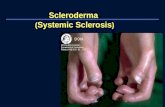
Scleroderma August 2012 Spectrum
Transcript of Scleroderma August 2012 Spectrum

August 2012
a clinical newsletter for medical professionals
Scleroderma
Spectrum
Inside This Issue:Future Options for Scleroderma Therapy • Challenges in Scleroderma Therapy • Novel Concepts and Target Sites

2 Scleroderma Spectrum
S C L E R O D E R M A S P E C T R U MAugust 2012. Text, photographs,
artwork, logo, and all other contents © 2010 The Scleroderma Foundation, Inc.
Published by The Scleroderma Foundation
300 Rosewood Drive, Danvers, MA 01923Tel: 978-463-5843 | Info Line: 800-722-HOPE
Fax: 978-463-5809 Web site: www.scleroderma.org
Scleroderma Spectrum medical editorial committee
robert lafyatis, m.d., Associate Professor of Medicine and Laboratory Director, Boston Univer-sity Medical Centerrobert Simms, m.d., Professor of Medicine, Sec-tion Chief, ad interim; Clinical Director; Rheu-matology Fellowship Program Director, Boston University Medical Centerterence trow, m.d., Director of the Pulmonary Hypertension Center, Assistant Professor of Medi-cine, Yale University School of MedicineJohn Varga, m.d., Professor, Division of Rheuma-tology, Northwestern University’s Feinberg School of MedicineSupportive medical writing services provided by Cactus Communications, Inc.
Board oF directorSJoseph P. Camerino, Ph.D., Chair
Carol Feghali-Bostwick, Ph.D., Vice ChairCos Mallozzi, Treasurer
Mary Blades Lee Roy Jones Kevin Boyanowski Robert Kacick Marie Coyle Greg Marion Kerry Hall Robert Slappey
medical adViSorY BoardSteering committee
John Varga, M.D., ChairMaureen D. Mayes, M.D.
Richard Silver, M.D. Virginia Steen, M.D.members
Carol Black, M.D. Laura Hummers, M.D.Lorinda Chung, M.D. Dinesh Khanna, M.D., M.S.Philip Clements, M.D. Thomas Medsger, Jr., M.D.Aryeh Fischer, M.D. Janet Pope, M.D.Tracy Frech, M.D. Robert F. Spiera, M.D.Daniel Furst, M.D. Sergio Jimenez, M.D.Jessica Gordon, M.D. Arnold Postlethwaite, M.D.
members emeritiFrank Arnett, M.D.
Michael Ellman, M.D.Frederick Wigley, M.D.
Scleroderma FouNdatioN StaFFRobert J. Riggs, Chief Executive Officer
Mary Ann Berman, Office AssistantRyan Burrill, Programs and Services CoordinatorKerri Connolly, Director of Programs and Services
Shenna Gianetta, Executive AssistantLaura Koumarianos, Staff AccountantLinda Norris, Database Administrator
Christina Relacion, Communications ManagerTracey O. Sperry, Director of Development and Research
Anne Sweeney, Chapter CoordinatorMaureen Zuluaga, Database Administrator
History and tax Status: Founded in 1998 by a merger between the United Scleroderma Foundation and the Scleroderma Federation, The Scleroderma Foundation is incorporated in Illinois as a 501(c)(3) nonprofit tax-exempt organization.
Future Options for Scleroderma Therapy
Challenges in Scleroderma TherapyNeed for biomarkersScleroderma is a multisystem, autoimmune, rheumatic disorder with coexisting pathologic processes such as immune system dysregulation and fibroproliferative alterations of the microvasculature (Castro and Jimenez, 2010; Hummers, 2010). The disease is also known for its wide heterogeneity in phenotype and outcome. Owing to the complexity of the disorder and limited knowledge of its etiology, identifying patients at a risk of developing adverse outcomes and determining patient response to current therapies remain a major challenge (Hummers, 2010). The most recent, innovative, and unbiased approaches to biomarker identification are microarray and gene expression analysis, and proteomics. Anti-Scl-70, anti-RNA pol I and III, and anti-nucleolar autoantibodies have already shown some clinical utility as aids for distinguishing diffuse systemic sclerosis (dcSSc) from limited cutaneous systemic sclerosis (lcSSc). Additionally, certain serum and plasma proteins are elevated in SSc, including biomarkers such as von Willebrand factor, adhesion molecules as well as vascular endothelial growth factors associated with vascular injury or activation such as endothelin-1, N-terminal pro-brain natriuretic peptide associated with pulmonary arterial hypertension (PAH) (Pendergrass et al., 2010), KL-6, pulmonary surfactants A and D, and pulmonary and activation regulated chemokine (PARC) associated with pulmonary fibrosis (Castro and Jimenez, 2010).The recent establishment of an online database by the European League Against Rheumatism Scleroderma Trials and Research Group (EUSTAR), which includes clinical data of 8200 patients and recommendations for standardizing the collection, storage, and distribution of SSc biospecimens, may facilitate biomolecular studies for the development of novel biomarkers for targeted therapy (Beyer et al., 2011). Moreover, similar efforts in the United States supported by a National Institutes of Health/National Institute of Arthritis, Musculoskeletal and Skin Disease Award to create National Core Centers (including Proteomic and Microarray Core Laboratories) will provide a platform for rapid advances in identification and validation of new biomarkers. The availability of well-validated biomarkers for SSc could potentially revolutionize both patient care and the identification of new therapeutics. This is particularly important because of the variability of disease progression in patients with SSc and the difficulty in predicting who will develop specific disease complications. Better biomarkers could potentially circumvent these currently vexing difficulties facing treating physicians. Biomarkers might also provide better ways to measure