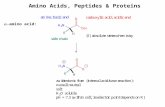
reaction - shodhganga.inflibnet.ac.inshodhganga.inflibnet.ac.in/bitstream/10603/15769/9/09_chapter...
Transcript of reaction - shodhganga.inflibnet.ac.inshodhganga.inflibnet.ac.in/bitstream/10603/15769/9/09_chapter...
Aza variant of intramolecular NCAL reaction Chapter4
4.1. Introduction
Polyhydroxylated pyrrolidines, piperidines (often called "aminosugars" or "aza sugars")
and their synthetic analogues have attracted considerable interest due to their ability to
mimic sugars in which the ring oxygen has been substituted for a nitrogen atom.1 Their
often potent inhibitory activity towards glycosidases and glycosyltransferases of
carbohydrate processing. enzymes make them useful in a wide range of potential
therapeutic strategies including the treatment of viral infections/ cancer,3 diabetes,4
tuberculosis,5 lysosomal storage diseases,6 and parasitic protozoa.7
0
?>" t:( "OH
OH
BCX-4208
OH 0
c!f'~
~ H OH
3-Hydroxy-2-hydroxymethyl pyrrolidines
OH
fa: C .. JOH
N H
4-( hydroxymethyl) piperidin-3-ol
OH
HO
OH
HO,,.~OH
lN~OH H
1-deoxygalactonojirimycin
HN~COOH s~o NH2
HN 0 0~
~~ OH
HO
tetrazomine cv: 0 Cycloth;and;ne
~ l~ N
isofebrifugine
Figure 1. Aza sugars
Hydroxylated piperidine structural framework is found in a wide variety of
natural and synthetic compounds of biomedical significance; for example cis-3-
hydroxypipecolic acid is an important constituent of a naturally occurring antibiotic
tertazomine8 whereas its acid reduced analogue is found in 1-deoxygalactonojirimycin
173
Aza variant of intramolecular NCAL reaction Chapter4
which has shown to be the strong inhibitors of a-galactosidase A and is currently in
undergoing preclinical trials as a potential therapy for Farby's disease, a severe
lysosomal storage disorder.9 Also (2R,3R}-3-hydroxy-2-hydroxymethylpiperidine is a
structural unit found in the antimalarial isofebrifugine.10 Amongst the pyrrolidine class,
cyclothialidine11 (a potent DNA-gyrase inhibitor}, slaframine/2 castanospermine13 and
detoxinine14 contains a cis-3-hydroxyproline structural motif whereas a literature search
revealed only one reported synthesis of its acid reduced congener, cis-3-hydroxy-2-
hydroxymethylpyrrolidine (the "azaDNA" analog} starting from serine.15 Included among
the polyhydroxylated pyrrolidine, 4-hydroxymethyl-pyrrolidin-3-ol is used as the
precursor for the BCX-4208, a clinical candidate evolved from the second generation of
PNB inhibitors (Fig. 1}.16-18
4.2. Basis of work and Retro-synthesis
,LHactones (2-oxetanones} have recently emerged as important synthetic targets owing
to their occurrence in a variety of natural products and their utility as versatile synthetic
intermediates in the synthesis of chiral products due to their masked aldol functionality
and their inherent reactivity. 19'20 In 1982, Wyn berg and co-workers successfully reported
the first catalytic, asymmetric [2+2] addition of ketene with highly electrophilic
aldehydes (eg. a~chlorinated} in the presence of cinchona alkaloids to yield ,8-lactones 3
in high yield (89%} and enantioselectivity (98% ee} (Scheme 1}.21
2
4moi%QD
PhMe, -25 °C
3
89%yield 98%ee
Scheme 1. Wynberg's ,8-lactone synthesis
174
QD
Aza variant of intramolecular NCAL reaction Chapter4
The proposed mechanism involves the formation of a zwitterionic ammonium
enolate intermediate, arising from the nucleophilic attack of the quinuclidine ring of the
catalyst on the ketene, followed by a stepwise addition to the aldehyde (Scheme 2). One
drawback associated with Wynberg and co-workers' original process was the
requirement of highly reactive aldehydes, presumably to compete with ketene
dimerization. As a result of this, only aldehydes containing electronwithdrawing groups
adjacent to the carbonyl were suitable coupling partners, and although the reaction
often returned good results, it was restricted in scope.22
Scheme 2. Mechanism of j3-lactone formation
Recently, Romo eta/. have reported a high enantioselective synthesis of a series
of bicyclic beta-lactones via intramolecular nucleophile-catalyzed aldol lactonization
(NCAL) of aldehyde acids, which provided the first strategy to beta-lactone synthesis
with unactivated aldehydes.23 The mechanism involves formation of the activated
carboxyl function (Scheme 3) from the carboxylic acid 4 (using the Mukaiyama salt, 5),
that can be subsequently intercepted with the nucleophilic catalyst, 0-acetylquinidine.
