less preferred, may be appropriate for some patients. Because penetrance is 80%, as many as 20% do not develop the phenotype. There-fore, at-risk individuals with no evidence of colonic disease should undergo frequent endoscopic surveillance. In select circumstances, prophylactic total abdominal colectomy may be a reasonable choice.
surgery for malignant polyps
The treatment of superfi cial carcinomas or malignant polyps depends on the location, depth of bowel invasion (if a focus of carcinoma is identifi ed), and probability of com-pletely removing the tumor endoscopically. The follow-up regimen for patients undergoing excision of pedunculated polyps, which, on histologic examination, reveal superfi cial carcinoma with clear margins and no high-risk features, often consists of close observation without formal colectom y. On the other hand, medically fi t patients with superfi cial tumors or polyps demonstrating positive margins, or high-grade pathologic features such as lymphovascular/perineu-ral invasion, poor differentiation, or single-cell infi ltrate, are at increased risk for regional nodal metastases. In these cases, formal intestinal resection is warranted.
local excision of rectal cancer
Local excision, transanal excision (TAE), and transanal endoscopic microsurgery (TEM) for rectal cancer continue to gain popularity. The appeal is considerable, and the advan-tages are signifi cant: rapid recovery from surgery, minimal morbidity, and preservation of bowel function. However,

Scientifi c American Surgery
09/14
gastro adenocarcinoma of the colon and rectum — 16
more recent data suggest that extreme caution should be exercised before taking this approach. Patients must be selected carefully, and a full discussion of potential risks and benefi ts is mandatory before surgery. Generally, these procedures are reserved for patients with superfi cial rectal adenocarcinoma (T1 or T2) occupying less than one third of the bowel circumference, with no palpable or radiologically identifi ed perirectal lymph nodes, and located within 10 cm of the anal verge. Although the early results of local excision were encouraging, more recent studies with long-term follow-up data consistently show high rates of recurrence and poorer survival than would be expected.88 For example, a report of 125 patients treated with local excision found that the rates of local recurrence were 17% and 28% for T1 and T2 rectal lesions, respectively—signifi cantly higher than the rates reported for radical resection of stage I rectal cancer.89 The explanation for such high relapse rates following local excision is multifactorial; however, it is clearly associated with the issue of regional lymph nodes. Local excision does not assess, resect, or treat potential lymph node metastases. Although candidates for local excision are screened via endorectal ultrasonography or MRI before undergoing resection, nodal metastases from superfi cial rectal lesions (T1 and T2) are generally very small and therefore diffi cult to detect preoperatively.90 Of even more concern, recent reports note that local recurrences are not always amenable to salvage surgery.91 In fact, the two largest series on salvage surgery for recurrent rectal cancer following local excision show that relapse is generally diagnosed when disease is at an advanced stage, necessitating an extended multiorgan resection.91,92 Overall survival after salvage surgery is disap-pointingly low, especially when one considers the early stage of the primary lesion.91 The results following TEM appear to be better than those following TAE, but recurrence remains high.93 One study comparing TEM with TME noted local recurrence rates of 24% with TEM compared with 0% with TME.94 Improved staging modalities are clearly needed if we are to accurately identify candidates for local excision. Until then, patients should be fully informed of the known risks and limitations.
minimally invasive surgery for crc
Minimally invasive techniques have been successfully employed in the surgical treatment of CRC. Prospective randomized series conclude that laparoscopic colectomy for cancer has oncologic equivalence to open surgery, with the signifi cant advantages of less pain and discomfort, speedier postoperative recovery, and smaller, more cosmetically appealing incisions.95–103 Overall, the data support minimally invasive surgery for colon cancer. However, it is important to note that all of the studies to date involve surgeons with specialty training and extensive experience performing lapa-roscopic colectomy. There is a steep learning curve. Some suggest that a surgeon must perform 30 to 50 cases before gaining enough profi ciency to avoid and/or effectively han-dle intraoperative complications or conversions and make the most effi cient use of operating time. These data stress the importance of a surgical mentor and hands-on participa-tion in laparoscopic-assisted procedures, including hand-assisted surgery.
There are growing data on minimally invasive rectal resection for cancer.104 Laparoscopic TME is a more demand-ing procedure technically because of the confi nes of the bony pelvis and the limitations of current stapling technol-ogy, especially when attempting sphincter preservation.105,106 The recently completed COlorectal cancer Laparoscopic or Open Resection II (COLOR II) trial reported important short-term outcome data in 1,103 patients randomized to minimally invasive and open rectal resection. Study cohorts had similar resection margins and completeness of resec-tion. However, short-term recovery was improved in the laparoscopic group.107 Robotic approaches have also been applied to CRC, offering the surgeon the potential benefi ts of improved visualization, retraction, and dexterity. This may improve the ability to resect tumors from the narrow pelvis with greater precision; however, studies are pending.108
obstructing and perforated cancers
Obstructing and perforated colon cancers are associated with a poor prognosis and with increased surgical morbid-ity (as a consequence of the need for emergency surgery). Perforation can occur either via direct erosion of the tumor through the wall of the colon or secondary to obstruction with resultant bowel distention proximal to the tumor. Patients with perforated colon cancer are managed with emergency laparotomy, washout, and resection of the primary lesion to prevent further soilage. A diverting stoma is usually indicated, with either a Hartmann pouch or a mucous fi stula constructed distally. Select patients may be managed by means of primary anastomosis, with or without a proximal diverting colostomy or ileostomy.
Obstructing right-sided cancers (up to the splenic fl exure) can usually be treated with resection and primary anasto-mosis. The traditional emergency treatment of obstructing left-sided colon cancers is a diverting colostomy, with or without resection of the lesion. In many such cases, the stoma is never taken down. Some surgeons advocate emer-gency treatment of these lesions with total abdominal colec-tomy and ileorectal anastomosis as a means of improving outcomes.109 Another treatment option is primary resection and anastomosis, with or without on-table intestinal lavage. Yet another option for managing obstructing left-sided colon and rectal cancers is the use of colorectal stents, with the goal of avoiding emergency surgery. Stents can serve as a bridge to defi nitive resection by decompressing the colon and thereby allowing subsequent bowel preparation. In patients with advanced disease, stents may also be employed for palliation as an alternative to surgical resection or a diverting stoma. A recent prospective randomized trial noted a high rate of complications with stents, indicating that patients with obstruction should be treated on a case-by-case basis.110
Choosing which pathway to take in an obstructed patient depends on the condition of the patient (comorbidities and hemodynamic stability), extent of the primary lesion, and available surgical expertise. Removal of the tumor is pre-ferred if it can be done safely and completely. In an unstable patient, diversion may be the best course of action. For a mid- to high rectal cancer where neoadjuvant therapy is indicated, diversion or stenting is preferred.

Scientifi c American Surgery
09/14
gastro adenocarcinoma of the colon and rectum — 17
synchronous primary crcs
The incidence of synchronous CRCs is reported to range from 3 to 5%111,112 but may be as high as 11%.113 Stage for stage, there appear to be no differences in survival between synchronous cancers and single primary cancers.114,115 Synchronous adenomatous polyps are present in as many as 35% of CRC patients undergoing surgical treatment.112,115 In one study, the presence of synchronous lesions made the surgical procedure for resection of the primary tumor more extensive than initially planned in 11% of patients.112
Most synchronous polyps are identifi ed on preoperative colonoscopy, and the colon can often be cleared of these lesions before operation. Management of adenomas not amenable to endoscopic resection and management of syn-chronous cancers are more challenging. Each primary cancer must be managed surgically according to sound oncologic principles. One option is to perform multiple segmental resections with multiple anastomoses. Another is to perform an extended resection that encompasses all of the lesions or even total abdominal colectomy if needed. The presence of a rectal cancer and a second synchronous lesion makes sur-gical treatment even more challenging, especially if sphinc-ter preservation and a low rectal or coloanal anastomosis are contemplated.
peritoneal carcinomatosis
Peritoneal carcinomatosis develops in approximately 13% of all CRC patients.116 The survival rate for patients who present with peritoneal carcinomatosis from CRC is gener-ally poor. In patients with stage IV disease, the presence of carcinomatosis is associated with a signifi cant reduction in survival (from 18.1 months to 6.7 months).117 Treatment has traditionally included systemic chemotherapy, with surgery reserved for palliation of symptoms such as bowel obstruc-tion. Contemporary chemotherapy regimens that include agents such as oxaliplatin may improve survival but are certainly not curative.
Peritoneal carcinomatosis is often associated with hema-togenous metastases, but in some 25% of patients, the peri-toneal cavity is the only site of disease. Several groups have advocated the use of cytoreductive surgery and hyperther-mic intraperitoneal chemotherapy (HIPEC) as a means of improving survival in these patients.118 This treatment, however, is associated with morbidity and mortality.119 A randomized trial from the Netherlands comparing cytore-ductive surgery plus HIPEC with systemic chemotherapy plus palliative surgery found that patients in the former group exhibited a statistically signifi cant improvement in median survival (22.3 months versus 12.6 months).120 At 8 years of follow-up, a signifi cant improvement in median disease-specifi c survival was maintained.121 Cytoreductive surgery plus HIPEC seems to be a viable option for the treat-ment of peritoneal carcinomatosis. Given the substantial morbidity and mortality associated with these aggressive procedures, patient selection remains a major issue.
synchronous metastatic (stage iv) disease
As many as 20% of CRC patients have metastatic disease at the time of the initial presentation. The need for surgical
intervention in this group is not well defi ned. Clearly, surgi-cal resection or diversion is indicated in patients who pres-ent with signifi cant bleeding, perforation, or obstruction. In asymptomatic patients with unresectable metastatic disease, the role of surgical resection of the primary lesion remains controversial. In patients with resectable metastatic disease (e.g., isolated liver or lung metastases), curative resection may be undertaken.
A recent review of 233 patients with synchronous stage IV colorectal cancer found that 217 patients (93%) never required surgical palliation of the primary tumor; 16 patients (7%) needed emergency surgery for obstruction or perfora-tion of the primary tumor; 10 patients (4%) were managed nonoperatively.68,122
Management of patients with synchronous resectable isolated liver metastases continues to evolve. Multiple studies have documented improved survival after liver resection in patients with metastatic disease confi ned to the liver. Patients presenting with synchronous lesions have a worse prognosis than those presenting with metachronous lesions.123 Many of these patients have been managed with staged resections of their primary cancers and the liver metastases. Several groups have reported that such com-bined procedures do not substantially increase surgical morbidity and mortality or compromise cancer survival.124,125 These combined procedures should be done only in care-fully selected patients, at specialized centers where there is signifi cant experience in resection of both CRCs and liver tumors.
Chemotherapy
adjuvant therapy of colon cancer
The fi rst defi nitive trial to show a benefi t of adjuvant therapy after resection of stage III colon cancer was the National Cancer Institute (NCI) cooperative Intergroup trial INT-0035. In this study, involving over 900 patients, those who received 1 year of 5-FU plus levamisole (an agent thought to have immunomodulatory effects but later shown to be inactive) showed a 33% risk reduction in the chance of death or recurrence within 5 years compared with those receiving surgery only.126 Further studies demonstrated that 6 months of chemotherapy had essentially the same benefi t at 12 months and that 5-FU plus leucovorin (folinic acid, LV) was as active as 5-FU plus levamisole or 5-FU plus levami-sole and LV. Thus, 6 months of 5-FU/LV became standard practice for all stage III patients who did not have a medical contraindication to this treatment.127
As discussed below, during the late 1990s and the early part of this decade, a number of drugs were shown to have clinically meaningful activity in metastatic CRC. This led to studies to evaluate whether incorporation of such agents into the adjuvant setting would be useful in increasing the cure rate. Of all the drugs tried thus far, however, only oxaliplatin has shown benefi t. The lack of benefi t demon-strated by other agents remains poorly understood but serves as an important cautionary note about the need to complete adjuvant trials before adopting new agents into the adjuvant setting.