the extent of disease, a key part of assessing patients’ response to medications in clinical or clinical trial settings.Several recent observations have provided new hope for biomarker development in each of these areas, particularly in relation to skin disease. Analysis of gene expression in the skin of SSc patients has shown that the degree of skin involvement in patients can be effectively measured by the degree of increased expression of 4 genes that are known targets of the cytokines transforming growth factor-beta (TGF-β) and interferon (IFN) (Farina et al., 2010). In addition, examining global gene expression in the skin of SSc patients has indicated that patients may be stratified into several different subgroups, possibly responding differently to medications (Milano et al., 2008). Newer diagnostic toolsApart from biomarkers, the future of effective treatment of scleroderma revolves around newer evolved tools for early diagnosis and effective prognostic evaluation. The modified Rodnan skin score (MRSS), which uses subjective,

August 2012 3
semi-quantitative skin scoring, is the most established method for skin assessment in scleroderma patients. A new method that assesses skin health by producing and analyzing surface waves in the skin to determine its viscoelastic properties might prove to be a promising tool in the future (Zhang et al., 2011b). Pulmonary arterial hypertension associated with SSc (PAH-SSc) has been found to have a poorer prognosis than the other forms of PAH (Campo et al., 2010). It has been shown that early therapeutic intervention, at World Health Organization (WHO) Functional Class (FC) II, can significantly improve patient survival (Galie et al., 2008). Evaluation of ventricular mass index (VMI) by magnetic resonance imaging (MRI), which correlates well with the mean pulmonary artery pressure, may play a role in predicting the presence or absence of PAH in patients in whom echocardiographic screening has failed to provide the correct diagnosis (Hagger et al., 2009). High-resolution computed tomography (HRCT) indices of alveolitis and fibrosis may be a new tool for evaluating the relationship between pulmonary involvement and systemic impairment in SSc (Bellia et al., 2009), although this technique has been validated in only a small number of SSc patients till date. Moreover, esophageal dilations visible on an HRCT scan of the chest can aid early diagnosis and specific treatment. Microvascular damage represents the earliest morphological and functional marker of SSc and is clinically mirrored by Raynaud phenomenon (RP). Nailfold videocapillaroscopy (NVC) enables the early differentiation between primary and secondary RP by imaging and identifying morphological patterns specific to various stages of SSc; NVC patterns could increase the sensitivity of the classification criteria for SSc (Cutolo et al., 2010).Newer diagnostic evaluators are being used for not only SSc but also localized scleroderma (LS). The Localized Scleroderma Cutaneous Assessment Tool is a promising skin scoring tool that can differentiate between activity and damage, is sensitive to change, and requires no additional equipment (Fett and Werth, 2011). Multiphoton laser scanning microscopy (MPLSM) can be used for discriminating between sclerodermatous skin and normal skin and can be used for the in vivo diagnosis and monitoring of LS (Lu et al., 2009).
Efficacy of available treatment optionsNone of the available treatment options for scleroderma have proven efficacy in preventing progression of disease, reversing fibrosis, and/or improving long-term outcome (Quillinan and Denton, 2009). A comparative analysis of the latest treatment recommendations by EULAR, EUSTAR, the German Network for Systemic Sclerosis, the European Respiratory Society, and the International Society of Heart and Lung Transplantation concluded that there is a need to clarify the definitive role of a number of new immunosuppressants and the effects of autologous stem cell transplantation in SSc because response to immunosuppressant therapy has been found to be usually weaker in SSc than in other connective tissue disorders
(Opitz et al., 2011). Opitz et al. also indicated that treatment of PAH associated with connective tissue disorders follows the same algorithm as that for idiopathic PAH; however, clinical differences in therapeutic response and outcome are known to exist between PAH-SSc and idiopathic PAH groups (Benza et al., 2010; Condliffe et al., 2009; Mathai and Hassoun, 2009; Zhang et al., 2011a). Owing to inadequate data to back clinical usage, it is not surprising that many of the treatment options used for scleroderma have been found to have limited efficacy in clinical trials. For instance, bosentan, an endothelin receptor antagonist, was recently tested in a randomized clinical trial (RCT) for the treatment of interstitial lung disease (ILD) secondary to SSc without regard to the presence of PAH. However, the authors did not find any improvement in exercise capacity between the 2 groups, thus concluding that there was no data to support the use of endothelin receptor antagonists as therapy for ILD secondary to SSc (Seibold et al., 2010). Moreover, although a number of drugs have been tested in RCTs so far, cyclophosphamide is the only drug that has demonstrated a significantly favorable effect on skin thickening associated with SSc (Tashkin et al., 2006); this shows that the available drug pool for the treatment of skin fibrosis has limited effectiveness.
