Fe 3+ (aq) + SCN - (aq) ⇆ FeSCN 2+ (aq) (blood red) EQUILIBRIUM CONSTANT DETERMINATION.
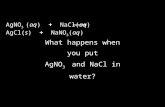
Warmup (3 minutes) Na 2 CO 3 (aq) + 2HCl (aq) 2NaCl (aq) + CO 2 (g) + H 2 O (l) 1) In the reaction...
-
Upload
theodore-wilkins -
Category
Documents
-
view
225 -
download
0
Transcript of Warmup (3 minutes) Na 2 CO 3 (aq) + 2HCl (aq) 2NaCl (aq) + CO 2 (g) + H 2 O (l) 1) In the reaction...
Warmup (3 minutes)
Na2CO3(aq) + 2HCl(aq) 2NaCl(aq) + CO2(g) + H2O(l)
1) In the reaction above, identify the compounds whose concentrations or pressures will increase during the course of the reaction, and the compounds whose amounts will decrease as the reaction progresses.
2) What is the purpose of refrigerating milk?
When a glow stick is snapped and shaken it gives off light! I’ll bet you have some questions about this. I sure do!
What’s inside that makes them light up?
How do they work?
•Hydrogen peroxide oxidizes the phenyl oxalate ester, making phenol and an unstable peroxyacid ester (POE). •Ester decomposes, forming a cyclic peroxy compound, which decomposes to CO2, a process which releases energy to the dye. •Electrons in the dye atoms jump to a higher level, then fall back down releasing energy (light).
What determines how long they last? Let’s discuss collision theory first…
Collision theory: molecules must hit each other hard and be under favorable conditions to react
Molecules are always colliding. Atoms SOMETIMES rearrange to form new products (ex. pool).
Changing any condition that forces molecules to collide more frequently will increase reaction rate
don’t draw
The amount of time that a lightstick lasts (AKA the rate of a reaction!) depends on:1. Temperature!Increasing temperature increases KE of particles, which move faster and have more frequent and harder collisions…..faster reaction!Extra energy accelerate the reaction brighter, (but for a shorter amount of time)Cool the light stick slow reaction light will dim.If you want to preserve your light stick for the next day, put it in the freezer -- it won't stop the process, but it will drag out the reaction considerably. 2. Particle Size/Structure of the compounds 3. Presence of a Catalyst? (more on this later)
4. Concentration (aq) or a change
in volume or pressure (g)
Any change that makes molecules
closer together will cause them to collide more
frequently
Which molecules in the lightstick “decide” to react first?• We recognize that we can't precisely predict the behavior of one
molecule but we CAN do this with groups molecules using statistics
Maxwell Boltzmann Distribution
As temperature increases, the curve will spread to the right but the area under the curve remains the same (always 100% molecules).As T increases, the fraction of molecules with energies greater than the red line increase.The molecules that “decide’ to react first are the ones with enough energy to react.
When molecules meet the threshold energy, they have enough energy to react
%
There are fancy and nonfancy ways to measure how much phenyl oxalate ester (POE) is left….don’t worry about this too much.
If 1/10 (10.0%) of the remaining chemical is used up every 10.0 minutes, calculate the amount of chemical remaining at the end of each 10.0 minute increment.
x20 = (x10 – 0.100x10) = 81.0
Time(min)
mass POE left (mg)
0.0 100.0
10.0
20.0
30.0
40.0
50.0
60.0
70.0
80.0
81.0
90.0
65.6
72.9
59.0
43.0
47.8
53.1
Time(min)
mass POE left (mg)
0.0 100.0
10.0
20.0
30.0
40.0
50.0
60.0
70.0
80.0
81.0
90.0
65.6
72.9
59.0
43.0
47.8
53.1
Go ahead and graph our data
What units should be used to describe the rate at which the POE is being used?
rate = ∆mg/∆min
What is the rate for the first ten minutes?
Rate = 10.0mg/ 10.0 min =
The POE is decomposing at a rate of 1.00 mg/min during the first 10 minutes
Can measure rate as:
1.Rate of disappearance of reactant (usually)
2. Rate of product appearance (sometimes)
Units = [ ]/time
We can calculate the reaction rate if the [reactants and products] is known
Time(min)
mass POE left
(mg)
Rate (∆mg/∆
min)
0.0 100.0 -------
10.0 90.0
20.0 81.0
30.0 72.9
40.0 65.6
50.0 59.0
60.0 53.1
70.0 47.8
80.0 43.0
0.900
What is the reaction rate for the second ten minutes?