Scientifi c American Surgery
09/14
gastro adenocarcinoma of the colon and rectum — 18
oxaliplatin regimens
Oxaliplatin is a platinum-based anticancer agent. Periph-eral sensory neurotoxicity, manifesting itself as cold sensi-tivity, numbness, and paresthesias, is the major toxicity of this agent. Fatigue, myelosuppression, and diarrhea are also part of its toxicity spectrum. Nausea and vomiting can be severe if inadequate prophylaxis is not used, but with proper prophylactic use of current antiemetic regimens, these toxicities are usually minor or nonexistent. In marked contradistinction to cisplatin, the fi rst platinum-based chemotherapy agent, oxaliplatin has no nephrotoxicity.
Oxaliplatin has minimal antitumor activity by itself but has shown greater activity when combined with other agents, such as 5-FU/LV or capecitabine. In the pivotal Multicenter International Study of Oxaliplatin, Fluorouracil, and Leucovorin in the Adjuvant Treatment of Colon Cancer (MOSAIC) trial, a total of 2,246 patients (of whom 60% were stage III and 40% stage II) were randomized to postopera-tive 5-FU/LV by 48-hour infusion every other week, plus/minus a 2-hour infusion of oxaliplatin.128,129 (This regimen of oxaliplatin, 5-FU, and LV, given every other week with a 2-day ambulatory infusion of 5-FU, is known by the acronym “FOLFOX.”) Five-year disease-free survival (DFS) was 73.3% for the FOLFOX group compared with 67.4% in the 5-FU/LV group (p = .003). Six-year overall survival rates were 72.9% for those receiving FOLFOX versus 68.7% for those receiving 5-FU/LV (p = .023). No improvement in overall survival was seen with the addition of oxaliplatin in patients with stage II disease, including those with stage II cancer having one or more high-risk factors.130 The outcome of this trial established FOLFOX as the (current) routine standard treatment for patients with stage III colon cancer.
Long-term neurotoxicity is a major concern in oxaliplatin therapy. The toxicities seen in the FOLFOX arm included a 12.5% incidence of grade 3 sensory neuropathy, which remained at grade 3 in 1.3% of patients at the time of their 1-year follow-up. Thirty percent of those receiving oxalipla-tin in the MOSAIC trial had some residual neurotoxicity 1 year after the end of treatment, and 15% had some degree of residual neurotoxicity after 4 years of follow-up, which, for all practical purposes, represents permanent neuropathy, of which 3.5% had grade 2 or greater neuropathy. The National Surgical Adjuvant Breast and Bowel Project (NSABP) C-07 trial evaluated the addition of oxaliplatin to a weekly schedule of 5-FU/LV by brief IV bolus injection (as opposed to infusion) and randomly assigned 2,407 patients with stage II or III colon cancer (71% with stage III disease) to half a year of weekly bolus 5-FU/LV, with or without oxaliplatin administered on weeks 1, 3, and 5 of every 8-week cycle (known as the FLOX regimen).131 The 5-year DFS in the FLOX arm was 69.4%, versus 64.2% in the 5-FU/LV arm (hazard ratio 0.82, p = .002). For the stage III patients, the 5-year DFS was improved by 6.6%, from 57.8 to 64.4%. However, with a median follow-up of 8 years, this trial of 2,409 stage II and III patients did not achieve a statistically signifi cant survival benefi t for the addition of oxaliplatin. There was virtually no overall survival benefi t seen in stage II patients. In the stage III patients, there was a 2.7% absolute improvement in median overall survival (73.8 years versus 76.5 years) with the addition of oxaliplatin, with a p value of
borderline signifi cance (p = .052). The weekly bolus schedule of 5-FU/LV used here is known to cause a substantial amount of diarrhea, and hospitalizations for diarrhea/dehy-dration were required for 5% of patients receiving FLOX and 3% receiving 5-FU/LV. This FLOX regimen is regarded as acceptable but less preferred than either FOLFOX or CapeOx (discussed below); thus, it should be used only when there are good reasons not to use these other options.
Studies of the oral fl uoropyrimidines capecitabine and tegafur-uracil (UFT) have demonstrated that each is an acceptable alternative to parenteral 5-FU/LV in the adjuvant setting. In a study powered to evaluate the noninferiority of capecitabine compared with the Mayo Clinic bolus 5-FU/LV schedule, noninferiority was demonstrated.132 Similar results (noninferiority of an oral fl uoropyrimidine versus Mayo Clinic 5-FU/LV) were shown in the NSABP C-06 trial of the oral agent UFT plus oral LV (although UFT is not commer-cially available in the United States).107 However, given that the standard regimen in the adjuvant setting is no longer 5-FU/LV alone, use of these oral agents is limited.
More recently, the combination of oral capecitabine plus IV oxaliplatin (CapeOx) has been compared with IV 5-FU/LV in an 1,866-patient phase III trial.133 The 3-year DFS was 70.9% in the CapeOx arm versus 66.5% with 5-FU/LV. The difference between arms in overall survival at 5 years favored the CapeOx arm, 77.6% versus 74.2%; however, this difference did not reach statistical signifi cance at the time of this analysis (p = .15486), and further analysis and follow-up are ongoing. It should be noted that no head-to-head comparisons of FOLFOX versus CapeOx have been, or will be, done in the adjuvant setting as such trials would require in excess of 10,000 patients for an adequately pow-ered noninferiority study. However, the regimens have been compared head to head in the metastatic setting and appear to be quite similar in effi cacy.134 Therefore, it is reasonable to regard FOLFOX and CapeOx as having similar effi cacy in the adjuvant setting, and each of these regimens is equally acceptable. It must be noted, however, that the oral admin-istration aspects of CapeOx put a lot of responsibility on the patient, so it is only appropriate for a highly competent, motivated, and reliable patient who is willing and able to comply with the complex oral medication schedule. Because CapeOx patients require IV oxaliplatin and oral capecitabi-ne, it is not a simple matter of oral versus IV chemotherapy. Whether parenteral FOLFOX or parenteral plus oral CapeOx is more convenient for the patient is a debatable point, and individual patient preferences and abilities regarding this will differ.
negative trials
As outlined in the section below on metastatic disease, irinotecan, bevacizumab, and cetuximab are all active agents in metastatic CRC. All of these, however, have failed to show benefi t in the adjuvant setting, and none have a role in treating anything but stage IV disease.
Irinotecan
Irinotecan is a topoisomerase I inhibitor, which has shown substantial antitumor activity in CRC in the metastatic setting. However, a 1,200-patient randomized phase III trial

Scientifi c American Surgery
09/14
gastro adenocarcinoma of the colon and rectum — 19
of weekly bolus 5-FU/LV with or without the addition of irinotecan failed to demonstrate any clinical benefi t with the addition of irinotecan and higher rates of early death and in grade 3 and 4 (serious and life-threatening) neutropenia.135 Similarly, disappointing results were seen with the FOLFIRI regimen (irinotecan plus biweekly 48-hour infusions of 5-FU/LV). Two studies done in Europe combining irinote-can with 48-hour 5-FU infusion demonstrated no benefi t.136,137 An additional study of 5-FU/LV plus/minus irinotecan in patients with resected liver metastases also showed no benefi t with irinotecan.137
Bevacizumab
A phase III trial in the metastatic setting using irinotecan, 5-FU, and LV plus/minus bevacizumab showed a 4.7-month median survival benefi t to the group receiving bevacizumab. Following this, the NSABP conducted a 2,673-patient randomized trial of FOLFOX versus FOLFOX plus bevaci-zumab in patients with stage II and III colon cancer.138 Although there was an early trend toward improved DFS at the 1.25-year mark, this had disappeared by the 3-year mark. There was no statistically signifi cant improvement in either DFS or overall survival for the study as a whole or for the stage III subset (overall survival for stage III hazard ratio 1.0, p = .99). A second trial randomized 3,451 stage II and III patients (2,867 with stage III) to one of three arms: FOLFOX, FOLFOX plus bevacizumab, or CapeOx plus bevacizumab. This, too, was a fully negative trial, with no indication of benefi t for the addition of bevacizumab. Bevacizumab did not improve DFS, and there was a trend toward decreased overall survival in the bevacizumab-containing arms relative to the FOLFOX control arm. Thus, there is no role for bevacizumab in the adjuvant treatment of stage II or III colon cancer.
Cetuximab
Cetuximab is an anti–epidermal growth factor receptor (EGFR) monoclonal antibody that has shown activity in treating metastatic CRC. Current data have demonstrated that it has potential for activity only in tumors that lack mutations in the K-ras gene. The North Central Cancer Treatment Group (NCCTG) conducted a phase III trial of FOLFOX versus FOLFOX plus cetuximab in 2,070 patients with stage III, K-ras–wild-type colon cancer. This study did not result in an improvement in either disease-free or overall survival, and the addition of cetuximab added considerable toxicity.139 Patients over age 70 had a statistically signifi cantl y worse 3-year DFS with the addition of cetuximab. Thus, cetuximab should not be used in the adjuvant setting for stage III patients. It would be reasonable to extrapolate this fi nding to panitumumab as well because this agent is also an anti-EGFR monoclonal antibody.
Stage II Colon Cancer
Stage II colon cancer has a reasonably good chance of sur-gical cure, with 72 to 85% overall survival. This is one of the most controversial areas in terms of treatment. In general, the evidence is not compelling that chemotherapy offers any meaningful improvement in the cure rate for good-risk
stage II colon cancer. For patients with “high-risk” stage II cancer—defi ned as the presence of either a T4 primary lesion, clinical obstruction, perforation, poorly differentiated histology, or inadequate lymph node sampling (< 12 nodes)—the risk of relapse is potentially higher than the risk of relapse for patients with stage IIIA disease. Therefore, high-risk stage II patients who do not have contraindica-tions to chemotherapy are routinely treated with adjuvant fl uoropyrimidine chemotherapy (either capecitabine or 5-FU/LV) chemotherapy. The question of inclusion of oxali-platin in the management of stage II patients is complex. Data are overwhelming that oxaliplatin adds no benefi t in good-risk stage II patients, and it does add considerable toxicity. In high-risk stage II patients, a preliminary analysis of the MOSAIC trial had suggested a trend toward improved outcome with oxaliplatin; however, a recent update of this trial showed no benefi t for the inclusion of oxaliplatin in the treatment of stage II patients, regardless of whether they were classifi ed as high risk.130
molecular markers
At the time of this writing, we do not have any fully vali-dated markers to assist us in selecting patients who either do or do not need chemotherapy, or specifi c chemotherapy agents, in the adjuvant setting. Several multigene assays have become available commercially; however, these assays are prognostic and not predictive. As such, they do not pro-vide a decision point in terms of who will or will not benefi t from chemotherapy. For this reason, their use has not been incorporated into NCCN guidelines, and their use is not recommended at this time.140 One factor that does have clear prognostic and predictive signifi cance is the presence of MMR defi ciency, as demonstrated either by the absence of immunohistochemical expression of these proteins or by PCR analysis for high MSI. The literature on this matter is complex, with some studies suggesting that tumors with high MSI, as detected either by direct measurement or by demonstration of defi cient MMR protein on immunohis-tochemical staining, do not benefi t or may even suffer a detriment from 5-FU/LV. However, an analysis of MSI from archival tissues in four NSABP adjuvant chemotherapy trials demonstrated no prognostic correlation and no trend toward a correlation between high MSI and overall survival (p = .67).141 More recently, Sargent and colleagues reported on 457 stage II or III colon cancer patients with available tumor samples who were randomized to 5-FU-based chemotherapy versus observation in NCI cooperative group trials.10 Fifteen percent of these patients were found to have MMR-defi cient tumors. In individuals with MMR-defi cient tumors, no benefi t from 5-FU in terms of DFS was seen. In the stage II patients with MMR-defi cient tumors, treatment with 5-FU appeared to be associated with decreased overall survival. These data support the use of MMR evaluation in stage II patients and suggest that single-agent fl uoropyrimi-dine should not be used in patients with MMR-defi cient tumors. Data do support the use of chemotherapy in stage III tumors that have MMR defi ciency, however. Sinicrope and colleagues demonstrated that although MMR defi ciency remains a favorable prognostic indicator in stage III patients, 5-FU is still able to improve prognosis.142 As oxaliplatin was