Side effects of available treatment optionsIt has been observed that the side effects associated with scleroderma therapies often outweigh their effectiveness. For example, UVA-1 phototherapy showed positive short- and long-term efficacy in patients with LS, with a reduction in sclerotic plaques, an increase in skin elasticity, and a reduction in lesional skin thickness (Andres et al., 2010). However, UVA-1 therapy is also linked to short-term side effects such as erythema, pruritus, xerosis cutis, tanning, and recrudescence of herpes simplex infection (Kroft et al., 2008). Similarly, high-dose corticosteroids have side effects that may lead to renal crisis. Hence, they need to be avoided in patients with diffuse SSc, and the dosage should not exceed 15 mg/day (Bussone et al., 2011; Opitz et al., 2011). According to EULAR expert opinion, “low dose of steroids are commonly used for the treatment of inflammatory arthritis in patients with SSc, but its efficacy is not substantiated by RCT” (Kowal-Bielecka et al., 2009). Although cyclophosphamide is the most commonly used therapy for SSc-associated ILD, the potential for side effects means that ideally it might be reserved for patients with progressive disease or disease refractory to other treatment (Yoo, 2010). Patients receiving disease-modifying antirheumatic drugs (DMARDs) such as methotrexate and D-penicillamine also have occasional serious side effects, leading to their discontinuation from treatment regimen (Derk et al., 2008; Kroft et al., 2009). High-dose immunosuppressive therapy with autologous hematopoietic cell transplantation, currently under investigation in both Europe and the US, may provide the greatest hope for patients with severe progressive scleroderma and its complications. Although found to be associated with high mortality in early studies, more recent experience suggests that most patients can be treated safely with this therapy (Nash et al., 2007).

4 Scleroderma Spectrum
Novel Concepts and Target Sites Drugs affecting signaling pathways The major profibrotic signaling pathways in SSc are initiated by TGF-β, platelet-derived growth factor (PDGF), endothelin-1 (ET-1), and connective tissue growth factor (CCN2/CTGF), which function through myofibroblast differentiation and pericyte recruitment in lesional SSc fibroblasts (Leask, 2010). The signaling cascades of the cytokines TGF-β and PDGF utilize tyrosine kinases, and recent studies suggested that tyrosine kinase inhibitors like imatinib, dasatinib, and nilotinib lead to decreased production of extracellular matrix proteins (Distler and Distler, 2010; Gordon and Spiera, 2010). However, an assessment of the safety, tolerability, and effectiveness of imatinib in the treatment of PAH in a randomized, double-blind, placebo-controlled study showed that there was no significant change in 6-min walk distance (6MWD) between the 2 groups. In addition, serious adverse events occurred in 11 imatinib recipients (39%) compared to 7 placebo recipients (23%). Therefore, the effectiveness of imatinib in the treatment of PAH is questionable (Ghofrani et al., 2010). Currently, an open label study of a monoclonal antibody blocking TGF-β (GC1008/fresolimumab) is underway, possibly leading to further development of this currently unapproved drug for scleroderma and its complications. CCN2 may be another important target, as a key downstream mediator of TGF-β. In a recent study, loss of CCN2 resulted in resistance to bleomycin-induced skin fibrosis in mice, indicating that drugs blocking CCN2 in vivo might add to the treatment armamentarium of scleroderma (Liu et al., 2011). Another signaling molecule implicated in scleroderma is Rho-associated kinase (Rock), which is the major cellular mediator of Rho GTPase and plays an important role in the organiza-tion of actin cytoskeleton. Rock inhibition is known to benefit vascular disease and a Rock inhibitor, fasudil, is presently being investigated in a clinical trial to test its efficacy and safety in the treatment of RP (ClinicalTrials.gov identifier: NCT00498615). Rock has also been found to stimulate the differentiation of resting fibroblasts into myofibroblasts; hence, Rock inhibitors might prove to be effective drugs for SSc-associated fibrosis (Akhmetshina et al., 2008).In comparison to healthy skin fibroblasts, dermal fibroblasts cultured from lesional areas of SSc patients have been shown to possess greater activity levels of Rac, another Rho GTPase. NSC23766, a Rac inhibitor, was shown to suppress the persistent fibrotic phenotype of lesional SSc fibroblasts (Liu et al., 2008; Xu et al., 2009). NSC23766 also blocked the elevated levels of Akt phosphorylation in SSc fibroblasts. Akt is a serine/threonine kinase that has been recently implicated in collagen regulation (Bujor et al., 2008). It has also been shown that inhibition of Akt upregulates basal matrix metalloproteinase 1 (MMP1) production and reverses the inhibitory effect of TGF-β on MMP1 gene expression; hence, Akt might be a potential therapeutic target. The expression and function of peroxisome proliferator-activated receptor-gamma (Ppar-γ), a nuclear orphan receptor that may have a role in TGF-β-dependent fibrogenesis, have been found to be impaired in SSc. Therefore, Ppar-γ agonists could potentially be used as therapy (Wei et al., 2010; Wu et al., 2009).
