REGULATION OF BLOOD pH: REVISITING THE “LACTATE ...
-
Upload
nguyencong -
Category
Documents
-
view
216 -
download
2
Transcript of REGULATION OF BLOOD pH: REVISITING THE “LACTATE ...

REGULATION OF BLOOD pH: REVISITING THE “LACTATE PARADOX””
Claudio Marconi
IBFM-Sect. of Muscle Physiology and Proteome, National Research Council, Milano, Italy;

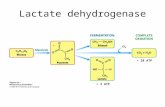
[ADP] oxidative phosphorilation
PCr + ADP + H+ ATP + Cr
LOHMANN REACTION
In normoxia, the transition from rest to exercise is characterized by rapid changes in muscle and blood [H+]
CK

0
10
20
30
40
50G
lyco
lytic
AT
P su
pply
, %
0
10
20
30
40
50G
lyco
lytic
AT
P su
pply
, %
PERCENTAGE OF ATP SUPPLY FROM GLYCOLYSIS (LACTATE GENERATION UNDER AEROBIC CONDITIONS)
wrist flexor tibialis ant quadriceps tailshaker
rattlesnake
ATP by an excess of pyruvate supply
ATP by glycogen shunt

The total metabolic proton production increases with time as the rate of PCrbreakdown declines and anaerobic glycolysis plays a progressively greater role.
In hypoxia, the mechanism involved in proton generation are the same as in normoxia, but anaerobic glycolysis appears to be regulated differently.

CO2 HYDRATION
CO2 + H2O [H2CO3] H+ + HCO3-
Henderson-Hasselbalch
pH = pK + log[HCO3
-]0.03 PCO2.
pK = 6.12

normal buffer line
HYPERVENTILATION PCO 2= 20
torr
PCO 2= 40 to
rr
urinary excretion of HCO3-
N
acute hypoxia
incomplete accl.
complete accl.
Cl reabsorption-HCO3 excretion-
[HCO3]p(mM)
-25
20
15
7.3 7.4 7.5 7.6 7.7 pH

BUFFER POWER IN BLOOD
β = Δ [H+]/ΔpH
-plasma proteins -19% (due to Ht >)-hemoglobin +20% -phosphate ions =-2,3 DPG +97% (due to Hb >, DPG/Hb>)
TOTAL NON BICARBONATES +35%
At 5,000 mβ

0
100
200
300
Buffer power, mM H+per unit pH change
0 2000 4000 6000
Altitude, m
total
Hb
2,3-DPG
plasma proteins
inorganic phosphates
TOTAL NON-BICARBONATE BUFFER

INCREASING NON-BICARBONATE BUFFER POWER HAS TWO CONTRASTING EFFECTS:
The pH shift secondary to a given change in the acid-base balance is less
The amount of acid or base needed to compensate that change is increased
The two corrections may not be equivalent

EVALUATION OF THE EFFECTS OF AN INCREASED NON-BICARBONATE
BUFFER POWER ON THE BICARBONATE β

0
10
20
30
40
50
60
70Bicarbonates, mM
6.9 7.0 7.1 7.2 7.3 7.4 7.5 7.6 7.7 7.8 7.9
pH
β=252.1
β=186.7
Start a
bb'
c
PCO2=40 mmHg
PCO2=21 mmHg (at 5,000 m)
Uncompensated
Fullycompensated
Increased non-bicarbonate β at altitude contributes to decrease alkalosis derived from hyperventilation
Metabolic acidosis

As the main producer and consumer of large amounts of lactic acid,
muscle plays a key role in the body acid-base balance
• proteins (imidazol groups of histidine residues)• inorganic phosphates and bicarbonate
Muscle buffer capacity depends on theconcentration of:
During heavy exercise lactic acid accounts for 85-95% of the total H+ load

Muscle buffer capacity ranges from 25 to 40 mmol H+· l-1 H2O · pH-1
and depends on: type, intensity, time course of muscle activity, and training.
• 8 weeks of sprint training increases β by 38% (likely as a consequence of increased levels of dipeptides such as carnosine, which is mainly present in white muscle)
• Endurance training has no effect on β• Altitude acclimatization alters β, mainly because of
changes in muscle protein concentration.