Scientifi c American Surgery
09/14
gastro adenocarcinoma of the colon and rectum — 20
not available and therefore was not used in the patients studied, these data do not comment on the role of MMR in impacting the effi cacy of FOLFOX or other oxaliplatin-containing regimens.
summary and clinical recommendations
The FOLFOX and CapeOx regimens are the current regi-mens of choice for stage III colon cancer patients, and the FLOX regimens appear to be somewhat less desirable but would be an acceptable alternative in a patient unable to receive either FOLFOX or CapeOx. Trials of irinotecan, bevacizumab, and cetuximab have been negative in the adjuvant setting, and these agents should therefore not be used in adjuvant treatment. In those patients to be treated with fl uoropyrimidine alone, 5-FU/LV and the oral fl uoro-pyrimidine capecitabine or UFT plus LV appear to be rea-sonable options. Adjuvant chemotherapy for stage II colon cancer remains a complex and controversial topic. All stage II patients should have a medical oncology consultation for frank discussion of the potential benefi ts and risks. High-risk stage II patients are typically recommended to receive treatment with 5-FU/LV or capecitabine. The role of oxali-platin in stage II is not supported by data. MMR protein status should be determined on all stage II patients, and those with MMR defi ciency should not receive adjuvant chemotherapy.
Rectal Cancer
The management of local and locoregionally advanced rectal cancer differs from the management of colon cancer in that radiation therapy plays a major role. Patient evaluation should include either endorectal ultrasonography or pelvic MRI as the initial treatment decision is based on the T stage determined by one of these modalities. In general, if the patient has a T1 or T2 rectal primary tumor without unequivocal evidence of nodal metastases, initial resection is warranted. The exception would be a patient with a distal tumor for whom a sphincter-sparing procedure might be facilitated by tumor regression, in which case, preoperative chemoradiotherapy would be indicated. If, however, the preoperative workup indicates either a T3 or T4 primary tumor and/or positive lymph nodes, preoperative chemora-diation is warranted. Many older, uncontrolled trials allowe d for some differences of opinion on this issue. However, the German Rectal Trial, published in 2004, provides Level I evidence in favor of preoperative chemoradiotherapy in anything other than T1 or T2 lesions, rendering previous arguments to the contrary moot.143 This trial, although show-ing no statistically signifi cant difference in overall survival, showed improved local control (which is the goal of radia-tion therapy) and decreased toxicity when preoperative rather than postoperative chemoradiotherapy was used.
Current data support the use of either single-agent 5-FU or oral capecitabine with concurrent radiation therapy. A large randomized study comparing infusional 5-FU with oral capecitabine showed these to be of equal effi cacy.144 (Multiple studies have indicated that concurrent use of oxaliplatin with radiation therapy adds to toxicity but not effi cacy, so this approach is not recommended.144,145)
A treatment plan including preoperative pelvic radiation therapy plus chemotherapy also includes postoperative chemotherapy, beginning 4 to 6 weeks after surgery and lasting approximately 4 months. It is imperative to note, and to discuss in advance with the patient, that absolutely no fi nding intraoperatively will obviate the necessity for post-operative chemotherapy. Even if a pathologic complete response is noted at operation, postoperative chemotherapy should be given as initially planned. Pathologic complete response is an indication of the results of radiation plus chemotherapy; it indicates nothing about the effect of che-motherapy on micrometastatic disease. To fully minimize the chance of death from distant metastatic disease, the full, planned course of postoperative chemotherapy must be given. Extrapolating from the data on colon cancer, postop-erative oxaliplatin-containing regimens such as FOLFOX are typically used in the setting of rectal cancer postoperatively. The data thus far do not support routine incorporation of oxaliplatin into concurrent chemoradiotherapy regimens.146
Although the use of chemoradiation as an initial maneu-ver has become standard practice, interest has been growing in the use of systemic oxaliplatin-containing chemotherapy as an initial treatment maneuver, followed by chemoradia-tion in locally advanced rectal cancer. Chau and colleagues published an initial series of patients with locally advanced poor-risk rectal cancer treated with CapeOx for 3 months, followed by capecitabine plus radiation therapy.147 The radiologic response rate was 88%, and 86% of patients achieved symptomatic response in a median of 32 days. A recent single-center retrospective report on the use of initial induction FOLFOX chemotherapy in patients with locally advanced rectal cancer demonstrated high tumor response rates to induction chemotherapy, with ability to deliver vir-tually all planned systemic chemotherapy without interfer-ence with ability to deliver planned chemoradiotherapy.148 Patients were routinely reevaluated by their surgeons after approximately 8 weeks of chemotherapy to ensure that local progression was not occurring, and no patient in this series had local progression at that examination. All patients undergoing TME surgery achieved an R0 resection.
In further support of this approach, a small pilot trial explored the use of initial therapy for locally advanced rectal cancer with FOLFOX-based chemotherapy without planned radiation therapy. Of the 30 patients treated with initial chemotherapy alone, eight (27%) achieved a pathologic complete response, with no viable tumor detectable in the resection specimen.149 The use of preoperative chemotherapy without routine use of radiation is an experimental approach that, at the time of this writing, is being evaluated in the NCI cooperative group Predictors of Response to CRT (PROSPECT) trial and is not an approach that should be routinely used outside a clinical trial at this time.
The concept of “chemotherapy fi rst,” with 4 months of FOLFOX or CapeOx chemotherapy, followed immediately by chemoradiation therapy, followed by TME, is a logical extrapolation from current practice and may be reasonably considered a standard care option. It is clear that there never will be an adequately powered, randomized phase III trial of “chemotherapy fi rst” versus the current routine “chemotherapy last”; however, there are numerous reasons

Scientifi c American Surgery
09/14
gastro adenocarcinoma of the colon and rectum — 21
why giving all planned therapy prior to resection would be expected to be advantageous. These include the ability to introduce best systemic therapy as early as possible into the patient’s treatment to maximally address the concern of distant micrometastatic disease, as well as the ability to more rapidly initiate chemotherapy and obtain rapid symptom-atic relief. Patients treated with chemotherapy as a fi rst maneuver have a high likelihood of receiving all planned chemotherapy, which is not the case when chemotherapy is planned postoperatively. Furthermore, no chemotherapy must be taken with a diverting ostomy, and diverting osto-mies can be closed substantially sooner than if postoperative chemotherapy were required.
Treatment of Systemic Metastatic (Stage IV) Disease
From the late 1950s until the mid-1990s, chemotherapy for CRC was limited to 5-FU. Between 1996 and 2004, fi ve new agents received regulatory approval (irinotecan, capecitabi-ne, oxaliplatin, cetuximab, and bevacizumab). We have been stuck in the doldrums of drug development for CRC ever since. With the exception of panitumumab, which differs only trivially from cetuximab and does not open up any novel treatment paradigms, nothing new has become available.
5-FU, patented in 1957, remains the single most active and important drug in the treatment of CRC. It is a sobering reality that all drugs developed since have essentially been failures when compared with preclinical expectations. All of these agents were designed to replace 5-FU. None, however, has demonstrated single-agent superiority. As a result, the fallback strategy of drug development has been employed: drugs that have failed to show any ability to displace 5-FU have been combined with it, and the resulting regimens show evidence of superiority over single-agent 5-FU alone.
The fi rst such agent to be developed was the topoisomer-ase I inhibitor irinotecan. A randomized trial of irinotecan/5-FU/LV (IFL) versus 5-FU/LV alone demonstrated modest superiority for the IFL regimen.150 This trial used 5-FU on a weekly bolus injection schedule. A parallel trial conducted in Europe used an every-other-week infusion schedule of 5-FU, with similar results.151
At the same time, investigators working with the third-generation platinum compound oxaliplatin showed that the combination of oxaliplatin plus 2-day infusions of 5-FU/LV (FOLFOX) every other week was superior to the 5-FU/LV regimen alone.152 These trials, however, although demon-strating improvements in response rate and progression-free survival, failed to show a statistically signifi cant survival benefi t; because of this, approval of oxaliplatin in the United States was delayed for several years.
Subsequently, a randomized intergroup trial (N9741) of irinotecan plus bolus 5-FU/LV (IFL), versus oxaliplatin plus infusional 5-FU (FOLFOX), showed a higher response rate and longer time to tumor progression for the FOLFOX arm.153 Survival on the FOLFOX arm was superior; however, the meaning of these survival data is diffi cult to interpret because second-line irinotecan was widely available to the FOLFOX patients, whereas second-line oxaliplatin was not widely available to the IFL patients (oxaliplatin was not
approved for use in the United States at that time). Second-line irinotecan has been shown to confer a survival advan-tage,154 and survival has been correlated with the availability of all active drugs.155
Overall, the N9741 trial did show the FOLFOX regimen to be preferable to the IFL regimen. Unfortunately, this was largely misinterpreted as evidence of the superiority of oxaliplatin over irinotecan, which is not a correct interpreta-tion. In fact, what the trial most likely shows is the superior-ity of infusional over bolus 5-FU. Two randomized studies comparing fi rst-line FOLFOX (infusional 5-FU/LV plus oxaliplatin) versus FOLFIRI (infusional 5-FU/LV plus irinotecan) indicate that response rate, time to tumor progression, and overall survival were virtually the same, regardless of which regimen was used fi rst.156,157 The conclu-sion of the aggregate of these trials is that FOLFOX and FOLFIRI are equally acceptable in the front-line managemen t of metastatic CRC.
A large phase III trial compared FOLFOX with CapeOx, demonstrating that these two regimens were comparable in effi cacy and similar in overall degree of toxicity.134 Thus, FOLFOX, FOLFIRI, and CapeOx are acceptable standard front-line combination regimens. Capecitabine has also been the focus of a large trial comparing sequential use of single agents with combinations. In this trial, patients treated with sequential single agents experienced overall survival similar to that achieved with combination regimens.158 The Fluoro-uracil, Oxaliplatin, CPT-11: Use and Sequencing (FOCUS) trial, which compared front-line infusional 5-FU/LV with front-line FOLFOX and FOLFIRI, also showed no survival benefi t by using combination therapy as initial treatment.159 These trials indicate the importance of individualization of care. Clearly, some patients can be spared the toxicity of combination regimens without detriment, whereas others, especially those with rapidly progressing and/or symptom-atic disease, are more likely to benefi t from the higher response rates achieved with combination therapies.
Bevacizumab is a monoclonal antibody that binds to vascular endothelial growth factor (VEGF). It was initially hoped that bevacizumab would have meaningful single-agent antitumor activity, but this turned out not to be the case. Bevacizumab does appear to add to the effi cacy of some chemotherapeutic regimens, however. In a random-ized, double-blind, placebo-controlled trial, IFL plus bevaci-zumab was superior to IFL plus placebo, with a 4.7-month improvement in overall survival, a 4.4-month improvement in progression-free survival, and a 35 to 45% improvement in response rate.160 The addition of bevacizumab to front-line oxaliplatin-based therapy has been somewhat less success-ful. In a very large, randomized, placebo-controlled trial of either CapeOx or FOLFOX plus either bevacizumab or pla-cebo,161 progression-free survival was signifi cantly improved statistically, but the incremental benefi t was a modest 1.4 months. Overall survival was also improved by 1.4 months, which did not reach statistical signifi cance. In addition, the response rate with bevacizumab was virtually identical to that of placebo.
Cetuximab and panitumumab are monoclonal antibodies that target the EGFR. Although they have never been (and likely never will be) compared head to head, they appear to