A pivotal role of the ADAM-17/Notch pathway in SSc following activation by reactive oxygen species has been elucidated, indicating that this pathway may represent a new treatment target for SSc (Kavian et al., 2010) as well. Gene targetsInvestigations to understand the gene regulation cascade in the pathogenesis of SSc revealed that in response to TGF-β, the friend leukemia integration-1 (Fli-1) gene is repressed through a series of post-translational modifications in which the tyrosine kinase c-Abl acts as an upstream regulator of the profibrotic protein kinase C-δ (PKC-δ)/phosphorylated-Fli-1 (P Fli1) pathway. Therefore, blocking the TGF-β/c-abl/PKC-δ/P-Fli1 pathway could be an attractive alternative approach for scleroderma therapy (Bujor et al., 2011). Rottlerin, a PKC-δ inhibitor, may also be effective as a therapeutic option (Li and Jimenez, 2011). Apart from Fli-1, Egr-1 is another transcription factor that has been shown to play an important role in physiologic and pathological connective tissue remodeling (Bhattacharyya et al., 2011). Egr-1-null mice are protected from fibrosis, and Egr-1 appears to be a promising putative target for the development of anti-fibrotic therapy. Immune modulatorsImmune modulators such as belimumab (approved by the US Food and Drug Administration [FDA] for systemic lupus erythematosus), which is a monoclonal antibody targeting the B-lymphocyte stimulator protein BLyS; abatacept, which inhibits the costimulation of T cells (In clinical trial, ClinicalTrials.gov identifier: NCT00442611); and peptide human TGF-β 1 type III receptor (p144) blocker (In clinical trial, ClinicalTrials.gov identifier: NCT00574613) are currently under investigation in clinical trials for alleviation of skin fibrosis. Miscellaneous drugs/drug targetsA few other drug targets and potential therapy options include the following: oral collagen I for relieving skin fibrosis (In clinical trial. ClinicalTrials.gov identifier: NCT00005675); caveolin-1 which at reduced levels in lung fibroblasts promote collagen overexpression and lung fibrosis through stimulation of TGF-β signaling (Del Galdo et al., 2008a; Del Galdo et al., 2008b; Tourkina et al., 2010); tumor necrosis factor-related weak inducer of apoptosis (TWEAK) which when elevated could be a protective factor against the development of pulmonary fibrosis (Yanaba et al., 2009); serum amyloid P (SAP) which has been shown to reduce bleomycin-induced pulmonary fibrosis in rats (Pilling et al., 2007); taurine, an antioxidant which inhibits the production of proinflammatory cytokines such as interleukin (IL)-1 and IL-6 along with the production of TGF-β (a major fibrogenic cytokine involved in scleroderma) (Fallahzadeh et al., 2010); microRNA-29 which when down regulated in SSc fibroblasts increased the levels of profibrotic genes (Maurer et al., 2010); synthetic cannabinoid receptor agonist WIN55,212-2, which prevented skin fibrosis in a bleomycin-induced mouse model of scleroderma (Balistreri et al., 2011); and retinoic acid, which can modulate connective tissue metabolism and exhibit anti-fibrotic activity, thereby improving the clinical symptoms of SSc (Xiao et al., 2011). N-acetylcysteine can ameliorate vascular renal function in patients with low disease severity (Rosato et al., 2009).

August 2012 5
A proliferation-inducing ligand (APRIL, a member of the tumor necrosis factor family), which plays an important role in the survival of peripheral B cells, may contribute to the pathogenesis of SSc through the upregulation of autoantibody production and maintenance of autoimmune phenomena (Bielecki et al., 2010).
Monotherapy versus combination therapyDespite absence of firm evidence from RCTs, combination therapies are often used for patients deteriorating on monotherapy (Keogh et al., 2011). Since a number of cellular pathways are implicated in SSc, many clinicians consider that simultaneous blockade of these pathways might produce better and longer-lasting results (Ramos-Casals et al., 2010). A recent (January 2005 to August 2009) Australian collaborative report on 112 patients with WHO-FC II-IV PAH who were deteriorating on monotherapy and therefore received a non-parenteral combination therapy, which included bosentan, or sitaxsentan, or ambrisentan, or iloprost, or sildenafil, showed that survival with dual therapy in patients with idiopathic PAH/familial PAH was 93% at 1 year and 79% at 2 years, and in patients with PAH-SSc, survival was 72% at 1 year and 48% at 2 years. In survivors, dual therapy reversed deterioration in FC, from 3.1 ± 0.6 on monotherapy to 2.2 ± 0.6 at 12 months. Similarly, dual therapy improved 6MWD, and sequential echocardiography demonstrated a fall in pulmonary artery systolic pressure and improved right ventricular function (Keogh et al., 2011). Commonly used dual therapy include prostacyclin analogs plus endothelin antagonists or phosphodiesterase inhibitors (McLaughlin et al., 2010; McLaughlin et al., 2006; Simonneau et al., 2008).
Comorbid hyperlipidemiaMild dyslipidemia and a high atherogenic ratio (LDL-C/HDL-C) found in SSc patients has led to hyperlipidemia being a risk factor for hemodynamic disturbances in these patients (Kozlova et al., 2001; Tsifetaki et al., 2010). Therefore, lipid profiling (especially measurement of lipoprotein[a]) in scleroderma patients has been suggested (Lippi et al., 2006). HMG-CoA reductase inhibitors (or statins), which lower cholesterol levels, were found to aid in treating RP and digital ulcers in SSc patients by retarding vascular injury (Abou-Raya et al., 2008). The lysophospholipids lysophosphatidic acid and sphingosine 1-phosphate levels are elevated in the sera of SSc patients and may be targeted in SSc therapy (Pattanaik and Postlethwaite, 2010; Tokumura et al., 2009).
