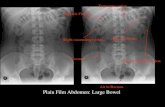
Serrated lesions of colon and rectum
-
Upload
dr-snehal-kosale -
Category
Health & Medicine
-
view
1.302 -
download
0
Transcript of Serrated lesions of colon and rectum

SERRATED LESIONS OF COLON AND RECTUM
PRESENTER- Dr. Snehal Kosale (Junior Resident)

Contents
Normal anatomy and histology of colon and rectum
Precancerous lesions Adenomas-
Clinical features,
Colonoscopy findings,
Histopathology
Adenoma Carcinoma sequence Classic
Serrated neoplastic pathways
Serrated polyps Classification
Types
Description
Clinical implications

ANATOMY

ANATOMY
The large bowel comprises the terminal 1–1.5 m of thegastrointestinal tract and is divided into the following regions: Cecum,
Ascending (right) colon,
Transverse colon,
Descending (left) colon,
Sigmoid colon, and
Rectum
The rectum forms the distal 8–15 cm Extraperitoneal
Lies within the pelvis
Ends at the anal canal

Histology
The large bowel wall is composed of four layers: Mucosa,
Submucosa,
Muscularis propria, and
Serosa (or, in the rectum, perimuscular tissues).
The mucosa has three components: Epithelium,
Lamina propria, and
Muscularis mucosae.
The mucosal surface is covered by a single layer of cuboidal to low columnar epithelium.

HISTOLOGY OF COLONIC MUCOSA
The mucosa consists of cells of two types: absorptive cells and mucus
secreting goblet cells arranged in closely packed straight tubular glands
or crypts, which extend down to sit on to the muscularis mucosae.

PRECANCEROUS LESIONS
BACKGROUND

Colon polyps
A polyp is any lesion that is elevated above themucosal surface of the bowel.
Polyps are most common in the colon But may occur in
Esophagus,
Stomach, or
Small intestine.
Polyps without stalks are referred to as sessile whilethose with stalks are termed pedunculated. Proliferation of cells adjacent to the polyp and the effects of
traction on the luminal protrusion, may combine to create astalk.

Colon polyps
In general, intestinal polyps can be classified
Nonneoplastic
Neoplastic.
Non-neoplastic colonic polyps can be further classifiedas
Inflammatory,
Hamartomatous, or
Hyperplastic.
The most common neoplastic polyp is the adenoma
Potential to progress to carcinoma

Adenomas
The most common and clinically important
neoplastic polyps are colonic adenomas
Majority of colorectal adenocarcinomas arise
from adenomas.
Colorectal adenomas are characterized by the
presence of epithelial dysplasia

Adenomas- Colonoscopy
Typical adenomas range from 0.3 to 10 cm in diameter
having a texture resembling velvet or a raspberry, due to
the abnormal epithelial growth pattern.

Adenomas- Histopathology
The hallmark of epithelial dysplasia is
Nuclear Hyperchromasia,
Nuclear Stratification
Nuclear Elongation.

Adenomas- Histopathology
Adenomas can be classified as (on the basis of
their architecture)
Tubular,
Tubulovillous, or
Villous
Villous adenomas have more malignant potential than
the other types.

Adenomas- Histopathology
Tubular adenoma
Closely packed tubular
crypts
<20% villous component
Tubulo-villous adenoma
Both tubular and villous
components
Villous component 20-80%
Hiromi Shinya., William 1. Wolff, Morphology, Anatomic Distribution and Cancer Potential of Colonic Polyps. ANNALS OF SURGERY 1979; Vol 190:6

Adenomas - Screening
As precursors to colorectal cancer (CRC),
All adults should undergo screening colonoscopystarting at age 50 years
Persons with a family history increased risk forCRC earlier in life
If family history is present, then screeningcolonoscopy should be started at least 10 yearsbefore the youngest age at which a relative wasdiagnosed.
Vinay Kumar, Abul K. Abbas, Jon C. Aster. Oral cavity and Gastrointestinal tract, 9 ed. Canada: Elsevier; 2013.

1. CLASSIC PATHWAY
2. MICROSATELLITE INSTABILITY
3. SERRATED NEOPLASTIC PATHWAY
ADENOMA CARCINOMA
SEQUENCE

Adenoma Carcinoma
Sequence - Classic
The classic adenoma-carcinoma sequence,
Accounts for as much as 80% of sporadic colon
tumors
Involves mutation of the APC tumor
suppressor on chromosome 5q early in the
neoplastic process.
For adenoma to develop,
Both copies of the APC gene must be functionally
inactivated.

Adenoma Carcinoma Sequence -
Classic
APC is a key negative regulator of β-catenin, acomponent of the WNT signaling pathway.
β-catenin accumulates and translocates to thenucleus, where it activates the transcription ofgenes which promote proliferation.
This is followed by additional mutations, includingactivating mutations in KRAS, SMAD2, andSMAD4, and the tumor suppressor gene TP53which also promote growth and prevent apoptosis.