Scientifi c American Surgery
09/14
gastro adenocarcinoma of the colon and rectum — 22
be extremely similar and have very similar activity profi les. Panitumumab is associated with a lower incidence of hypersensitivity reactions.
Initial studies of cetuximab demonstrated a 22.5% response rate in irinotecan-refractory CRC when cetuximab was given in combination with continued irinotecan162 and a single-agent response rate of 10.5%.162 These activity levels were subsequently confi rmed in a randomized trial. Panitu-mumab was also shown to have a 10% response rate as a single agent in a similar patient population.163 Subsequently, it was demonstrated that both of these agents had potential effi cacy only in cancers characterized by a wild-type (nonmutated) K-ras gene. Analysis of the K-ras gene for mutations in exon 2 has now become standard practice, and neither cetuximab nor panitumumab should be offered to patients whose tumors have mutated K-ras.164,165 More recent data indicate that all ras mutations, including non–exon 2 K-ras mutations and N-ras mutations, confer resistance to anti-EGFR therapy, so treatment with cetuximab or panitu-mumab is limited to the approximately 50% of CRC patients whose tumors have wild-type ras.166 The major side effect of both of these anti-EGFR monoclonal antibodies is an acnelike skin rash. Studies have now consistently shown that, for reasons that remain unclear, skin rash is tightly linked to antitumor activity, and only those patients with moderate to severe skin rashes benefi t from these agents. Management of the skin rash is diffi cult, however. Oral antibiotics appear to improve it somewhat, but topical agents other than moisturizers have not demonstrated convincing usefulness thus far.
Studies using either cetuximab or panitumumab with fi rst-line FOLFOX or FOLFIRI regimens have now been reported.167–169 Activity, as expected, is confi ned to those patients with wild-type K-ras tumors; in several studies, patients with mutated K-ras who receive anti-EGFR agents have a worse outcome than the control arm.168,169 Given that only those patients who react with substantial skin rash benefi t from this approach, its practical utility in fi rst-line management is somewhat limited. Furthermore, several studies have now failed to show benefi t for the addition of cetuximab to oxaliplatin-based fi rst-line regimens, and such an approach is no longer recommended.170,171 Of note, the Panitumumab Randomized Trial in Combination with Chemotherapy for Metastatic Colorectal Cancer to Deter-mine Effi cacy (PRIME) trial, evaluating the addition of pani-tumumab to FOLFOX, did show a modest but statistically signifi cant benefi t for the panitumumab-containing arm.166 As discussed above, however, the only patients who benefi t are those with a substantial skin rash, and this makes selection of a panitumumab-containing front-line regimen problematic.
Given the demonstrated activity of bevacizumab and the anti-EGFR agents with chemotherapy, there was logical enthusiasm for combining anti-VEGF and anti-EGFR strate-gies. A small, randomized phase II study demonstrated the feasibility of this strategy162; however, two large randomized trials, one with CapeOx-bevacizumab plus/minus cetux-imab172 and one with FOLFOX-bevacizumab plus/minus panitumumab,173 each showed that there was not only no benefi t to adding an anti-EGFR agent to a regimen of chemotherapy plus bevacizumab but also that there was
actually a harmful effect: the dual antibody regimens had worse outcomes than the control arms. Therefore, concur-rent use of bevacizumab plus anti-EGFR agents is not recommended.
Chemotherapy with Surgery for Metastatic Disease
Resection of stage IV disease confi ned to the liver or lung has become accepted standard practice. The role of chemo-therapy in such surgical strategies continues to be defi ned. As noted earlier, a randomized trial of 5-FU/LV plus/minus irinotecan after liver resection showed no benefi t for the irinotecan-containing arm.136 However, this study did not address the merits of chemotherapy versus no chemotherap y. In the fi rst trial to address this issue with reasonable power, patients with clinically resectable liver metastases were randomly assigned to surgery alone versus surgery with 3 months of preoperative FOLFOX and 3 months of postop-erative FOLFOX.174 Of the patients who actually underwent an R0 resection, those who received chemotherapy had a statistically signifi cant improvement in 3-year DFS; how-ever, the actual improvement was 9.2%, a smaller margin than had been hoped for. Nevertheless, in patients who have not been previously exposed to FOLFOX chemotherapy, the use of FOLFOX in the perioperative setting appears to be appropriate.
Previously, the assumption had been made that any ther-apy demonstrating activity in unresected stage IV disease would be a reasonable adjuvant therapy in resected stage IV disease. This concept requires reexamination, however. As noted above, irinotecan,135,136,167 bevacizumab,138 and cetux-imab139 (and, by extrapolation, panitumumab) have all been shown to be ineffective in resected stage III patients: none eradicated micrometastases in these patients. As it is diffi -cult to see how these agents could be expected to eradicate micrometastases in the stage IV setting with any greater effi cacy than in stage III, it would seem diffi cult to justify their use following resection of stage IV disease. In terms of preoperative therapy, it is important to distinguish between (1) the true neoadjuvant setting (in which disease is clearly resectable, and in which case, these agents would not appear to have any more role than they would in postoperative use) versus (2) disease that is not believed to be resectable but rather potentially convertible to resectability in the setting of a suffi cient antitumor response. In such cases, use of an anti-EGFR agent with chemotherapy, use of an irinotecan-containing regimen, and use of an irinotecan-containing regimen with bevacizumab would appear to be reasonable considerations. A cautionary note, however, was seen in the Erbitux Plus Chemotherapy vs Chemotherapy Alone for Operable Metastatic Colorectal Cancer (EPOC) 2 trial (thus far presented only in abstract form), in which the addition of cetuximab to perioperative FOLFOX chemotherapy resulted in a markedly inferior progression-free survival for those patients in the cetuximab-containing arm (20.5 months versus 14.1 month, hazard ratio 1.49, p = .03).175 It should be noted that bevacizumab did not improve the response rate or degree of tumor shrinkage in a 1,400-patient randomized trial with oxaliplatin-based chemotherapy.161 Bevacizumab does appear to improve the response rate with irinotecan-based therapy, so if shrinkage of gross metastatic disease