ConclusionThe plethora of drug targets and treatment options being tested for scleroderma raise hope for more effective, targeted therapies to emerge in the near future. Furthermore, as most of the molecular targets for drug development are still being tested in cell lines and bleomycin-induced mouse models, there is an urgent need to translate the results obtained at the bench to the clinic. There is also a need to discover newer drugs for known target sites and also newer disease-specific target sites for efficacious drug development with minimal side effects.
References• Abou-Raya, A., Abou-Raya, S., and Helmii, M. (2008). Statins: potentially useful in therapy of systemic sclerosis-related Raynaud’s phenomenon and digital ulcers. J Rheumatol 35, 1801-1808.
• Akhmetshina, A., Dees, C., Pileckyte, M., Szucs, G., Spriewald, B.M., Zwerina, J., Distler, O., Schett, G., and Distler, J.H. (2008). Rho-associated kinases are crucial for myofibroblast differentiation and production of extracellular matrix in scleroderma fibroblasts. Arthritis Rheum 58, 2553-2564.
• Andres, C., Kollmar, A., Mempel, M., Hein, R., Ring, J., and Eberlein, B. (2010). Successful ultraviolet A1 phototherapy in the treatment of localized scleroderma: a retrospective and prospective study. Br J Dermatol 162, 445-447.
• Balistreri, E., Garcia-Gonzalez, E., Selvi, E., Akhmetshina, A., Palumbo, K., Lorenzini, S., Maggio, R., Lucattelli, M., Galeazzi, M., and Distler, J.W. (2011). The cannabinoid WIN55, 212-2 abrogates dermal fibrosis in scleroderma bleomycin model. Ann Rheum Dis 70, 695-699.
• Bellia, M., Cannizzaro, F., Scichilone, N., Riili, M., Triolo, G., Midiri, M., and Lagalla, R. (2009). HRCT and scleroderma: semiquantitative evaluation of lung damage and functional abnormalities. Radiol Med 114, 190-203.
• Benza, R.L., Miller, D.P., Gomberg-Maitland, M., Frantz, R.P., Foreman, A.J., Coffey, C.S., Frost, A., Barst, R.J., Badesch, D.B., Elliott, C.G., et al. (2010). Predicting survival in pulmonary arterial hypertension: insights from the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL). Circulation 122, 164-172.
• Beyer, C., Distler, J.H., Allanore, Y., Aringer, M., Avouac, J., Czirjak, L., Cutolo, M., Damjanov, N., Del Galdo, F., Fligelstone, K., et al. (2011). EUSTAR biobanking: recommendations for the collection, storage and distribution of biospecimens in scleroderma research. Ann Rheum Dis 70, 1178-1182.
• Bhattacharyya, S., Wu, M., Fang, F., Tourtellotte, W., Feghali-Bostwick, C., and Varga, J. (2011). Early growth response transcription factors: Key mediators of fibrosis and novel targets for anti-fibrotic therapy. Matrix Biol. doi:10.1016/j.matbio.2011.03.005.
• Bielecki, M., Kowal, K., Lapinska, A., Bernatowicz, P., Chyczewski, L., and Kowal-Bielecka, O. (2010). Increased production of a proliferation-inducing ligand (APRIL) by peripheral blood mononuclear cells is associated with antitopoisomerase I antibody and more severe disease in systemic sclerosis. J Rheumatol 37, 2286-2289.
• Branley, H.M., du Bois, R.M., Wells, A.U., and Jones, H.A. (2008). PET scanning of macrophages in patients with scleroderma fibrosing alveolitis. Nucl Med Biol 35, 901-909.
• Bujor, A.M., Asano, Y., Haines, P., Lafyatis, R., and Trojanowska, M. (2011). The c-Abl tyrosine kinase controls protein kinase Cdelta-induced Fli-1 phosphorylation in human dermal fibroblasts. Arthritis Rheum 63, 1729-1737.
• Bujor, A.M., Pannu, J., Bu, S., Smith, E.A., Muise-Helmericks, R.C., and Trojanowska, M. (2008). Akt blockade downregulates collagen and upregulates MMP1 in human dermal fibroblasts. J Invest Dermatol 128, 1906-1914.
• Bussone, G., Noel, L.H., and Mouthon, L. (2011). [Renal involvement in patients with systemic sclerosis.]. Nephrol Ther. doi:10.1016/j.nephro.2011.03.006.
• Campo, A., Mathai, S.C., Le Pavec, J., Zaiman, A.L., Hummers, L.K., Boyce, D., Housten, T., Champion, H.C., Lechtzin, N., Wigley, F.M., et al. (2010). Hemodynamic predictors of survival in scleroderma-related pulmonary arterial hypertension. Am J Respir Crit Care Med 182, 252-260.
• Castro, S.V., and Jimenez, S.A. (2010). Biomarkers in systemic sclerosis. Biomark Med 4, 133-147.
• Condliffe, R., Kiely, D.G., Peacock, A.J., Corris, P.A., Gibbs, J.S., Vrapi, F., Das, C., Elliot, C.A., Johnson, M., DeSoyza, J., et al. (2009). Connective tissue disease-associated pulmonary arterial hypertension in the modern treatment era. Am J Respir Crit Care Med 179, 151-157.
• Cutolo, M., Sulli, A., and Smith, V. (2010). Assessing microvascular changes in systemic sclerosis diagnosis and management. Nat Rev Rheumatol 6, 578-587.
