Sodium hydroxide and hydrobromic acid. NaOH (aq) +HBr (aq) NaBr (aq) +H 2 O (l)
-
Upload
jamari-hinks -
Category
Documents
-
view
263 -
download
2
Transcript of Sodium hydroxide and hydrobromic acid. NaOH (aq) +HBr (aq) NaBr (aq) +H 2 O (l)

Sodium hydroxide and hydrobromic acid

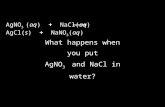
NaOH(aq)+HBr(aq)NaBr(aq)+H2O(l)

OH-(aq)+H+
(aq)H2O(l)

Propane combusts

2C4H10(g)+O2(g)8CO2(g)+ 10H2O(l)

2C4H10(g)+O2(g)8CO2(g)+ 10H2O(l)

Potassium and water

2K(s)+2H20(l)2KOH(aq)+H2(g)

2K(s)+2H20(l)2K+(aq)+2OH-
(aq) +H2(g)

Magnesium and hydrobromic acid

Mg(s)+2HBr(aq)MgBr2(aq)+H2 (g)

Mg(s)+2H+(aq)Mg2+
(aq)+H2(g)

Copper and hydrobromic acid

Cu(s)+HBr(aq)no rxn

Cu(s)+HBr(aq)no rxn

Sodium carbonate decomposes

Na2CO3(aq)Na2O(aq)+CO2(g)

CO32-
(g)O2-(g)
+ CO2(g)

Aluminum reacts with air

4Al(s)+302(g)2Al2O3(s)

4Al(s)+302(g)2Al2O3(s)

Hydrochloric acid and sodium carbonate

2HCl(aq)+Na2CO3(aq)H2O(l)+ CO2(g)+2NaCl(aq)

2H+(aq)+ CO3
2-(aq)H2O(l)+ CO2(g)

Iron(III) chloride and sodium hydroxide

FeCl3(s)+3NaOH(aq)3NaCl(aq) +Fe(OH)3(s)

Fe3+(s)+3OH-
(aq)Fe(OH)3(s)

Lithium hydroxide and potassium chloride

LiOH(aq)+KCl(aq)no rxn

LiOH(aq)+KCl(aq)no rxn

Hydrobromic acid and potassium sulfide

HBr(aq)+K2S(aq)2KBr(aq)+H2S (g)

2H+(aq) + S2-
(aq) H2S(g)