Regulation of muscle pH at rest
• At rest pH ranges from 7.10 to 7.15• It depends on the balance between:
H+ metabolic production and H+ inflow, Transport of H+ out (Na+/H+ exchange) or HCO3
- in (HCO3- /Cl- exchange)

-180 -60 60 180 300 420 540 6600
1
2
3
Time (sec)
VO2 (
L/m
in)
Regulation of muscle pH at exercise
ATP + CrPCr + ADP + H+CK
CO2 and HCO3-
Lactic acid

05
1015202530
-5 0 5 10 15
6,66,76,86,97,07,17,2
-5 0 5 10 15
Rest Ex
(min)
pH
[La]mmol·kg-1
Recovery
05
1015202530
-5 0 5 10 15
6,66,76,86,97,07,17,2
-5 0 5 10 15
Rest Ex
(min)
pH
[La]mmol·kg-1
Recovery
Quadriceps muscle

Schematic model of pH regulation
a: prevailing mechanism in moderate exercise (it may be responsible for the greater accumulation of H+ than La- in the extracellular space after short bouts of supramaximal exercise)b: prevailing mechanisms in maximal exercise
H+CO2 + H2OHCO3
-
Lactic acid Lactate
Fuels Metabolism Intracellular buffers
Passive fluxes Transport systems
La-
H+
Na+
H+a
Na+
HCO3-
Cl-H+
bb MCT

ACID-BALANCE DURINGSUPRAMAXIMAL EXERCISE
[Lab] = 25 mMpHb < 7.0HCO3
- buffers ~75% of protonsNon-bicarbonate buffer system plays an increasing role for [Lab] > 15 mM
[Lam] = 40 mM pHm = 6.6

MCT : a family of monocarboxylate La-/H+ transporters
• MCT are membrane proteins and are pH sensitive• MCT1 is more expressed in slow-twitch fibers
(they need a fast MCT transport because theyproduce La- for long time periods) and myocardial fibers than in fast-twitch fibers.
• MCT4 is not correlated to fiber type• It is suggested that MCT1 is specialized for
uptake, whereas MCT4 is specialized for efflux.
MCT and HYPOXIAThere are relatively few data supporting a role of MCT in regulating the acid-base balance in hypoxia.

ACID-BASE BALANCE AT EXERCISE DURING ALTITUDE ACCLIMATIZATION

pHa in acclimatized lowlanders:literature survey
7.3
7.4
7.5
7.6
7.7
7.8
0 2000 4000 6000 8000 10000
Altitude, m
Cerretelli unpublishedLahiri 1967Winslow 1984Winslow estimatedSutton 1988, chamberBender 1989Kayser 1993
pHa in acclimatized lowlanders:literature survey
7.3
7.4
7.5
7.6
7.7
7.8
0 2000 4000 6000 8000 10000
Altitude, m
Cerretelli unpublishedLahiri 1967Winslow 1984Winslow estimatedSutton 1988, chamberBender 1989Kayser 1993
7.3
7.4
7.5
7.6
7.7
7.8
0 2000 4000 6000 8000 10000
Altitude, m
Cerretelli unpublishedLahiri 1967Winslow 1984Winslow estimatedSutton 1988, chamberBender 1989Kayser 1993

Samaja et al Acta Physiol. Scand. 1997 * P<0.05 from Caucasians
7.40
7.45
7.50
7.55pHa
3400 5050 6450
Caucasiansafter 3 weeks
SherpasCaucasians
0
10
20
30
40PaCO 2, mmHg
3400 5050 6450
* * * * **
0102030405060
PaO2, mmHg
3400 5050 6450
Altitude, m
Samaja et al Acta Physiol. Scand. 1997 * P<0.05 from Caucasians
7.40
7.45
7.50
7.55pHa
3400 5050 6450
Caucasiansafter 3 weeks
SherpasCaucasians
0
10
20
30
40PaCO 2, mmHg
3400 5050 6450
* * * * **
0102030405060
PaO2, mmHg
3400 5050 6450
Altitude, m

Hb-O2 equilibrium curve
PO2 , mmHg
SO2
, %
0
20
40
60
80
100
0 25 50 75 100
pH 7.4PCO2=40 mmHg[2,3-DPG]/[Hb]=0.8
P50
H+
CO22,3-DPG
Hb-O2 equilibrium curve
PO2 , mmHg
SO2
, %
0
20
40
60
80
100
0 25 50 75 100
pH 7.4PCO2=40 mmHg[2,3-DPG]/[Hb]=0.8
P50
H+
CO22,3-DPG

Blood-O2 equilibrium curve
0%
25%
50%
75%
100%
0 20 40 60 80
Hb-
O2
satu
ratio
n, o
r SO
2
PO2 , mmHg
pH 7.4, PCO2 = 40 mmHg[2,3-DPG] / [Hb] = 0.8
pH 7.6
[2,3-DPG] / [Hb] = 1.2
P50
Blood-O2 equilibrium curve
0%
25%
50%
75%
100%
0 20 40 60 80
Hb-
O2
satu
ratio
n, o
r SO
2
PO2 , mmHg
pH 7.4, PCO2 = 40 mmHg[2,3-DPG] / [Hb] = 0.8
pH 7.6
[2,3-DPG] / [Hb] = 1.2
P50


DURING EXERCISE, THE OVERALL BENEFICIAL EFFECT OF ALKALOSIS IS QUESTIONABLE
PROLONGED ALKALOSIS IS NOT COMPATIBLE WITH NORMAL BODY HOMEOSTATSIS
AT REST, THE NEED TO OXYGENATE TISSUES CONFLICTS WITH THE NEED TO MAINTAIN H+ HOMEOSTASIS