• Del Galdo, F., Lisanti, M.P., and Jimenez, S.A. (2008a). Caveolin-1, transforming growth factor-beta receptor internalization, and the pathogenesis of systemic sclerosis. Curr Opin Rheumatol 20, 713-719.
• Del Galdo, F., Sotgia, F., de Almeida, C.J., Jasmin, J.F., Musick, M., Lisanti, M.P., and Jimenez, S.A. (2008b). Decreased expression of caveolin 1 in patients with systemic sclerosis: crucial role in the pathogenesis of tissue fibrosis. Arthritis Rheum 58, 2854-2865.
• Derk, C.T., Huaman, G., and Jimenez, S.A. (2008). A retrospective randomly selected cohort study of D-penicillamine treatment in rapidly progressive diffuse cutaneous systemic sclerosis of recent onset. Br J Dermatol 158, 1063-1068.
• Distler, J.H., and Distler, O. (2010). Tyrosine kinase inhibitors for the treatment of fibrotic diseases such as systemic sclerosis: towards molecular targeted therapies. Ann Rheum Dis 69 Suppl 1, i48-51.
• Fallahzadeh, M.K., Namazi, M.R., and Gupta, R.C. (2010). Taurine: a potential novel addition to the anti-systemic sclerosis weaponry. Arch Med Res 41, 59-61.
• Farina, G., Lafyatis, D., Lemaire, R., and Lafyatis, R. (2010). A four-gene biomarker predicts skin disease in patients with diffuse cutaneous systemic sclerosis. Arthritis Rheum 62, 580-588.
• Fett, N., and Werth, V.P. (2011). Update on morphea: part II. Outcome measures and treatment. J Am Acad Dermatol 64, 231-242; quiz 243-234.
(continued)

6 Scleroderma Spectrum
• Galie, N., Rubin, L., Hoeper, M., Jansa, P., Al-Hiti, H., Meyer, G., Chiossi, E., Kusic-Pajic, A., and Simonneau, G. (2008). Treatment of patients with mildly symptomatic pulmonary arterial hypertension with bosentan (EARLY study): a double-blind, randomised controlled trial. Lancet 371, 2093-2100.
• Ghofrani, H.A., Morrell, N.W., Hoeper, M.M., Olschewski, H., Peacock, A.J., Barst, R.J., Shapiro, S., Golpon, H., Toshner, M., Grimminger, F., et al. (2010). Imatinib in pulmonary arterial hypertension patients with inadequate response to established therapy. Am J Respir Crit Care Med 182, 1171-1177.
• Gordon, J.K., and Spiera, R.F. (2010). Targeting tyrosine kinases: a novel therapeutic strategy for systemic sclerosis. Curr Opin Rheumatol 22, 690-695.
• Hachulla, E., and Denton, C.P. (2010). Early intervention in pulmonary arterial hypertension associated with systemic sclerosis: an essential component of disease management. Eur Respir Rev 19, 314-320.
• Hagger, D., Condliffe, R., Woodhouse, N., Elliot, C.A., Armstrong, I.J., Davies, C., Hill, C., Akil, M., Wild, J.M., and Kiely, D.G. (2009). Ventricular mass index correlates with pulmonary artery pressure and predicts survival in suspected systemic sclerosis-associated pulmonary arterial hypertension. Rheumatology (Oxford) 48, 1137-1142.
• Hummers, L.K. (2010). The current state of biomarkers in systemic sclerosis. Curr Rheumatol Rep 12, 34-39.
• Kavian, N., Servettaz, A., Mongaret, C., Wang, A., Nicco, C., Chereau, C., Grange, P., Vuiblet, V., Birembaut, P., Diebold, M.D., et al. (2010). Targeting ADAM-17/notch signaling abrogates the development of systemic sclerosis in a murine model. Arthritis Rheum 62, 3477-3487.
• Keogh, A., Strange, G., Kotlyar, E., Williams, T., Kilpatrick, D., Macdonald, P., Brown, K., Pidoux, A., Kermeen, F., Steele, P., et al. (2011). Survival after the initiation of combination therapy in patients with pulmonary arterial hypertension: an Australian collaborative report. Intern Med J 41, 235-244.
• Kowal-Bielecka, O., Landewe, R., Avouac, J., Chwiesko, S., Miniati, I., Czirjak, L., Clements, P., Denton, C., Farge, D., Fligelstone, K., et al. (2009). EULAR recommendations for the treatment of systemic sclerosis: a report from the EULAR Scleroderma Trials and Research group (EUSTAR). Ann Rheum Dis 68, 620-628.
• Kozlova, L.K., Tamgina, T.F., Nuzhdina, T.V., Antonenko, T.V., and Alekhina, E.M. (2001). [Echocardiographic functional assessment of the heart and lipid metabolism in patients with systemic scleroderma and systemic lupus erythematosus]. Ter Arkh 73, 33-36.
• Kroft, E.B., Berkhof, N.J., van de Kerkhof, P.C., Gerritsen, R.M., and de Jong, E.M. (2008). Ultraviolet A phototherapy for sclerotic skin diseases: a systematic review. J Am Acad Dermatol 59, 1017-1030.
