WAO Global Hereditary Angioedema (HAE) Practice Parameter
description
Transcript of WAO Global Hereditary Angioedema (HAE) Practice Parameter

WAO Global Hereditary Angioedema (HAE) Practice Parameter
Timothy J. Craig, DOProfessor of Medicine and Pediatrics
Distinguished EducatorChief, Allergy, Asthma, and Immunology
Program Director Director of Clinical Allergy and Respiratory Research
Pennsylvania State University College of MedicineHershey, Pennsylvania, USA

WAO Global Hereditary Angioedema Practice ParameterChair: Tim Craig, USA
General Advisor: Richard F. Lockey, USA Steering Committee Members: Konrad Bork, Germany Tom Bowen, Canada Henrik Boysen, Belgium Marco Cicardi, Italy Henriette Farkas, Hungary Anete Grumach, Brazil (SLAAI) Connie Katelaris, Australia (APAAACI) Hilary Longhurst, UK William Lumry, USA (ACAAI) Marcus Maurer, Germany (EAACI) Bruce Ritchie, Canada Bruce Zuraw, USA (AAAAI) Emel Aygören Pürsün, Germany Inmaculada Martinez-Saguer, Germany

Sponsors of the WAO HAE ProgramCompany Financial Supporter
CSL Behring XX
Dyax XX
Viropharma XX
Shire XX (via Jerini)
Pharming (did not yet have a product on the market)

WAO Global Hereditary Angioedema Practice Parameter
OBJECTIVES:1.Produce an evidence based guideline for care of HAE patients throughout the world2.Develop a document that would be a reference for HCP3.To develop a document that could be used at the bedside4.Have a document that could be utilized in all countries5.Develop a guideline that would be approved by the global allergy community6.Develop a power point program for use by all members of the WAO7.Lastly, and most importantly, to improve care for the patients with Hereditary Angioedema and to improve access of therapies to all patients, in all countries around the world.

Is there a need for this?

ActiveCleaved: Inactive

What Is C1-Inhibitor?
Key regulator of fourbiochemical pathways
1. Complement 2. Contact 3. Fibrinolytic4. Coagulation
Human plasma protein …that mediates inflammation
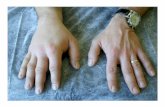
C1-Inhibitor deficiency
can cause:– debilitating pain– disfiguring swelling– asphyxiation &
death

Autosomal Dominant Defect
Crowder JR, Crowder TR. Five generations of angioneurotic edema. Arch Inter Med 1917; 20:840-52

Bissler JJ, et al. Proc Assoc Am Physicians. 1997;109:164-173.Davis AE 3rd. Annu Rev Immunol. 1988;6:595-628.Verpy E, et al. Am J Hum Genet. 1996;59:308-319.Zuraw BL, Herschbach J. J Allergy Clin Immunol. 2000;105:541-546.
HAE Is Caused By C1 Inhibitor Mutations

C1-INH involved in 3 systems → C1-INH depletion
Kallikrein
HMW-K
Factor XII
Prekallikrein
Contact SystemContact System
C4C2
C1
Complement Complement SystemSystem
Increased vascular
permeability ANGIOEDEMA
C1-
INH
C1-
INH
C1-INH C1-INH C
1-IN
H
C
1-IN
H
C1-
INH
C1-
INH
Bradykinin
FibrinolyticFibrinolyticSystemSystem
Factor XIIa
Plasmin
Plasminogen
C1rs
C1-INH

C1INH gene +/+ +/+ -/- -/- -/-B2BKR gene +/+ +/+ +/+ +/+ -/-Evans blue No Yes Yes Yes YesC1INH therapy No No No Yes No
C1INH Null Mice and Vascular Permeability
Adapted from Han ED, et al. J Clin Invest. 2002;109:1057-1063.

In Vivo Generation of Kinins in HAE
From Nussberger J, et al. J Allergy Clin Immunol. 1999;104:1321-1322; with permission.

Actin stress fibersVE-cadherin
Nonstimulated
Stimulated
Increased vascular permeability
From Tiruppathi C, et al. Vascul Pharmacol. 2003;39:173-185; with permission.
How Does BK Cause Angioedema?

Common triggers of HAE attacks
Trauma Menstruation
Infection
Stress
Medications
AngioedemaAngioedema attack





• Conceptually divide intothree categories– Long-term prophylaxis
• Minimize attack frequency and severity
• Prevent hospitalizations and emergency room visits
– Short-term prophylaxis• Prevent attacks after trauma• Prevent attacks during
important life events
– Treatment of acute attacks• Terminate ongoing attack• Prevent morbidity and
mortality
Treatment of HAE

PK
Therapeutic Implications
Adapted from Zuraw BL. Immunol Allergy Clin North Am. 2006;26:691-708.

Drug Advantages Disadvantages Best use Status
Plasma-derivedC1-INH
• Extensive clinical experience
• Corrects the fundamental defect
• long half-life
• Infectious risk• Needs IV access• Limited supply
• Acute attacks• Short-term• Long-term
prophylaxis• Prodromes
• Berinert P: approved in EU, USA, Canada, Argentina
• Cinryze: approved in EU, USA
• Cetor in the EU, Turkey
RecombinantC1-INH
• Corrects the fundamental defect
• No human virus risk
• Scalable supply
• Needs IV access• Short half-life• Potential for
allergic reactions
• Acute attacks• Short
prophylaxis• Prodrome?
• Rhucin: approved in the EU
Ecallantide • More potent than C1-INH
• No infectious risk• Subcutaneous
administration
• Antibodies may cause allergic reaction or neutralization
• Short half-life
• Acute attacks in office
• Kalbitor: approved in the USA
Icatibant • No infectious risk
• Stable at room temperature
• Subcutaneous
• Short half-life• Local pain or
irritation
• Home treatment of acute attacks?
• Firazyr: approved in EU, USA, Brazil





In Summary
Global document is now being evidence based
From here it will go to the steering committee for approval
Than it will go to WAO leadership Finally out to all the Allergy
Associations for their approval Power Point slides will go through the
same process

Thank [email protected]