Microsatellite Instability
Pathway Occurs in patients with DNA mismatch repair
deficiency.
Mutations in microsatellite sequences incoding/promoter regions Type II TGF-β receptor- uncontrolled cell proliferation
(inhibits epithelial proliferation)
Pro-apoptotic protein BAX- inhibits survival.
Mutations in the oncogene BRAF along withhypermethylation of CpG islands within genepromoters- CpG island methylator phenotype(CIMP) also occur.

Microsatellite Instability
Pathway
Thus, signature of this pathway of
carcinogenesis is the combination of
Microsatellite instability,
BRAF mutation, and
Methylation of specific targets (MLH1)

The pathways for left sided and right sided colon differ and are
now cumulatively called
‘THE SERRATED NEOPLASTIC PATHWAY’

Left colon
Traditional SNP
Distal Hyperplastic Polyps
Traditional serrated adenoma
Silencing of tumor suppressor genes MSS (CIMP-L)
Serrated adenocarcinoma
The Serrated Neoplastic
Pathway
Right colon
Sessile SNP
Proximal HyperplasticPolyps
Sessile serrated Lesion leading to Serrated
Adenoma
MLH1 hypermethylationMSI (CIMP-H)
Serrated adenocarcinoma
K-ras mutation BRAF mutation

SERRATED POLYPS

Serrated Polyps
Serrated polyp is a general term for any polyp
that shows a serrated (sawtooth or stellate)
architecture of the epithelial compartment.
It is a heterogeneous group of lesions that
mainly include
Hyperplastic polyp,
Sessile serrated lesions
Traditional serrated adenoma

Serrated Polyps -Why so
important?
High frequency in proximal colon:
Missed in colonoscopy
Flat/SessileMorphology:
Easily Overlooked
Ill-defined Borders:
Incomplete Resection
Putative rapidgrowth of MSI
cancers
Aggressive biological behavior
and poorer outcomes of BRAF, MSS
cancer

SERRATED LESIONS

WHO Classification of Serrated
Lesions (2010)
Hyperplastic polyp (HP) Microvesicular HP (MVHP)
Goblet cell rich HP (GCHP)
Mucin poor HP (MPHP)
Sessile serrated adenoma/polyp (SSA/P) SSA/P with atypia
Adenomatous
Serrated
SSA/P without atypia
Traditional serrated adenoma (TSA)
Snover D et al. WHO classification of tumours. Pathology and genetics. Tumours of the digestive system. 4th edition. Berlin: Springer-Verlag. 2010.

HYPERPLASTIC POLYPS
(HP)

Hyperplastic Polyps-
Introduction Formerly called
metaplastic polyps
More common on leftside of colon and inmales
Characteristics Small <10mm
Sessile
K-ras mutation morecommon
Otori K, Yoda Y, Sugiyama K et al. High frequency of K-ras mutations in human colorectal hyperplastic polyps. Gut 1997; 40:6660-663

Hyperplastic Polyps-
Progression
Inhibition of Apoptosis
Epithelial Cell Accumulation
Luminal Inbudding
Serrated or saw tooth
Appearance
NO genuine nuclear dysplasia. Only minor nuclear enlargement with reactive
changes

Immunohistochemistry
Immunohistochemistry shows regularly
expanded proliferative and luminal
compartments with Ki67 observed in the crypt
bases

Hyperplastic Polyps-
Subtypes Microvesicular Hyperplastic Polyp (MVHP)
Have microvesicular mucin
Goblet Cell rich Hyperplastic Polyp (GCHP) Mostly have goblet cells and less serrations
Small in size
Mucin poor hyperplastic polyp
Although pathological and molecular differences, NO clinical implication.

Microvesicular HP
Mostly right colon
Cells havemicrovesicular mucindroplets that impart ahazy, basophilicquality to thecytoplasm
Minimal nuclearatypia and mildnuclear stratificationoccurs in crypts andsurface
H/E stained sections showing hypermucinous
surface cells with few goblet cells and papillary
tufting
Hamilton S, Aoltonen L. Pathology and genetics of tumours of the digestive system (WHO classification of tumours). Internayional agency for research on
cancer, 2000:314

HYPERPLASTIC POLYP –MICROVESICULAR SUBTYPE
Serration and goblet cells are generally limited to the
superficial half of the crypt

Goblet Cell Rich HP
Found in the left colon
Crowded crypts containing a
disproportionately high number of mature
goblet cells
Minimal serrations
Hamilton S, Aoltonen L. Pathology and genetics of tumours of the digestive system (WHO classification of tumours). Internayional agency for research on
cancer, 2000:314