Scientifi c American Surgery
09/14
gastro adenocarcinoma of the colon and rectum — 23
for the purpose of converting a patient from unresectable to resectable is the goal of treatment, the use of an irinotecan plus bevacizumab–containing combination would seem reasonable. It should also be noted that bevacizumab does impede wound healing, and its half-life is approximately 21 days. Therefore, it is recommended that bevacizumab be discontinued at least 6 weeks prior to any planned major surgery.
CRC Post–Resection Follow-up
Eighty percent of patients who recur after curative resec-tion of colon and rectal carcinomas do so within 3 years. Therefore, any posttreatment plan should include regular follow-up during at least these 3 years. An additional biologic precept in designing follow-up should take into account the effi cacy of therapy once recurrent disease is identifi ed. It is important to note that the role of surveillance is to identify recurrent disease that can be resected with true curative intent; early identifi cation of asymptomatic, incur-able disease is exceedingly unlikely to improve outcome as there are neither data nor a compelling rationale to believe that outcome would be improved by earlier institution of noncurative treatment, such as systemic chemotherapy.176
In general, if a patient is a candidate for resection of recur-rent disease (e.g., hepatic resection), serum carcinoembry-onic antigen (CEA) testing should be performed every 3 to 6 months for 2 years and then every 6 months for 5 years after resection of the primary tumor. Chest, abdomen, and pelvis CT is recommended annually for 3 years for patients at high risk for recurrence. PET-CT is not recommended in NCCN, ASCO, or CCO guidelines and should not be used for the purpose of postoperative surveillance. Colonoscopy should be performed 1 year after surgery or 3 to 6 months after surgery if not performed preoperatively due to an obstructing lesion, and then 3 years later, and then every 5 years, unless fi ndings or specifi c risk factors dictate more frequent evaluations.
Another important aspect of follow-up is instruction of the patient. Specifi c and nonspecifi c signs and symptoms that should initiate a patient’s return for routine history, physical examination, and appropriate diagnostic studies should be detailed. When these signs and symptoms are not present, and because palliation is generally the only goal in treating most recurrences, there is little benefi t in attempting to defi ne recurrence early. Nevertheless, it is suggested that a clinical history and physical examination be performed every 3 to 6 months for the fi rst 3 postoperative years and at least annually for the next 2 years. Routine oncologic follow-up and surveillance are typically considered completed after approximately 5 years, after which time, surveillance scanning and CEA monitoring should be discontinued.
Colonoscopic surveillance, however, is routinely recom-mended to continue indefi nitely. This is because the ratio-nale for colonoscopy in perioperative staging and follow-up is not to identify recurrent cancer, and the yield in diagnos-ing isolated suture line recurrence by either endoscopy or guaiac testing of stool is low. The major reason for using colonoscopy is to identify synchronous or metachronous bowel tumors, usually polyps. As patients are exposed more uniformly to follow-up endoscopy after primary CRC
resection, the incidence of metachronous lesions seems to be increasing.176–178 Whatever the ultimate incidence of meta-chronous bowel lesions, however, patients with sentinel colorectal carcinomas are unquestionably at signifi cant risk for developing metachronous polyps. If these polyps are dis-covered and removed, the risk of subsequent development of colon and rectal cancer decreases. Adequate screening to rule out synchronous lesions at the time of primary surgery or later and serial follow-up every 3 to 5 years to ensure a cancer- and polyp-free colon should be a mandatory part of any postoperative surveillance program for CRC patients.
Tumor Markers
CEA remains the prototypical solid tumor marker. Despite its lack of specifi city, if used correctly, CEA testing is a valuable addition to the process of clinical decision mak-ing in patients diagnosed with colon or rectal carcinoma. However, it is not an appropriate screening test. Whether sampled once or serially, CEA cannot be used in the differ-ential diagnosis of an unknown-but-suspected bowel prob-lem or malignancy. Nevertheless, when CEA concentrations are determined before primary tumor resection, they may be of additional prognostic value; this is particularly true in patients with stage II disease, for whom elevated preopera-tive CEA is a poor prognostic marker and may infl uence the decision regarding whether to administer adjuvant chemotherapy.
Serial CEA values obtained postoperatively are a poten-tially effective means of monitoring response to therapy. A postoperative CEA titer serves as a measure of the complete-ness of tumor resection. It should be remembered, however, that the half-life of CEA is 7 to 14 days; therefore, postop-erative baselines are best established several weeks after resection. If a preoperatively elevated CEA value does not fall to normal within 2 to 3 weeks after surgery, it is likely that (1) the resection was incomplete or (2) occult metastases are present. A rising trend in serial CEA values from a normal postoperative baseline (< 5 ng/mL) may predate any other clinical or laboratory evidence of recurrent disease by 6 to 9 months.
Serial CEA values tend to roughly parallel tumor regres-sion or progression during treatment for metastatic dis-ease.179 The majority of patients who respond to treatment show a decline in CEA levels. Rising CEA levels are usually incompatible with tumor regression.180 However, the actual utility of these measurements is limited as decisions to continue or discontinue a chemotherapy regimen should rarely (if ever) be made on the basis of a rising CEA alone.
There are no data to guide an optimal schedule of CEA monitoring after potentially curative colorectal resection. A reasonable strategy is to obtain CEA levels every 3 to 6 months for the fi rst 3 years postoperatively and every 6 months for the fourth and fi fth postoperative year. The available data do not suggest that continued CEA monitor-ing after 5 years is of benefi t, and this should not be done.
A newly elevated CEA should fi rst be repeated at an interval of approximately 4 to 6 weeks to confi rm the fi nding or rule out laboratory error. Litvak and colleagues recently reported a 10-year experience evaluating new elevations in CEA in patients under surveillance following complete

Scientifi c American Surgery
09/14
gastro adenocarcinoma of the colon and rectum — 24
resection of CRC.181 They found that 49% of elevations between 5 and 10 ng/mL were false positive elevations that either regressed or remained stable without evidence of active cancer for a year or more. Thus, serial monitoring of such low-level CEA abnormalities, with further evaluation only on clear progression, would be reasonable. The major-ity of CEA elevations above 10 ng/mL were found to be indicative of active cancer, representing recurrent disease in most cases, although in 5% of cases, the CEA elevation was indicative of a new, noncolorectal primary cancer. Over this 10-year experience, only two CEAs repeated and confi rmed above 20 ng/mL, and none over 35 ng/mL, were found to be false positives. Thus, a CEA greater than 35 ng/mL is virtually diagnostic of active cancer of one sort or another.
A confi rmed new CEA elevation should be further evalu-ated with a full-body CT scan and, if this is negative, a colo-noscopy. In considering workup of an elevated CEA, it is important to keep in mind that the goals of this screening are to identify potentially curable patients: individuals with surgically resectable metastatic disease. Isolated liver, lung, or ovarian metastases are potentially curable with surgery, as are some local anastomotic recurrences. Identifi cation of asymptomatic but incurable disease, such as peritoneal metastases or retroperitoneal lymph nodes, is of essentially no benefi t and does not contribute to the patient’s overall well-being. There is no evidence that early initiation of sys-temic chemotherapy for incurable metastatic disease results in a better outcome. Thus, the role of CEA monitoring and postoperative imaging is to help identify patients with resectable (and therefore curable) disease.
“Second-look” surgery in the absence of an identifi ed cur-able lesion on imaging studies is no longer recommended because it is extremely unlikely to show curable disease.182 In later application of radioimmunologic scanning techniques using either external or intraoperative gamma-scanning,183 the weak link remains the lack of any effective systemic therapy even when recurrent disease is found early.
Serial CEA rise will show the steepest slope if the liver or lung is the fi rst or only site of recurrence. Specifi c diagnostic tests to confi rm recurrence in the liver or lung are now pref-erable to so-called blind, CEA-directed second-look proce-dures.184 At present, only patients manifesting recurrence of CRC as defi ned, isolated liver, lung, ovarian, or anastomotic metastases should undergo surgery. Therefore, it is recom-mended that postoperative monitoring of CEA be reserved for patients who would be candidates for resection of these potentially curable metastases should they occur. As is the case with other follow-up testing, the optimal frequency of serial CEA monitoring has not been established.184,185
PET scanning has been recommended by some as a tool for workup of elevated CEA. It should be remembered, however, that identifi cation of asymptomatic unresectable disease is unlikely to improve long-term outcome and may actually increase the patient’s anxiety. In the setting of negative, high-quality, current CT and/or MRI scans, most surgeons would be reluctant to operate on the basis of a positive PET scan alone. For this reason, the true contribu-tion of PET to management of an elevated postresection CEA is highly questionable.
Financial Disclosures: Martin R. Weiser, MD, has no relevant fi nancial relationships to disclose. Leonard B. Saltz, MD, is a consultant for Roche, Genentech, Pfi zer, and Bayer. This topic review was previously authored by Bruce M. Brenner, MD, FACS, and David M. Ota, MD, FACS, with disclosure made at the time of initial publica-tion. This topic review has been reviewed, updated, and rereleased by the authors listed.
References
1. Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statis-tics, 2002. CA Cancer J Clin 2005;55:74–108.
2. Kolonel LN, Wilkens LR. Migrant studies. In: Schottenfeld D, Fraumeni JF, editors. Cancer epidemiology and preven-tion. Philadelphia: WB Saunders; 1982. p. 194–207.
3. Jessup JM, McGinnis LS, Steele GD Jr, et al. The National Cancer Data Base. Report on colon cancer. Cancer 1996;78:918–26.
4. Jemal A, Tiwari RC, Murray T. American Cancer Society. Cancer statistic, 2004. CA Cancer J Clin 2004;54:8–29.
5. Jemal A, Siegel R, Xu J, Ward E. Cancer statistics. CA Cancer J Clin 2010;60:277–300.
6. Akerley WL 3rd, Moritz TE, Ryan LS, et al. Racial com-parison of outcomes of male Department of Veterans Affairs patients with lung and colon cancer. Arch Intern Med 1993;153:1681–8.
7. Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell 1990;61:759–67.
8. Jones S, Chen WD, Parmigiani G, et al. Comparative lesion sequencing provides insights into tumor evolution. Proc Natl Acad Sci U S A 2008;105:4283–8.
9. Ribic CM, Sargent DJ, Moore MJ, et al. Tumor microsatellite-instability status as a predictor of benefi t from fl uorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med 2003;349:247–57.
10. Sargent DJ, Marsoni S, Monges G, et al. Defective mis-match repair as a predictive marker for lack of effi cacy of fl uorouracil-based adjuvant therapy in colon cancer. J Clin Oncol 2010;28:3219–26.
11. Grady WM. WCIMP and colon cancer gets more compli-cated. Gut 2007;56:1498–500.
12. Lipton L, Halford SE, Johnson V, et al. Carcinogenesis in MYH-associated polyposis follows a distinct genetic pathway. Cancer Res 2003;63:7595–9.
13. Sandler RS. Epidemiology and risk factors for colorectal cancer. Gastroenterol Clin North Am 1996;25:717–35.
14. Atkin WS, Morson BC, Cuzick J. Long-term risk of colorec-tal cancer after excision of rectosigmoid adenomas. N Engl J Med 1992;326:658–62.
15. Markowitz A. Screening and surveillance. In: Saltz LB, editor. Colorectal cancer: multimodality management. Totowa (NJ): Humana Press; 2002. p. 65–80.
16. Heald RJ. Synchronous and metachronous carcinoma of the colon and rectum. Ann R Coll Surg Engl 1990;72:172–4.
17. Solomon MJ, Schnitzler M. Cancer and infl ammatory bowel disease: bias, epidemiology, surveillance, and treat-ment. World J Surg 1998;22:352–8.
18. PM, M. The cancer risk in ulcerative colitis. Lancet 1966;2:655.
19. Lavery IC, Chiulli RA, Jagelman DG, et al. Survival with carcinoma arising in mucosal ulcerative colitis. Ann Surg 1982;195:508–12.

