Theoretical Study of the Flame Synthesis of Titanium Dioxide Nanoparticles Kui Ting Lam, Doug...
-
Upload
prosper-leonard -
Category
Documents
-
view
218 -
download
4
Transcript of Theoretical Study of the Flame Synthesis of Titanium Dioxide Nanoparticles Kui Ting Lam, Doug...

Theoretical Study of the Flame Synthesis of Titanium Dioxide NanoparticlesKui Ting Lam, Doug DePrekel, Kevin Ngo, Phu Vo, and Yingbin Ge
Department of Chemistry, Central Washington University, Ellensburg, WA 98926
Introduction
• Titanium dioxide (TiO2) nanoparticles are widely used in our daily lives. For example, TiO2 nanoparticles are used as whitening agent, coating for polymers, surface support for photocatalysis and for solar energy conversion.
• Compared to the sol-gel synthesis of the TiO2 nanoparticles, the flame synthesis is a cheaper and faster alternative. The mechanism of the flame synthesis of the TiO2 nanoparticles, however, is not well understood. In this research, we will study theoretically the chemical mechanism of the flame synthesis of the TiO2
nanoparticles, which involves both gas-phase and surface reactions.• Density functional theory methods will be used to model these gas-phase and surface
reactions. We aim to propose the mechanism of the flame synthesis of the TiO2 nanoparticles through the density functional theory study. The ultimate goal is to provide theoretical guidance on the size and surface control of the flame-synthesized TiO2 nanoparticles.
References
1. Sung, Y.; Raman, V.; Fox, R. O.; Chem. Eng. Sci., 2011, 66, 4370. 2. Wang, T.; Dixon, D. A.; Navarrete-lo, A. M.; Li, S.; Gole, J. L., J. Phys. Chem.
A, 2010, 114, 7561. 3. Zhao, W-N; Liu Z-P, Chem. Sci., 2014, 5, 2256.
Production of TiO2 nanoparticles
-200
-100
0
100
200
300
400
42 DFT methods vs benchmark coupled-clusters calculations (for 28 Ti-O-Cl species)
MADMSD
Ato
miz
ation
Ene
rgy
Dev
iati
on (k
J/m
ol)
(Mean Absolute Deviation)(Mean Signed Deviation)
Computational methods
• The coupled-cluster and density function theory (DFT) methods were used to study small TixOyClz species theoretically.
• The coupled-cluster method is highly accurate but extremely slow.• DFT methods are in general less accurate but much faster than the coupled-cluster
method. We need to first assess the accuracy of the DFT methods for the Ti-O-Cl species before studying the mechanism of the flame synthesis of TiO2.
Future Studies
• Kinetic modeling of reactions between the Ti-O-Cl gas phase species using the B98 or B97-1 method..
• Kinetic modeling of reactions between the gas phase species and the surfaces of the rutile, brookite, and anatase TiO2 solid surfaces.
Conclusions
• Accurate thermodynamic data of 28 Ti-O-Cl species were obtained at the coupled-cluster level of theory.
• Calculations of 42 density functional theory (DFT) methods were compared with the coupled-cluster results. B98 and B97-1 methods were found to be the most accurate among the 42 DFT methods.
• The calculations of the 42 DFT methods were compared to Dixon’s coupled-cluster calculations of the Ti-O-Cl-H species. B98 and B97-1 are still the most accurate among all assessed DFT methods.
• For molecular complexes, the inclusion of the empirical description of the dispersion energy may enhance the accuracy of the calculations.
Dye sensitized solar cell Toothpaste (whitening agent)
Sol-gel methodby mixing CH3CH2OH, CH3COOH, Ti[OCH(CH3)2]4.
cheaper faster
Self-cleaning: with vs. without TiO2 coating
Results and Discussion
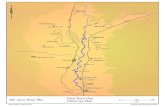
Reactions between the gas-phase species and the TiO2 surfaces.3
Flame synthesis1
TiCl4+ O2 + CH4 → TiO2 (solid) + CO2 + H2O + HCl
B2PLYP
B2PLYP-D3BJ
B3LYP
B3LYP-D
3
B3LYP-D
3BJ
B3P86
B3PW
91
B3PW
91-D3B97-1
B97-2
B97-D
B97-D
3BJ
ωB97X
ωB97XD B9
8
BHandH
BHandHLYPBLYP
BMKBP86
BPBE
BPW91
CAM-B3LYPHCTHτHCTH
τHCTHhyb
M06
M06-2x
M06-D3
M06-HF
M06-LOLYP PB
EPBE0PW
91
mPW2PLYP
mPWLYP
mPWPW
91SVWNTPSSTPSShX3LYP
-300
-250
-200
-150
-100
-50
0
50
100
150
200
250
300
350
400
450
500
42 DFT methods vs. Dixon's coupled-cluster results2 (for Ti-O-Cl-H species)
MADMSD
Ato
miz
ation
Ene
rgy
Dev
iatio
n (k
J/m
ol)
(Mean Absolute Deviation)(Mean Signed Deviation)
Acknowledgements
• K.T. Lam thanks the CWU School of Graduate Studies and Research for the Undergraduate Research Fellowship.
• Department of Chemistry, Central Washington University.