(Edwards,1936)Altitude (km)
0 1 2 3 4 5 6 70
1
2
3
4
5
6
7
8
9
10continuousintermittent[Lab]
(mM)
LACTATE PARADOX

Work load (watt)0 50 100 150 200 250 300 350 400
0
2
4
6
8
10
12
14
[La b
] (m
M)
SL1ALT1ALT2ALT3

resting level
0
3
6
9
12
15
0 1 2 3 4 5 6 7 8 9
Altitude (km)
[La b
]max
(mM
)Operation Everest IICaucasians (different sources)Altitude natives (different sources)Caucasians (Marconi, pers.comm.)Sherpas (Marconi, pers.comm.)Caucasians (Marconi et al., 1998)
resting level
0
3
6
9
12
15
0 1 2 3 4 5 6 7 8 9
Altitude (km)
[La b
]max
(mM
)Operation Everest IICaucasians (different sources)Altitude natives (different sources)Caucasians (Marconi, pers.comm.)Sherpas (Marconi, pers.comm.)Caucasians (Marconi et al., 1998)
Operation Everest IICaucasians (different sources)Altitude natives (different sources)Caucasians (Marconi, pers.comm.)Sherpas (Marconi, pers.comm.)Caucasians (Marconi et al., 1998)

(Cerretelli, 1976)
Buffer capacity of the whole body
1) At altitude, the buffer capacity of acclimatized lowlanders is lower than at sea level
2) The highest Δ[H+] is similar at altitude and at sea level, and is independent of [Lab]
1
2

Simultaneous measurements of arterial pH and [La] in a group of acclimatized lowlanders after 9 weeks at 5,260 m did not show any significant difference in total body buffer capacity, compared to sea level, despite the reduction in bicarbonate buffer. (Van Halle et al, 2001; Wagner et al, 2002)
This implies that only non-bicarbonate buffers play a substantial role in the regulation of acid-base balance during anaerobic exercise at altitude

048
121620
SL ALT
[La b
] pea
k(m
M)
Kayser et al, 1993
0
10
20
30
40
ControlBicarbonate
Δ[H
+ ] (n
M)
SL ALT
*
*

0
100
200
300
* *
0
3
6
9
12
15
*** *
Wpe
ak(W
)[L
a b] p
eak
(mM
)
Grassi et al, 1996
SL Alt1
Alt1
-O2
Alt5
Alt5
-O2
·

0
3
6
9
12
15 SL
ALT
Grassi et al, 2001
[La b
] pea
k(m
M)
10 s 30 s Increm. ex
Lactate paradox is at least in part consequence of transient reduced working capacity and/or exercise mode

Until year 2000 there was consensuson the occurrence and persistence of a lactate paradox, even though the origin of the phenomenon was poorly understood.
An interesting feature of lactate paradox was its slow reversal upon restoring normoxia

[Lab]p (mM)
0
3
6
9
12
15
*
* **
*SL
1
ALT
1
ALT
2
ALT
3
SL2
SL3
SL4
SL5
SL6
[Lab]p (mM)
0
3
6
9
12
15
*
* **
*SL
1
ALT
1
ALT
2
ALT
3
SL2
SL3
SL4
SL5
SL6
5,050 m

0
4
8
12
16
0 25 50 75 100
Tib 1
Tib 2
Nep
[La](mM)
% VO2 peak.
Hochachka et al (1991) hypothesized that lactate paradox is permanent in Quechuas transferred to sea level.

24
68
101214
0 2 4 6 8 10Altitude exposure (weeks)
[La b
]max
(mM
)
Lundby et al ., 2000 Grassi et al.,1995 van Hall et al., 2001 Grassi et al.,1996 Grassi et al.,2001Kayser et al.,1993b Kayser et al., 1993a Personal Data Personal Data
Evolution of the “lactate paradox” as a function of exposure duration at altitudes ranging from 5,000 to 5,500 m. There is a fast drop of [La]max within 2-5 weeks from the onset of hypoxia and a progressive recovery thereafter to pre-exposure levels

• It is assumed that a better coupling between ATPsupply and demand may be responsible for lactate paradox.
• The progressive disappearance of lactate paradox during prolonged acclimatization means a loss of an acquired adaptive feature, as a consequence of a progressive impairment of muscle function.
• Lactate paradox may underlie a complex gene-based “reorganization” of muscle metabolism occurring in hypoxia.

Time, weeksHypoxia
Lactate paradox
HIF-1
Enzymes
Deterioration
ROS
Antioxidants
0 5 10 Time, weeksHypoxia
Lactate paradox
HIF-1
Enzymes
Deterioration
ROS
Antioxidants
0 5 10
Proposed time courses of the main events that occur during a period of hypoxia and after return to sea level (from Cerretelli and Samaja, EJAP 2003)
1
2glycolytic
3