∆min = 20.0 – 10.0 min∆mg = 90.0 – 81.0 = 9.0mgRate = 9.0mg/10.0 min = 0.900
mg/minFinish filling in the 3rd column.What is the average rate during the
first 50 minutes of the reaction?Rate = 41.0mg/50.0 min = 0.820
mg/minWhat is the average rate during the
course of the whole reaction?Rate = 57.0mg/80.0min = 0.713
mg/minThe point? The reaction rate
changes during the course of a reaction (curve!)
1.00
0.730
0.660
0.590
0.530
0.480
0.810
Catalysts:
• allow bonds in the reactants to break and form more readily
Transition-state theoryAs molecules approach each other, their
orbitals distort each other and bonds weaken
This allows bonds to break and reform with other atoms
Ene
rgy
Reaction coordinate
Reactants
Products
Increase of energy as reactants approach each other
Maximum energy when they collide
Decrease in energy as products recoil
Ene
rgy
Reaction coordinate
Reactants
Products
Activation Energy: minimum energy to make the reaction happen
Reaction rate depends on the Ea in this potential energy profile
Activated Complex or Transition State: where old bond is half-broken and new bond is half-formed
Ene
rgy
Reaction coordinate
Reactants
Products
Catalyst: substance that speeds up reaction without being used up.
The reaction goes faster.
The overall energy is the same!
Ea
It gives reaction a new path, with a lower activation energy.
Pt surface
HH
HH
C HH C
HH
Catalysts can be enzymes (biology!), or atoms or ions which like to give away or accept electrons. Platinum is
the catalyst in our vehicles to break down gas emissions into “greener gases.”
Pt surface
HH
HH
C HH C
HH
The double bond breaks and bonds to the catalyst.
Catalysts can be enzymes (biology!), or atoms or ions which like to give away or accept electrons. Platinum is
the catalyst in our vehicles to break down gas emissions into “greener gases.”
Pt surface
HH
HH
C HH C
HH
The hydrogen atoms bond with the carbon
Catalysts can be enzymes (biology!), or atoms or ions which like to give away or accept electrons. Platinum is
the catalyst in our vehicles to break down gas emissions into “greener gases.”
Pt surface
H
C HH C
HH
H HH
Catalysts can be enzymes (biology!), or atoms or ions which like to give away or accept electrons. Platinum is
the catalyst in our vehicles to break down gas emissions into “greener gases.”
123 Blastoff! demonstration
One of the reactions in rocket propulsion is the reduction of potassium permanganate. Reduction is when a species (ion, compound, etc) gains electrons. Two possible permanganate reduction reactions are shown below:
Reaction #1: 8H3O+ + MnO4- + 5Fe2+ 5Fe3+ + Mn2+ + 12H2O
reduction by iron(II)
Reaction #2: 16H3O+ + 2MnO4- + 5O2
2- 2Mn2+ + 5O2 + 24H2Oreduction by peroxide
Materials
Two 6g FeSO4 solid samples 200ml 3% liquid H2O2
distilled water 20 ml 3.0M MnSO4 50ml 0.02M KMnSO4
three 10ml graduated cylinders four 125ml Erlenmeyer flasks
Part 1: H2SO4 as a Catalyst
1.Into both Erlenmeyer flasks, dissolve 4g iron(II)sulfate in a few ml of 0.5M H2SO4.
2.Fill one flask to 100ml with distilled water. Fill the other with the sulfuric acid.
3.Measure 10 ml MnO4- solution into two
different graduated cylinders. Add the MnO4-
into each flask simultaneously.
3. Observe and record results.
Does the reduction of MnO4- to Mn2+ proceed
faster or slower in the presence of H2SO4?
Part 2: MnSO4 as a Catalyst
1.Add 100 ml H2O2 solution into two Erlenmeyer flasks.
2.Measure 10 ml MnO4- solution into two
different graduated cylinders. Add the MnO4-
into each flask simultaneously.
3. Using the third graduated cylinder, add 10 ml of Mn2+ solution to just one of the flasks.
Does the reduction of MnO4- to Mn2+ proceed
faster or slower in the presence of MnSO4?




































![Section 7.6: Solubility Equilibria and the Solubility Product ...Write the solubility product constant equation. K sp=[Ag +(aq)][I!(aq)] [Ag+(aq)]=[I!(aq)] K sp=[Ag +(aq)]2 Step 3.](https://static.fdocuments.us/doc/165x107/6123f8ac1375fc2ea57b63da/section-76-solubility-equilibria-and-the-solubility-product-write-the-solubility.jpg)