Scientifi c American Surgery
09/14
gastro adenocarcinoma of the colon and rectum — 25
20. Ransohoff DF, Riddell RH, Levin B. Ulcerative colitis and colonic cancer. Problems in assessing the diagnostic usefulness of mucosal dysplasia. Dis Colon Rectum 1985;28:383–8.
21. Sachar DB. Cancer in Crohn’s disease: dispelling the myths. Gut 1994;35:1507–8.
22. Fuchs CS, Giovannucci EL, Colditz GA, et al. A prospec-tive study of family history and the risk of colorectal cancer. N Engl J Med 1994;331:1669–74.
23. Lipkin M, Blattner WA, Gardner EJ, et al. Classifi cation and risk assessment of individuals with familial polyposis, Gardner’s syndrome, and familial non-polyposis colon cancer from [3H]thymidine labeling patterns in colonic epithelial cells. Cancer Res 1984;44:4201–7.
24. Mulvihill JJ. The frequency of hereditary large bowel cancer. In: Ingall JRF, Mastromarino AJ, editors. Preven-tion of hereditary large bowel cancer. New York: Alan R. Liss; 1983. p. 61.
25. Powell SM, Petersen GM, Krush AJ, et al. Molecular diag-nosis of familial adenomatous polyposis. N Engl J Med 1993;329:1982–7.
26. Nugent KP, Spigelman AD, Phillips RK. Life expectancy after colectomy and ileorectal anastomosis for familial adenomatous polyposis. Dis Colon Rectum 1993;36:1059–62.
27. Brensinger JD, Laken SJ, Luce MC, et al. Variable pheno-type of familial adenomatous polyposis in pedigrees with 3’ mutation in the APC gene. Gut 1998;43:548–52.
28. Lynch HT, Smyrk TC, Watson P, et al. Genetics, natural history, tumor spectrum, and pathology of hereditary nonpolyposis colorectal cancer: an updated review. Gastroenterology 1993;104:1535–49.
29. Marra G, Boland CR. Hereditary nonpolyposis colorectal cancer: the syndrome, the genes, and historical perspec-tive. J Natl Cancer Inst 1995;87:1114–25.
30. Vasen HF, Mecklin JP, Khan PM, Lynch HT. Hereditary non-polyposis colorectal cancer. Lancet 1991;338:877.
31. Aarnio M, Sankila R, Pukkala E, et al. Cancer risk in muta-tion carriers of DNA-mismatch-repair genes. Int J Cancer 1999;81:214–8.
32. Muir EG, Bell AJ, Barlow KA. Multiple primary carcino-mata of the colon, duodenum, and larynx associated with kerato-acanthomata of the face. Br J Surg 1967;54:191–5.
33. Lindor NM, Rabe K, Petersen GM, et al. Lower cancer inci-dence in Amsterdam-I criteria families without mismatch repair defi ciency: familial colorectal cancer type X. JAMA 2008;293:2028–30.
34. Scott RJ, McPhillips M, Meldrum CJ, et al. Hereditary nonpolyposis colorectal cancer in 95 families: differences and similarities between mutation-positive and mutation-negative kindreds. Am J Hum Genet 2001;68:118–27.
35. Shashidharan M, Smyrk T, Lin KM, et al. Histologic com-parison of hereditary nonpolyposis colorectal cancer asso-ciated with MSH2 and MLH1 and colorectal cancer from the general population. Dis Colon Rectum 1999;42:722–6.
36. Haggitt RC, Reid BJ. Hereditary gastrointestinal polyposis syndromes. Am J Surg Pathol 1986;19:871–87.
37. Wirtzfeld DA, Petrelli NJ, Rodriguez-Bigas MA. Hamarto-matous polyposis syndromes: molecular genetics, neoplas-tic risk, and surveillance recommendations. Ann Surg Oncol 2001;8:319–27.
38. Giardiello FM, Welsh SB, Hamilton SR, et al. Increased risk of cancer in the Peutz-Jeghers syndrome. N Engl J Med 1987;316:1511–4.
39. Jass JR, Williams CB, Bussey HJ, Morson BC. Juvenile polyposis—a precancerous condition. Histopathology 1988;13:619–30.
40. JM., C. Other polyposis syndromes. Semin Colon Rectal Surg 1995;6:61–6.
41. Laken SJ, Petersen GM, Gruber SB, et al. Familial colorectal cancer in Ashkenazim due to a hypermutable tract in APC. Nat Genet 1997;17:79–83.
42. Prior TW, Chadwick RB, Papp AC, et al. The I1307K poly-morphism of the APC gene in colorectal cancer. Gastroen-terology 1999;116:58–63.
43. Rozen P, Shomrat R, Strul H, et al. Prevalence of the I1307C APC gene variant in Israeli Jews of differing ethnic origin and risk for colorectal cancer. Gastroenterology 1999;116:54–7.
44. Sieber OM, Lipton L, Crabtree M, et al. Multiple colorectal adenomas, classic adenomatous polyposis, and germ-line mutations in MYH. N Engl J Med 2003;348:791–9.
45. Hyman NH, Anderson P, Blasyk H. Hyperplastic polypo-sis and the risk of colorectal cancer. Dis Colon Rectum 2004;47:2101–4.
46. Le Marchand L, Wilkens LR, Kolonel LN, et al. Associa-tions of sedentary lifestyle, obesity, smoking, alcohol use, and diabetes with the risk of colorectal cancer. Cancer Res 1997;57:4787–94.
47. Baron JA, Beach M, Mandel JS, et al. Calcium supplements for the prevention of colorectal adenomas. Calcium Polyp Prevention Study Group. N Engl J Med 1999;340:101–7.
48. Yiu HY, Whittemore AS, Shibata A. Increasing colorectal cancer incidence rates in Japan. Int J Cancer 2004;109:777–81.
49. Winawer SJ, Zauber AG, Ho MN, et al. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med 1993;329:1977–81.
50. Hixson LJ, Fennerty MB, Sampliner RE, et al. Prospective study of the frequency and size distribution of polyps missed by colonoscopy. J Natl Cancer Inst 1990;82:1769–72.
51. Zauber AG, Winawer S, Bond J. Guidelines for colonos-copy surveillance after polypectomy [abstract]. Gastroen-terology 1997;112:A50.
52. Pickhardt PJ, Choi JR, Hwang I, et al. Computed tomo-graphic virtual colonoscopy to screen for colorectal neoplasia in asymptomatic adults. N Engl J Med 2003;349:2191–200.
53. Cotton PB, Durkalski VL, Pineau BC, et al. Computed tomographic colonography (virtual colonoscopy): a multi-center comparison with standard colonoscopy for detec-tion of colorectal neoplasia. JAMA 2004;291:1713–9.
54. Deenadayalu VP, Rex DK. Fecal-based DNA assays: a new, noninvasive approach to colorectal cancer screening. Cleve Clin J Med 2004;71:497–503.
55. Imperiale TF, Ransohoff DF, Itzkowitz SH, et al. Colorectal Cancer Study Group. Fecal DNA versus fecal occult blood for colorectal-cancer screening in an average-risk population. N Engl J Med 2004;351:2704–14.
56. Delbeke D, Martin WH. PET and PET-CT for evaluation of colorectal carcinoma. Semin Nucl Med 2004;34:209–23.

Scientifi c American Surgery
09/14
gastro adenocarcinoma of the colon and rectum — 26
75. Enker WE, Thaler HT, Cranor ML, Polyak T. Total meso-rectal excision in the operative treatment of carcinoma of the rectum. J Am Coll Surg 1995;181:335–46.
76. Enker WE. Potency, cure, and local control in the operative treatment of rectal cancer. Arch Surg 1992;127:1396–401; discussion 1402.
77. Heald RJ, Moran BJ, Ryall RD, et al. Rectal cancer: the Bas-ingstoke experience of total mesorectal excision, 1978–1997. Arch Surg 1998;133:894–9.
78. Quirke P, Durdey P, Dixon MF, Williams NS. Local recur-rence of rectal adenocarcinoma due to inadequate surgical resection: histopathological study of lateral tumour spread and surgical excision. Lancet 1986;2:969–9.
79. Schrag D, Cramer LD, Bach PB, et al. Infl uence of hospital procedure volume on outcomes following surgery for colon cancer. JAMA 2000;284:3028–35.
80. Schrag D, Panageas KS, Riedel E, et al. Surgeon volume compared to hospital volume as a predictor of outcome following primary colon cancer resection. J Surg Oncol 2003;83:68–78.
81. Weiser MR, Quah HM, Shia J, et al. Sphincter preservation in low rectal cancer is facilitated by preoperative chemora-diation and intersphincteric dissection. Ann Surg 2009;249:236–42.
82. Rullier E, Laurent C, Bretagnol F, et al. Sphincter-saving resection for all rectal carcinomas: the end of the 2-cm distal rule. Ann Surg 2005;241:465–9.
83. Temple LK, Bacik J, Savatta SG, et al. The development of a validated instrument to evaluate bowel function after sphincter-preserving surgery for rectal cancer. Dis Colon Rectum 2005;48:1353–65.
84. Goligher JC, Dukes CE, Bussey HJ. Local recurrences after sphincter saving excisions for carcinoma of the rectum and rectosigmoid. Br J Surg 1951;39:199–211.
85. Wilson SM, Beahrs OH. The curative treatment of carci-noma of the sigmoid, rectosigmoid, and rectum. Ann Surg 1976;183:556–65.
86. Moore HG, Riedel E, Minsky BD, et al. Adequacy of 1-cm distal margin after restorative rectal cancer resection with sharp mesorectal excision and preoperative combined-modality therapy. Ann Surg Oncol 2003;10:80–5.
87. Rodriguez-Bigas MA, Petrelli NJ. Management of hereditary colon cancer syndromes. In: Saltz LB, editor. Colorectal cancer: multimodality management. Totowa (NJ): Humana Press; 2002. p. 99–114.
88. Gimbel MI, Paty PB. A current perspective on local exci-sion of rectal cancer. Clin Colorectal Cancer 2004;4:26–35.
89. Blumberg D, Paty PB, Picon A, et al. Stage I rectal cancer: identifi cation of high-risk patients. J Am Coll Surg 1998;186:574–9.
90. Landmann RG, Wong WD, Hoepfl J, et al. Limitations of early rectal cancer nodal staging may explain failure after local excision. Dis Colon Rectum 2007;50:1520–5.
91. Weiser MR, Landmann RG, Wong WD, et al. Surgical salvage of recurrent rectal cancer after transanal excision. Dis Colon Rectum 2005;48:1169–75.
92. Friel CM, Cromwell JW, Marra C, et al. Salvage radical surgery after failed local excision for early rectal cancer. Dis Colon Rectum 2002;45:875–9.
93. Madoff RD. Total mesorectal neglect in the age of total mesorectal excision. J Clin Oncol 2013;31:4273–5.
57. Jen J, Kim H, Piantadosi S, et al. Allelic loss of chromo-some 18q and prognosis in colorectal cancer. N Engl J Med 1994;331:213–21.
58. Shibata D, Reale MA, Lavin P, et al. The DCC protein and prognosis in colorectal cancer. N Engl J Med 1996;335:1727–32.
59. Goldstein NS. Lymph node recoveries from 2427 pT3 colorectal resection specimens spanning 45 years: recom-mendations for a minimum number of recovered lymph nodes based on predictive probabilities. Am J Surg Pathol 2002;26:179–89.
60. Compton CC, Greene FL. The staging of colorectal cancer: 2004 and beyond. CA Cancer J Clin 2004;54:295–308.
61. Waldman SA, Hyslop T, Schulz S, et al. Association of GUCY2C expression in lymph nodes with time to recur-rence and disease-free survival in pN0 colorectal cancer. JAMA 2009;301:745–52.
62. Saha S, Wiese D, Badin J, et al. Technical details of sentinel lymph node mapping in colorectal cancer and its impact on staging. Ann Surg Oncol 2000;7:82–4.
63. Bertagnolli M, Miedema B, Redston M, et al. Sentinel node staging of resectable colon cancer: results of a multicenter study. Ann Surg 2004;240:624–8.
64. O’Connell JB, Maggard MA, Ko CY. Colon cancer survival rates with the new American Joint Committee on Cancer sixth edition staging. J Natl Cancer Inst 2004;96:1420–5.
65. Minsky BD, Mies C, Recht A, et al. Resectable adenocarci-noma of the rectosigmoid and rectum. II. The infl uence of blood vessel invasion. Cancer 1988;61:1417–24.
66. Minsky BD, Mies C, Rich TA, Recht A. Lymphatic vessel invasion is an independent prognostic factor for survival in colorectal cancer. Int J Radiat Oncol Biol Phys 1989;17:311–8.
67. Temple LK, Hsieh L, Wong WD, et al. Use of surgery among elderly patients with stage IV colorectal cancer. J Clin Oncol 2004;22:3475–84.
68. Poultsides GA, Servais EL, Saltz LB, et al. Outcome of primary tumor in patients with synchronous stage IV colorectal cancer receiving combination chemotherapy without surgery as initial treatment. J Clin Oncol 2009;27:33790–84.
69. Herter FP, Slanetz CA. Patterns and signifi cance of lym-phatic spread from cancer of the colon and rectum. In: Weiss L, Gilbert HA, Ballon SC, editors. Lymphatic system metastasis. Boston: G.K. Hall Medical Publishers; 1980. p. 275–307.
70. Pezim ME, Nicholls RJ. Survival after high or low ligation of the inferior mesenteric artery during curative surgery for rectal cancer. Ann Surg 1984;200:729–33.
71. Grinnell RS. Results of ligation of inferior mesenteric artery at the aorta in resections of carcinoma of the descending and sigmoid colon and rectum. Surg Gynecol Obstet 1965;120:1031–6.
72. Botoman VA, Pietro M, Thirlby RC. Localization of colonic lesions with endoscopic tattoo. Dis Colon Rectum 1994;37:775–6.
73. Askin MP, Waye JD, Fiedler L, Harpaz N. Tattoo of colonic neoplasms in 113 patients with a new sterile carbon compound. Gastrointest Endosc 2002;56:339–42.
74. Heald RJ, Husband EM, Ryall RD. The mesorectum in rectal cancer surgery—the clue to pelvic recurrence? Br J Surg 1982;69:613–6.