• Kroft, E.B., Creemers, M.C., van den Hoogen, F.H., Boezeman, J.B., and de Jong, E.M. (2009). Effectiveness, side-effects and period of remission after treatment with methotrexate in localized scleroderma and related sclerotic skin diseases: an inception cohort study. Br J Dermatol 160, 1075-1082.
• Leask, A. (2010). Towards an anti-fibrotic therapy for scleroderma: targeting myofibroblast differentiation and recruitment. Fibrogenesis Tissue Repair 3, 8.
• Li, Z., and Jimenez, S.A. (2011). Protein kinase C delta and the c-Abl kinase are required for transforming growth factor-beta induction of endothelial-mesenchymal transition in vitro. Arthritis Rheum. doi:10.1002/art.30317.
• Lippi, G., Caramaschi, P., Montagnana, M., Salvagno, G.L., Volpe, A., and Guidi, G. (2006). Lipoprotein[a] and the lipid profile in patients with systemic sclerosis. Clin Chim Acta 364, 345-348.
• Liu, S., Kapoor, M., Shi-wen, X., Kennedy, L., Denton, C.P., Glogauer, M., Abraham, D.J., and Leask, A. (2008). Role of Rac1 in a bleomycin-induced scleroderma model using fibroblast-specific Rac1-knockout mice. Arthritis Rheum 58, 2189-2195.
• Liu, S., Shi-wen, X., Abraham, D.J., and Leask, A. (2011). CCN2 is required for bleomycin-induced skin fibrosis in mice. Arthritis Rheum 63, 239-246.
• Lu, K., Chen, J., Zhuo, S., Zheng, L., Jiang, X., Zhu, X., and Zhao, J. (2009). Multiphoton laser scanning microscopy of localized scleroderma. Skin Res Technol 15, 489-495.
• Mathai, S.C., and Hassoun, P.M. (2009). Therapy for pulmonary arterial hypertension associated with systemic sclerosis. Curr Opin Rheumatol 21, 642-648.
• Maurer, B., Stanczyk, J., Jungel, A., Akhmetshina, A., Trenkmann, M., Brock, M., Kowal-Bielecka, O., Gay, R.E., Michel, B.A., Distler, J.H., et al. (2010). MicroRNA-29, a key regulator of collagen expression in systemic sclerosis. Arthritis Rheum 62, 1733-1743.
• McLaughlin, V.V., Benza, R.L., Rubin, L.J., Channick, R.N., Voswinckel, R., Tapson, V.F., Robbins, I.M., Olschewski, H., Rubenfire, M., and Seeger, W. (2010). Addition of inhaled treprostinil to oral therapy for pulmonary arterial hypertension: a randomized controlled clinical trial. J Am Coll Cardiol 55, 1915-1922.
• McLaughlin, V.V., Oudiz, R.J., Frost, A., Tapson, V.F., Murali, S., Channick, R.N., Badesch, D.B., Barst, R.J., Hsu, H.H., and Rubin, L.J. (2006). Randomized study of adding inhaled iloprost to existing bosentan in pulmonary arterial hypertension. Am J Respir Crit Care Med 174, 1257-1263.
• Milano, A., Pendergrass, S.A., Sargent, J.L., George, L.K., McCalmont, T.H., Connolly, M.K., and Whitfield, M.L. (2008). Molecular subsets in the gene expression signatures of scleroderma skin. PLoS One 3, e2696.
• Nash, R.A., McSweeney, P.A., Crofford, L.J., Abidi, M., Chen, C.S., Godwin, J.D., Gooley, T.A., Holmberg, L., Henstorf, G., LeMaistre, C.F., et al. (2007). High-dose immunosuppressive therapy and autologous hematopoietic cell transplantation for severe
systemic sclerosis: long-term follow-up of the US multicenter pilot study. Blood 110, 1388-1396.
• Opitz, C., Klein-Weigel, P.F., and Riemekasten, G. (2011). Systemic sclerosis - a systematic overview: part 2 - immunosuppression, treatment of SSc-associated vasculopathy, and treatment of pulmonary arterial hypertension. Vasa 40, 20-30.
• Pattanaik, D., and Postlethwaite, A.E. (2010). A role for lysophosphatidic acid and sphingosine 1-phosphate in the pathogenesis of systemic sclerosis. Discov Med 10, 161-167.
• Pendergrass, S.A., Hayes, E., Farina, G., Lemaire, R., Farber, H.W., Whitfield, M.L., and Lafyatis, R. (2010). Limited systemic sclerosis patients with pulmonary arterial hypertension show biomarkers of inflammation and vascular injury. PLoS One 5, e12106.
• Pilling, D., Roife, D., Wang, M., Ronkainen, S.D., Crawford, J.R., Travis, E.L., and Gomer, R.H. (2007). Reduction of bleomycin-induced pulmonary fibrosis by serum amyloid P. J Immunol 179, 4035-4044.
• Quillinan, N.P., and Denton, C.P. (2009). Disease-modifying treatment in systemic sclerosis: current status. Curr Opin Rheumatol 21, 636-641.
• Ramos-Casals, M., Fonollosa-Pla, V., Brito-Zeron, P., and Siso-Almirall, A. (2010). Targeted therapy for systemic sclerosis: how close are we? Nat Rev Rheumatol 6, 269-278.
• Rosato, E., Zardi, E.M., Barbano, B., Menghi, G., Cianci, R., Amoroso, A., Afeltra, A., Pisarri, S., and Salsano, F. (2009). N-acetylcysteine infusion improves hepatic perfusion in the early stages of systemic sclerosis. Int J Immunopathol Pharmacol 22, 763-772.
