Water Facts: Formula = H 2 O Three phases – Solid, liquid, and gas Freezing point = 0˚C Boiling...
-
Upload
ciara-peardon -
Category
Documents
-
view
216 -
download
1
Transcript of Water Facts: Formula = H 2 O Three phases – Solid, liquid, and gas Freezing point = 0˚C Boiling...
- Slide 1
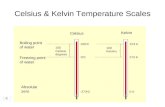
Slide 2 Water Facts: Formula = H 2 O Three phases Solid, liquid, and gas Freezing point = 0C Boiling point = 100C Inorganic molecule (aka: non- living, does not contain Carbon) Polar molecule Universal solvent About 70% of human body is water Slide 3 Polarity Polar molecules: slightly positive at one end and slightly negative on the other end Water is a polar molecule!!!!!! Draw a picture definition of polarity on your note sheet in the empty box. Slide 4 Non-polar molecules: No charge Polar molecules dissolve in water Non-polar molecules do not dissolve in water Like dissolves like Slide 5 Universal Solvent Solvent substance that does the dissolving Solute substance dissolved in a solution Solution mixture of solvent and solute http://programs.northlandcollege.edu/biology/Biology1111/a nimations/dissolve.html Slide 6 Magic Sand Slide 7 Slide 8 Cohesion Attraction between substances of the same kind Ex: Water forming drops/beading Caused by hydrogen bonds between water molecules Slide 9 Surface tension Prevents the surface of water from stretching or breaking easily Molecules at the surface of water are linked by hydrogen bonds Result of cohesion Slide 10 Natures use of Surface Tension Slide 11 Drops on a penny Slide 12 Adhesion Attraction between different polar substances Ex: things getting wet Slide 13 Capillary Action Property in which water can travel up tubes Powered by adhesion Ex: plants, trees Attraction between walls and water is greater than effect of gravity Slide 14 Celery Stalk Slide 15 Slide 16 Density Ratio of the mass of an object to its volume Density = Mass (g)___ Volume (ml) The density of water = 1.0 Density less than 1.0 = floats Density greater than 1.0 = sinks Ice floats, yet cold water sinks in ice, molecules spread out (ex: soda can in freezer) Cold water moves slower than warm water, causing it to sink Slide 17 Soda Can Slide 18 Slide 19 What causes water to do the amazing things it does?