HYPERPLASTIC POLYP –GOBLET CELL RICH SUBTYPE

Mucin Poor HP
Rare Hyperplastic polyp
Show a relative lack of gobletcells and microvesicularmucin
Epithelial cells are smallerwith less cytoplasm
Uniform and prominentserrations with amicropapillary pattern may beseen
Nuclear hyperchromasia andanisocytosis may beprominent

Hyperplastic Polyposis
Syndrome (HPS)
Familial clustering of multiple/ large (>3 cm) hyperplastic polyps
Predominantly distal location in colon
Usually asymptomatic
Associated with an increased risk (approx. 25%) of CRC with MSI.
Williams GT et al. Metaplastic polyps and polyposis of the colorectum. Histopathology 1980;40:155-170.
Legett BA et al, Hyperplastic polyposis: association with colorectal cancer. Am J Surg Pathol 2001;25:177-184.

Definition of HPS
Greater than five hyperplastic polyps proximal to the sigmoid of which two are >10 mm.
Greater than 30 hyperplastic polyps of anysize, proximal to the sigmoid colon.
Any number of hyperplastic polyps with a firstdegree relative with known HPS.

SESSILE SERRATED LESIONS
(SSL)

Sessile Serrated Lesions
(SSL) According to European
guidelines of 2011, SSL
More common in the rightside of colon and infemales
Endoscopically- Large
Sessile
Yellow in color
May have a mucus capwhich when present maybe missed
Quirke P et al. Quality assurance in pathology in colorectal cancer screening and diagnosis-European recommendations. Virchow Arch 2011;
4581:1-19

SSL - Progression
Inhibition of Apoptosis
Epithelial Cell Accumulation
Luminal Inbudding
Serrated or saw tooth
Appearance

SSL without atypia
Distorted architecture due to altered proliferative zone
Proliferative zone oftendisplaced from base toside
Crypts may herniatethrough muscularismucosae k/a invertedgrowth pattern
Pronounced serrationreaching upto the bases

SSL without atypia
Crypts are dilated
and branched with L
or inverted T shaped
Crypts are irregular,
branched
Dilatation of crypt
bases

SSL with atypia-
adenomatous More commonly found in the
cecum and ascending colon
Similar to conventional adenomatous polyps
An abrupt transition to dysplastic crypts resembling adenomatous crypts
Showing relatively small rounded and fairly uniform nuclei
Suggestion of residual serration

SSL with atypia- serrated
Glands retain a
serrated architecture
Have ample
eosinophilic
cytoplasm
Nuclei are typically
Vesicular
Basally located

Criteria For
Serrated Lesions
Serrated appearance at the base of the crypts
Horizontalisationof crypts with
branching
Dilatation of the crypts
Increase in epithelium-stroma ratio
> 50%
Mitoses on the surface of the crypts
Cellular atypia: Enlarged nucleus, overproduction of
mucin
2 of 6 criteria must be present for diagnosing sessile serrated
lesions

TRADITIONAL SERRATED
ADENOMAS

Traditional Serrated Adenoma-
TSA
Rare subtype (<6% of serrated polyps)
Occur in older adults particularly females
Located in the left colon
Endosopically-
Polypoidal lesions: Reddish discoloration and granulo-lobular appearance
Sessile lesions: Larger, proximally located, white mucosa
Torkalovic EE, Gomez DK et al. Sessile serrated adenoma (SSA) vs traidtional serrated adenoma (TSA). Am JSUrg Pathol 2008; 32:21-29

Traditional Serrated Adenoma-
Progression
Fully developed TSA with multiple
ectopic crypts lining villi.
Early TSA- an outward
growth creates ectopic
growth

Traditional Serrated Adenoma-
TSA
Prominent serration
Diffuse low grade dysplasia
(10% high grade dysplasia)
Luminal infoldings
perpendicular to the main
axis of the crypts called
‘ectopic crypt foci’

Traditional Serrated Adenoma-
TSA
Abundant eosinophilic cytoplasm
Centrally placed, pencillate nuclei
Nuclearstratification
Nuclear hyperchromasia

Features of Serrated Adenomas
WHO
Classification
Prevalence Shape Distribution Malignant
Potential
Hyperplastic polyp Very
common
Sessile/ flat Mostly distal Very low
Sessile serrated
adenoma/polyp
Common Sessile/ flat 80% proximal Significant
Traditional serrated
adenoma
Rare Sessile/
Pedunculated
Mostly distal Significant

SERRATED
ADENOCARCINOMA

Serrated Adenocarcinoma
End point of the serrated pathway
Reported to account for 7.5% of all colorectal carcinomas
More common in right colon
Left sided occur from microsatellite stable (MSS) or show low microsatellite instability (MSI) and arise from TSA
Right sided occur from high frequency of microsatellite instability (MSI) and arise from SSL

SERRATED ADENOCARCINOMA

Serrated Adenocarcinoma
Cells are cuboidal to
short columnar
Moderate
eosinophilic
cytoplasm
Very large nuclei
Open chromatin
Prominent nucleoli.