Scientifi c American Surgery
09/14
gastro adenocarcinoma of the colon and rectum — 27
94. De Graaf EJ, Doornebosch PG, Tollenaar RA, et al. Trans-anal endoscopic microsurgery versus total mesorectal excision of T1 rectal adenocarcinomas with curative intention. Eur J Surg Oncol 2009;35:1280–5.
95. Hermsen PE, Nonner J, De Graaf EJ, Doornebosch PG. Recurrences after transanal excision or transanal endo-scopic microsurgery of T1 rectal cancer. Minerva Chir 2010;65:213–23.
96. Delgado S, Lacy AM, García Valdecasas JC, et al. Could age be an indication for laparoscopic colectomy in colorec-tal cancer? Surg Endosc 2000;14:22–6.
97. Lacy AM, García-Valdecasas JC, Delgado S, et al. Laparos-copy-assisted colectomy versus open colectomy for treat-ment of non-metastatic colon cancer: a randomised trial. Lancet 2002;359:2224–9.
98. Weeks JC, Nelson H, Gelber S, et al. for the Clinical Outcomes of Surgical Therapy (COST) Study Group: Short-term quality-of-life outcomes following laparo-scopic-assisted colectomy vs. open colectomy for colon cancer: A randomized trial. JAMA 2002;287:321–8.
99. The Clinical Outcomes of Surgical Therapy Study Group. A comparison of laparoscopically assisted and open colec-tomy for colon cancer. N Engl J Med 2004;350:2050–9.
100. Fleshman J, Sargent DJ, Green E, et al. for The Clinical Out-comes of Surgical Therapy Study Group: Laparoscopic colectomy for cancer is not inferior to open surgery based on 5-year data from the COST Study Group Trial. Ann Surg 2007;246:655–64.
101. Cleary P, Shorvon S, Tallis R. Laparoscopic resection of rectosigmoid carcinoma: prospective randomised trial. Lancet 2004;363:1184–6.
102. Guillou PJ, Quirke P, Thorpe H, et al. for the MRC CLASICC trial group. Short-term endpoints of conven-tional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial. Lancet 2005;365:1718–26.
103. Jayne DG, Guillou PJ, Thorpe H, et al. Randomized trial of laparoscopic-assisted resection of colorectal carcinoma: 3-year results of the UK MRC CLASICC Trial Group. J Clin Oncol 2007;25:3061–8.
104. Peterson CY, Weiser MR. Minimally invasive surgery for locally-advanced rectal cancer: recent advances and future developments. Curr Colorectal Cancer Rep 2014. [In press]
105. Weiser MR, Milsom JW. Laparoscopic total mesorectal excision with autonomic nerve preservation. Semin Surg Oncol 2000;19:396–403.
106. Morino M, Parini U, Giraudo G, et al. Laparoscopic total mesorectal excision: a consecutive series of 100 patients. Ann Surg 2003;237:335–42.
107. van der Pas MH, Haglind E, Cuesta MA, et al. COlorectal cancer Laparoscopic or Open Resection II (COLOR II) Study Group. Laparoscopic versus open surgery for rectal cancer (COLOR II): short-term outcomes of a randomised, phase 3 trial. Lancet Oncol 2013;14:210–8.
108. Peterson CY, Weiser MR. Robotic colorectal surgery. J Gastrointest Surg 2014;18:398–403.
109. Arnaud JP, Bergamaschi R. Emergency subtotal/total col-ectomy with anastomosis for acutely obstructed carcinoma of the left colon. Dis Colon Rectum 1994;37:685–8.
110. van Hooft JE, Fockens P, Marinelli AW, et al. Early closure of a multicenter randomized clinical trial of endoscopic
stenting versus surgery for stage IV left-sided colorectal cancer. Endoscopy 2008;40:184–91.
111. Fante R, Roncucci L, Di Gregorio C, et al. Frequency and clinical features of multiple tumors of the large bowel in the general population and in patients with hereditary colorectal carcinoma. Cancer 1996;77:2013.
112. Arenas RB, Fichera A, Mhoon D, Michelassi F. Incidence and therapeutic implications of synchronous colonic pathology in colorectal adenocarcinoma. Surgery 1997;122:706–9; discussion 709–10.
113. Cunliffe WJ, Hasleton PS, Tweedle DE, Schofi eld PF. Incidence of synchronous and metachronous colorectal carcinoma. Br J Surg 1984;71:941–3.
114. Passman MA, Pommier RF, Vetto JT. Synchronous colon primaries have the same prognosis as solitary colon cancers. Dis Colon Rectum 1996;39:329–34.
115. Chen HS, Sheen-Chen SM. Synchronous and “early” meta-chronous colorectal adenocarcinoma: analysis of prognosis and current trends. Dis Colon Rectum 2000;43:1093–9.
116. Jayne DG, Fook S, Loi C, Seow-Choen F. Peritoneal carcinomatosis from colorectal cancer. Br J Surg 2002;89:1545–50.
117. Rosen SA, Buell JF, Yoshida A, et al. Initial presentation with stage IV colorectal cancer: how aggressive should we be? Arch Surg 2000;135:530–4; discussion 534–5.
118. Elias DM, Pocard M. Treatment and prevention of perito-neal carcinomatosis from colorectal cancer. Surg Oncol Clin N Am 2003;12:543–59.
119. Stephens AD, Alderman R, Chang D, et al. Morbidity and mortality analysis of 200 treatments with cytoreductive surgery and hyperthermic intraoperative intraperitoneal chemotherapy using the coliseum technique. Ann Surg Oncol 1999;6:790–6.
120. Verwaal VJ, van Ruth S, de Bree E, et al. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemo-therapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol 2003;21:3737–43.
121. Verwaal VJ, Bruin S, Boot H, et al. 8-year follow-up of randomized trial: cytoreduction and hyperthermic intra-peritoneal chemotherapy versus systemic chemotherapy in patients with peritoneal carcinomatosis of colorectal cancer. Ann Surg Oncol 2008;15:2426–32.
122. Scoggins CR, Meszoely IM, Blanke CD, et al. Nonoperative management of primary colorectal cancer in patients with stage IV disease. Ann Surg Oncol 1999;6:651–7.
123. Scheele J, Stangl R, Altendorf-Hofmann A, Gall FP. Indica-tors of prognosis after hepatic resection for colorectal secondaries. Surgery 1991;110:13–29.
124. Martin R, Paty P, Fong Y, et al. Simultaneous liver and colorectal resections are safe for synchronous colorectal liver metastasis. J Am Coll Surg 2003;197:233–41; discus-sion 241–2.
125. Chua HK, Sondenaa K, Tsiotos GG, et al. Concurrent vs. staged colectomy and hepatectomy for primary colorectal cancer with synchronous hepatic metastases. Dis Colon Rectum 2004;47:1310–6.
126. Laurie JA, Moertel CG, Fleming TR, et al. Surgical adju-vant therapy of large bowel carcinoma: an evaluation of levamisole and the combination of levamisole and fl uorouracil. J Clin Oncol 1989;7:1447–56.