• Seibold, J.R., Denton, C.P., Furst, D.E., Guillevin, L., Rubin, L.J., Wells, A., Matucci Cerinic, M., Riemekasten, G., Emery, P., Chadha-Boreham, H., et al. (2010). Randomized, prospective, placebo-controlled trial of bosentan in interstitial lung disease secondary to systemic sclerosis. Arthritis Rheum 62, 2101-2108.
• Simonneau, G., Rubin, L.J., Galie, N., Barst, R.J., Fleming, T.R., Frost, A.E., Engel, P.J., Kramer, M.R., Burgess, G., Collings, L., et al. (2008). Addition of sildenafil to long-term intravenous epoprostenol therapy in patients with pulmonary arterial hypertension: a randomized trial. Ann Intern Med 149, 521-530.
• Tashkin, D.P., Elashoff, R., Clements, P.J., Goldin, J., Roth, M.D., Furst, D.E., Arriola, E., Silver, R., Strange, C., Bolster, M., et al. (2006). Cyclophosphamide versus placebo in scleroderma lung disease. N Engl J Med 354, 2655-2666.
• Tokumura, A., Carbone, L.D., Yoshioka, Y., Morishige, J., Kikuchi, M., Postlethwaite, A., and Watsky, M.A. (2009). Elevated serum levels of arachidonoyl-lysophosphatidic acid and sphingosine 1-phosphate in systemic sclerosis. Int J Med Sci 6, 168-176.
• Tourkina, E., Richard, M., Oates, J., Hofbauer, A., Bonner, M., Gooz, P., Visconti, R., Zhang, J., Znoyko, S., Hatfield, C.M., et al. (2010). Caveolin-1 regulates leucocyte behaviour in fibrotic lung disease. Ann Rheum Dis 69, 1220-1226.
• Tsifetaki, N., Georgiadis, A.N., Alamanos, Y., Fanis, S., Argyropoulou, M.I., and Drosos, A.A. (2010). Subclinical atherosclerosis in scleroderma patients. Scand J Rheumatol 39, 326-329.
• Wei, J., Ghosh, A.K., Sargent, J.L., Komura, K., Wu, M., Huang, Q.Q., Jain, M., Whitfield, M.L., Feghali-Bostwick, C., and Varga, J. (2010). PPARgamma downregulation by TGFss in fibroblast and impaired expression and function in systemic sclerosis: a novel mechanism for progressive fibrogenesis. PLoS One 5, e13778.
• Wu, M., Melichian, D.S., Chang, E., Warner-Blankenship, M., Ghosh, A.K., and Varga, J. (2009). Rosiglitazone abrogates bleomycin-induced scleroderma and blocks profibrotic responses through peroxisome proliferator-activated receptor-gamma. Am J Pathol 174, 519-533.
• Xiao, R., Yoshida, N., Higashi, Y., Lu, Q.J., Fukushige, T., Kanzaki, T., and Kanekura, T. (2011). Retinoic acids exhibit anti-fibrotic activity through the inhibition of 5-lipoxygenase expression in scleroderma fibroblasts. J Dermatol 38, 345-353.
• Xu, S.W., Liu, S., Eastwood, M., Sonnylal, S., Denton, C.P., Abraham, D.J., and Leask, A. (2009). Rac inhibition reverses the phenotype of fibrotic fibroblasts. PLoS One 4, e7438.
• Yanaba, K., Yoshizaki, A., Muroi, E., Hara, T., Ogawa, F., Usui, A., Hasegawa, M., Fujimoto, M., Takehara, K., and Sato, S. (2009). Elevated circulating TWEAK levels in systemic sclerosis: association with lower frequency of pulmonary fibrosis. J Rheumatol 36, 1657-1662.
• Yoo, W.H. (2010). Successful treatment of steroid and cyclophosphamide-resistant diffuse scleroderma-associated interstitial lung disease with rituximab. Rheumatol Int. doi:10.1007/s00296-009-1347-z.
• Zhang, R., Dai, L.Z., Xie, W.P., Yu, Z.X., Wu, B.X., Pan, L., Yuan, P., Jiang, X., He, J., Humbert, M., et al. (2011a). Survival of Chinese patients with pulmonary arterial hypertension in the modern treatment era. Chest 140, 301-309.
• Zhang, X., Osborn, T.G., Pittelkow, M.R., Qiang, B., Kinnick, R.R., and Greenleaf, J.F. (2011b). Quantitative assessment of scleroderma by surface wave technique. Med Eng Phys 33, 31-37. doi:10.1016/j.medengphy.2010.08.016.
This is a publication of the Scleroderma Foundation and is made possible by unrestricted grants from
Pfizer, Inc. and Actelion Pharmaceuticals US.

August 2012 7

Scleroderma Foundation300 Rosewood Drive
Suite 105Danvers, MA 01923
800-722-HOPEwww.scleroderma.org
www.echossc.orgwww.facebook.com/sclerodermaUS
www.twitter.com/scleroderma
300 Rosewood DriveSuite 105Danvers, MA 01923
ADDRESS SERVICE REQUESTED
NON PROFIT ORG.U.S. POSTAGE
PAIDGALLERY