CLINICAL IMPLICATIONS

Clinical Implications
Irrespective of size SSLs may progress
rapidly to MSI carcinomas
Polyp size >2 cmThorough examination of
resection margins
Polyp morphology-
Serrated Polyps
Comment on margins as free/involved

Clinical Implications
Distal serrated adenocarcinomas have a
worse prognosis compared with non-serrated
and proximal cancers.
Therefore, important to distinguish SSLs and
TSAs
Associated malignancies may have a different
prognosis and treatment.
Mäkinen MJ. Colorectal serrated adenocarcinoma. Histopathology 50, 131–150 (2007)
Laiho P, Kokko A, Vanharanta S et al. Serrated carcinomas form a subclass of colorectal cancer with distinct molecular basis. Oncogene 26, 312–
320 (2007).
Garcia-Solano J, Perez-Guillermo M, Conesa-Zamora P et al. Clinicopathologic study of 85 colorectal serrated adenocarcinomas: further insights
into the full recognition of a new subset of colorectal carcinoma. Hum. Pathol.41, 1359–1368 (2010).

Clinical Implications
The presence of large, serrated polyps (>1
cm) are an independent risk factor for the
presence of CRC
The data by Goldstein et al suggest that
surveillance colonoscopy intervals for SSAs
should be at least as frequent as those
recommended for conventional adenomas.
Hiroaka S, Kato J, Fujiki S et al. The presence of large serrated polyps increases the risk of colorectal cancer.Gastroenterology 139,
1503–1510 (2010)
Schriener MA, Weiss DG, Lieberman DA et al. Proximal and large hyperplastic and nondysplastic serrated polyps detected by
colonoscopy are associated with neoplasia. Gastroenterology 139, 1497–1502 (2010).

Guidelines for endoscopic
surveillanceSerrated Lesion Lesion Size Recommended
Surveillance
(years)
Hyperplastic Polyp <10 mm 10
Sessile Serrated Lesion <10 mm 5
Sessile Serrated Lesion >10 mm 3
Mixed Hyperplastic/
Adenomatous Polyp
Mixed serrated/ adenoma
Any size 1
Traditional Serrated Adenoma Any size 1
Hyperplastic/ Serrated Polyposis Any size 1

References
Snover D et al. WHO classification of tumours. Pathology and genetics. Tumours of the digestive system. 4th edition. Berlin: Springer-Verlag. 2010.
Vinay Kumar, Abul K. Abbas, Jon C. Aster. Oral cavity and Gastrointestinal tract, 9 ed. Canada: Elsevier; 2013.
Hiromi Shinya., William 1. Wolff, Morphology, Anatomic Distribution and Cancer Potential of Colonic Polyps. ANNALS OF SURGERY 1979; Vol 190:6
Hamilton S, Aoltonen L. Pathology and genetics of tumours of the digestive system (WHO classification of tumours). Internayional agency for research on cancer, 2000:314
Hamilton S, Aoltonen L. Pathology and genetics of tumours of the digestive system (WHO classification of tumours). Internayional agency for research on cancer, 2000:314
Torkalovic EE, Gomez DK et al. Sessile serrated adenoma (SSA) vs traidtional serrated adenoma (TSA). Am JSUrg Pathol 2008; 32:21-29
Torkalovic EE, Gomez DK et al. Sessile serrated adenoma (SSA) vs traidtional serrated adenoma (TSA). Am JSUrg Pathol 2008; 32:21-29
Mäkinen MJ. Colorectal serrated adenocarcinoma. Histopathology 50, 131–150 (2007)
Laiho P, Kokko A, Vanharanta S et al. Serrated carcinomas form a subclass of colorectal cancer with distinct molecular basis. Oncogene 26, 312–320 (2007).
Garcia-Solano J, Perez-Guillermo M, Conesa-Zamora P et al. Clinicopathologic study of 85 colorectal serrated adenocarcinomas: further insights into the full recognition of a new subset of colorectal carcinoma. Hum. Pathol.41, 1359–1368 (2010).
Hiroaka S, Kato J, Fujiki S et al. The presence of large serrated polyps increases the risk of colorectal cancer.Gastroenterology 139, 1503–1510 (2010)
Schriener MA, Weiss DG, Lieberman DA et al. Proximal and large hyperplastic and nondysplasticserrated polyps detected by colonoscopy are associated with neoplasia. Gastroenterology 139, 1497–1502 (2010).
Torlakovic E, Snover DC. Serrated adenomatous polyposis in humans. Gastroenterology 110, 748–755 (1996)