Scientifi c American Surgery
09/14
gastro adenocarcinoma of the colon and rectum — 28
141. Kim GP, Colangelo LH, Wieand HS, et al. Prognostic and predictive roles of high-degree microsatellite instability in colon cancer: a National Cancer Institute-National Surgical Adjuvant Breast and Bowel Project Collaborative Study. J Clin Oncol 2007;25:767–72.
142. Sinicrope FA, Foster NR, Thibodeau SN, et al. DNA mismatch repair status and colon cancer recurrence and survival in clinical trials of 5-fl uorouracil-based adjuvant therapy. J Natl Cancer Inst 2011;103:863–75.
143. Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004;351:1731–40.
144. Roh MS, Yothers GA, O’Connell MJ, et al. The impact of capecitabine and oxaliplatin in the preoperative multimo-dality treatment in patients with carcinoma of the rectum: NSABP R-04 [abstract]. J Clin Oncol 2011;29(15 Suppl):3503.
145. Aschele C, Pinto C, Cordio S; on behalf of STAR Network Investigators. Preoperative fl uorouracil (FU)-based chemo-radiation with and without weekly oxaliplatin in locally advanced rectal cancer: pathologic response analysis of the Studio Terapia Adiuvante Retto (STAR)-01 randomized phase III trial [abstract]. J Clin Oncol 2009;27(18 Suppl):CRA4008.
146. Gerard JP, Azria D, Gourgou-Bourgade S, et al. Compari-son of two neoadjuvant chemoradiotherapy regimens for locally advanced rectal cancer: results of the phase III trial ACCORD 12/0405-Prodige 2. J Clin Oncol 2010;28:1638–44.
147. Chau I, Brown G, Cunningham D, et al. Neoadjuvant capecitabine and oxaliplatin followed by synchronous chemoradiation and total mesorectal excision in magnetic resonance imaging-defi ned poor-risk rectal cancer. J Clin Oncol 2006;24:668–74.
148. Cercek A, Goodman KA, Hajj C, et al. Neoadjuvant chemotherapy fi rst, followed by chemoradiation and then surgery, in the management of locally advanced rectal cancer. J Natl Compr Canc Netw 2014;12:513–9.
149. Schrag D, Weiser MR, Goodman KA, et al. Neoadjuvant chemotherapy without routine use of radiation therapy for patients with locally advanced rectal cancer: a pilot trial. J Clin Oncol 2014;32:513–8.
150. Saltz LB, Cox JV, Blanke C, et al. Irinotecan plus fl uoroura-cil and leucovorin for metastatic colorectal cancer. Irinote-can Study Group. N Engl J Med 2000;343:905–14.
151. Douillard JY, Cunningham D, Roth AD, et al. Irinotecan combined with fl uorouracil compared with fl uorouracil alone as fi rst-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet 2000;355:1041–7.
152. de Gramont A, Figer A, Seymour M, et al. Leucovorin and fl uorouracil with or without oxaliplatin as fi rst-line treat-ment in advanced colorectal cancer. J Clin Oncol 2000;18:2938–47.
153. Goldberg RM, Sargent DJ, Morton RF, et al. A randomized controlled trial of fl uorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol 2004;22:23–30.
154. Cunningham D, Pyrhonen S, James R, et al. Randomised trial of irinotecan plus supportive care versus supportive
127. Haller DG, Catalano PJ, Macdonald JS, et al. Phase III study of fl uorouracil, leucovorin, and levamisole in high-risk stage II and III colon cancer: fi nal report of Intergroup 0089. J Clin Oncol 2005;23:8671–8.
128. Andre T, Boni C, Mounedji-Boudiaf L, et al. Oxaliplatin, fl uorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med 2004;350:2343–51.
129. Andre T, Boni C, Navarro M, et al. Improved overall survival with oxaliplatin, fl uorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC Trial. J Clin Oncol 2009;27:3109–16.
130. Tournigand C, Andre T, Bonnetain F, et al. Adjuvant ther-apy with fl uorouracil and oxaliplatin in stage II and elderly patients (between ages 70 and 75 years) with colon cancer: subgroup analyses of the Multicenter International Study of Oxaliplatin, Fluorouracil, and Leucovorin in the Adjuvant Treatment of Colon Cancer trial. J Clin Oncol 2012;30:3353–60.
131. Yothers G, O’Connell MJ, Allegra CJ, et al. Oxaliplatin as adjuvant therapy for colon cancer: updated results of NSABP C-07 trial, including survival and subset analyses. J Clin Oncol 2011;29:3768–74.
132. Twelves C, Wong A, Nowacki MP, et al. Capecitabine as adjuvant treatment for stage III colon cancer. N Engl J Med 2005;352:2696–704.
133. Haller DG, Taberno J, Maroun J, et al. Capecitabine plus oxaliplatin compared with fl uorouracil and folinic acid as adjuvant therapy for stage III colon cancer. J Clin Oncol 2011;29:1465–71.
134. Cassidy J, Clarke S, Díaz-Rubio E, et al. Randomized phase III study of capecitabine plus oxaliplatin compared with fl uorouracil/folinic acid plus oxaliplatin as fi rst-line therapy for metastatic colorectal cancer. J Clin Oncol 2008;26:2006–12.
135. Saltz LB, Niedzwiecki D, Hollis D, et al. Irinotecan fl uoro-uracil plus leucovorin is not superior to fl uorouracil plus leucovorin alone as adjuvant treatment for stage III colon cancer: results of CALGB 89803. J Clin Oncol 2007;25:3456–61.
136. Ychou M, Raoul JL, Douillard JY, et al. A phase III randomised trial of LV5FU2 + irinotecan versus LV5FU2 alone in adjuvant high-risk colon cancer (FNCLCC Accord02/FFCD9802). Ann Oncol 2009;20:674–80.
137. Van Cutsem E, Labianca R, Bodoky G, et al. Randomized phase III trial comparing biweekly infusional fl uorouracil/leucovorin alone or with irinotecan in the adjuvant treat-ment of Stage III colon cancer: PETACC-3. J Clin Oncol 2009;27:3117–25.
138. Allegra CJ, Yothers G, O’Connell MJ, et al. Bevacizumab in stage II-III colon cancer: 5-year update of the National Surgical Adjuvant Breast and Bowel Project C-08 trial. J Clin Oncol 2013;31:359–64.
139. Alberts SR, Sargent DJ, Nair S, et al. Effect of oxaliplatin, fl uorouracil, and leucovorin with or without cetuximab on survival among patients with resected stage III colon cancer: a randomized trial. JAMA 2012;307:1383–93.
140. Kerr D, Gray R, Quirke P, et al. A quantitative multigene RT-PCR assay for prediction of recurrence in stage II colon cancer: selection of the genes in four large studies and results of the independent, prospectively designed QUASAR validation study [abstract]. J Clin Oncol 2009;27:15S (May 20 Supplement):4000.

Scientifi c American Surgery
09/14
gastro adenocarcinoma of the colon and rectum — 29
169. Bokemeyer C, Bondarenko I, Makhson A, et al. Fluoroura-cil, leucovorin, and oxaliplatin with and without cetux-imab in the fi rst-line treatment of metastatic colorectal cancer. J Clin Oncol 2009;27:663–71.
170. Maughan TS, Adams RA, Smith CG, et al. MRC COIN Trial Investigators. Addition of cetuximab to oxaliplatin-based fi rst-line combination chemotherapy for treatment of advanced colorectal cancer: results of the randomised phase 3 MRC COIN trial. Lancet 2011;377:2103–14.
171. Tveit KM, Guren T, Glimelius B, et al. Phase III trial of cetuximab with continuous or intermittent fl uorouracil, leucovorin, and oxaliplatin (Nordic FLOX) versus FLOX alone in fi rst-line treatment of metastatic colorectal cancer: the NORDIC-VII study. J Clin Oncol 2012;30:1755–62.
172. Tol J, Koopman M, Cats A, et al. Chemotherapy, bevaci-zumab, and cetuximab in metastatic colorectal cancer. N Engl J Med 2009;360:563–72.
173. Hecht JR, Mitchell E, Chidiac T, et al. A randomized phase IIIB trial of chemotherapy, bevacizumab, and panitu-mumab compared with chemotherapy and bevacizumab alone for metastatic colorectal cancer. J Clin Oncol 2009;27:672–80.
174. Nordlinger B, Sorbye H, Glimelius B, et al. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup Trial 40983): a randomised controlled trial. J Clin Oncol 2008;22:1007–16.
175. Primrose JN, Falk S, Finch-Jones M, et al. A randomized clinical trial of chemotherapy compared to chemotherapy in combination with cetuximab in k-RAS wild-type patients with operable metastases from colorectal cancer: the new EPOC study [abstract]. J Clin Oncol 2013;31(15 Suppl):3504.
176. Nava HR, Pagana TJ. Postoperative surveillance of colorec-tal carcinoma. Cancer 1982;49:1043–7.
177. Nivatvongs S, Fryd DS. How far does the proctosigmoido-scope reach? A prospective study of 1000 patients. N Engl J Med 1980;303:380–2.
178. Reasbeck PG. Colorectal cancer: the case for endoscopic screening. Br J Surg 1987;74:12–7.
179. Mayer RJ, Garnick MB, Steele GD Jr, Zamcheck N. Carcinoembryonic antigen (CEA) as a monitor of chemo-therapy in disseminated colorectal cancer. Cancer 1978;42(3 Suppl):1428–33.
180. Bronstein BR, Steele GD Jr, Ensminger W, et al. The use and limitations of serial plasma carcinoembryonic antigen (CEA) levels as a monitor of changing metastatic liver tumor volume in patients receiving chemotherapy. Cancer 1980;46:266–72.
181. Litvak AM, Cercek A, Segal NH, et al. False-positive elevations of carcinoembryonic antigen in patients with a history of resected colorectal cancer [abstract]. J Clin Oncol 2014;32(3 Suppl):403.
182. Andrews CW Jr, O’Hara CJ, Goldman H, et al. Sucrase-isomaltase expression in chronic ulcerative colitis and dysplasia. Hum Pathol 1992;23:774–9.
183. Tuttle SE, Jewell SD, Mojzisik CM, et al. Intraoperative radioimmunolocalization of colorectal carcinoma with a hand-held gamma probe and MAb B72.3: comparison of
care alone after fl uorouracil failure for patients with metastatic colorectal cancer. Lancet 1998;352:1413.
155. Grothey A, Sargent D, Goldberg RM, Schmoll HJ. Survival of patients with advanced colorectal cancer improves with the availability of fl uorouracil-leucovorin, irinotecan, and oxaliplatin in the course of treatment. J Clin Oncol 2004;22:1209–14.
156. Tournigand C, Andre T, Achille E, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorec-tal cancer: a randomized GERCOR study. J Clin Oncol 2004;22:229–37.
157. Colucci G, Gebbia V, Paoletti G, et al. Phase III random-ized trial of FOLFIRI vs. FOLFOX4 in the treatment of advanced colorectal cancer: a multicenter study of the Grupo Oncologico Italia Meridonale. J Clin Oncol 2005;23:4866–75.
158. Koopman M, Antonini NF, Douma J, et al. Sequential versus combination chemotherapy with capecitabine, irinotecan, and oxaliplatin in advanced colorectal cancer (CAIRO): a phase III randomised controlled trial. Lancet 2007;370:135–42.
159. Seymour MT, Maughan TS, Ledermann JA, et al. Different strategies of sequential and combination chemotherapy for patients with poor prognosis advanced colorectal cancer (MRC FOCUS): a randomised controlled trial. Lancet 2007;370:143–52.
160. Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevaci-zumab plus irinotecan, fl uorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004;350:2335–42.
161. Saltz LB, Clarke S, Diaz-Rubio E, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as fi rst-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol 2008;26:2013–9.
162. Saltz LB, Meropol NJ, Loehrer PJ Sr, et al. Phase II trial of cetuximab in patients with refractory colorectal cancer that expresses the epidermal growth factor receptor. J Clin Oncol 2004;22:1201–8.
163. Hecht JR, Patnaik A, Berlin J, et al. Panitumumab mono-therapy in patients with previously treated metastatic colorectal cancer. Cancer 2007;110:980–8.
164. Amado RG, Wolf M, Peeters M, et al. Wild-type KRAS is required for panitumumab effi cacy in patients with metastatic colorectal cancer. J Clin Oncol 2008;26:1626–34.
165. Karapetis CS, Khambata-Ford S, Jonker DJ, et al. K-ras mutations and benefi t from cetuximab in advanced colorec-tal cancer. N Engl J Med 2008;349:1757–65.
166. Douillard JY, Oliner KS, Siena S, et al. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med 2013;369:1023–34.
167. Van Cutsem E, Kohne CH, Hitre E, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med 2009;360:1408–17.
168. Douillard JY, Siena S, Cassidy J, et al. Randomized, phase III trial of panitumumab with infusional fl uorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as fi rst-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol 2010;28:4697–705.

Scientifi c American Surgery
09/14
gastro adenocarcinoma of the colon and rectum — 30
187. Edge SB, Byrd DR, Compton CC, et al, editors. AJCC cancer staging manual. 7th ed. New York: Springer-Verlag; 2010.
Acknowledgments
The authors gratefully acknowledge the editorial assistance of Jen Levin.Figure 1 Alice Y. ChenFigures 8, 9, and 10 Christine Kenney
in vivo gamma probe counts with in vitro MAb radiolocal-ization. Int J Cancer 1988;42:352–8.
184. Staab HJ, Anderer FA, Hornung A, et al. Doubling time of circulating CEA and its relation to survival of patients with recurrent rectal cancer. Br J Cancer 1982;46:773–81.
185. Desch CE, Benson AB 3rd, Smith TJ, et al. Recommended colorectal cancer surveillance guidelines by the American Society of Clinical Oncology. J Clin Oncol 1999;17:1312.
186. Jones S, Chen WD, Parmigiani G, et al. Comparative lesion sequencing provides insights into tumor evolution. Proc Natl Acad Sci U S A 2008;105:4283.