The acyl ammonium intermediate forms the Cl ammonium enolate, and an
175
Aza variant of intramolecular NCAL reaction Chapter4
intramolecular aldol reaction proceeds through a similar pathway to that outlined in
Scheme 2 to form bicyclic ,8-lactone (6) in high ee and moderate-to-good yields using 10
mol % of the catalyst. Both enantiomers are accessible using either the Ac-QD (0-acetyl
quinidine) or Ac-Q (0-acetyl quinine) catalysts. In a modification to their original
process, Rome and co-workers showed that an analogue of the Mukaiyama salt (TfO- or
BF4- counter ion instead of r) can be used to improve the output from the reaction. The
new salts are more soluble as well as prevent side reactions associated with the r
attacking the electrophilic pyridinium leading to a deactivated system.24
;--/COOH
~CHO
4
j +NR*3 (= AcQUIN)
+
10 mol% AcQUI N i-Pr 2NEt (4.0 equiv) Cl
®N 8 Cl I X
5 {3.0 equiv) 82% yield, 92% ee if X= OTf 54% yield, 92% ee if X= I
® -H
OMe Z" ~.,
0-Acetyl Quinidine
8 c))®.
NR 3
J
H
CWO 6
t
Scheme 3. Intramolecular nucleophile catalyzed aldollactonization
Literature reports thus clearly make obvious the utility of azasugars and this area
has seen resurgence in synthetic efforts directed towards convenient and efficient
synthetic routes to cis-aza sugars and their synthetic analogues. However many of these
strategies utilizing either chemical and enzymatic resolution 25, or by the use of amino
acids,26 or carbohydrates27 as chiral pool, have multi steps and/or selectivity deficient.
176
Aza variant of intramolecular NCAL reaction Chapter4
We, therefore, thought it prudent to develop new short catalytic synthetic strategy for
chiral synthesis of this class of compounds.
Derivatives of cinchona alkaloids have shown great promise as catalyst for a
broad range of asymmetric transformations thereby providing access to chiral products
of high enantiopurity.28 1nspired from Ramo's eta/. enantioselective synthesis of a series
bicyclic beta-lactones via intramolecular nucleophile-catalyzed aldol lactonization
(NCAL) of aldehyde acids, we envisaged a general synthetic strategy towards the chiral
synthesis of several cis-aza sugars and related molecules by utilizing formal
intramolecular NCAL reaction of particularly inexpensive achiral amino acid derivatives
via intermediary beta-lactone fused pyrrolidines and piperidines (intermediate A), as
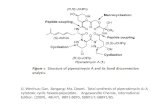
depicted in the retrosynthetic scheme. (Fig. 2)
aza-sugars c::::::> Cbz -GJ:t c::::::> 0
intermediate A
Figure 2. Retro-synthesis
4.3. Synthesis of aldehyde-acid precursors
c::::::> a ch ira I amino acids
Achiral amino acids 9a-d required as the starting point for intramolecular NCAL
reactions were prepared either by N-allylation of Cbz-GABA (7a) or Cbz-AiaOH (7b)
ROUTEB
8 <±> Br--(CH2Vm If HN co Me /'.... ~"'m "-..../" 2 Cl H3N C02Me ------- 'l' \'7.,.
ROUTE A
K2C03, CH3CN (m=2,3)
m=2; 7c (73.3%) m=3; 7d (68.1%)
J Cbz-CI, TEA, CH2CI2
then LiOH.H 20, THF:Hp;
Cbz H allylbromide, NaH, DMF I N Co H ~ vmNxvn C02H
0 3,-78 ° C, Me2S
80-82% / XV 2 -:;...- \'1rr \In Cbz nn
n=2;7a n=3; 7b
n=2, m=l; Sa (80.8%) n=3, m=l; Sb (68.6%) n=l, m=2; 8c (90.6%) n=l, m=3; 8d (78.5%)
Scheme 4. Synthesis of aldehyde-acid 177
n=2, m=l; 9a n=3, m=l; 9b n=l, m=2; 9c n=l, m=3; 9d
Aza variant of intramolecular NCAL reaction Chapter4
followed by ozonolysis to give aldehyde-acid 9a-b (Route A) or via employed a three
step procedure comprising of (i) N-w-alkenylation of glycine methyl ester (7c-d) (ii) one
pot Cbz protection, hydrolysis of ester group (Se-d) and (iii) subsequent ozonolysis
(Route B), to give aldehyde-acids 9c-d {Scheme 4).
4.3.1. ROUTE A:
N-Cbz amino acids 7a-b were prepared from ,8-alanine and GABA respectively by
treating them with Cbz-CI and NaOH, almost in quantitative yields according to the
method reported in literature.29 Allylation of 7a-b with allyl bromide, employing NaHas
a base in DMF, afforded the intermediates Sa-bin good yields {69-81%) which were fully
characterized by spectroscopic methods using NMR, IR and MS. Both products gave
their molecular ion peak at m/z corresponds to their molecular weights (Sa, m/z 264;
Sb, m/z 278) in their MS spectra. Compound 7a gave broad carbonyl absorption at 1689
cm-1 in IR spectrum corresponding to carboxylic acid whereas carbonyl absorption
appears at 1692 cm-1 in the IR spectrum of Sb. The terminal olefinic bond of Sa-b was
oxidised by bubbling ozone to their solution in CH2CI2 at -78 octo give the desired key
intermediate 9a-b possessing both the aldehyde and acid functionalities.
4.3.2. ROUTE 8:
Glycine methyl ester hydrochloride was prepared by treating glycine with thionyl
chloride in methanol and was confirmed by comparison of their melting point with that
of reported data.30 Treatment of methyl glycinate.HCI (1.2 equiv.) with K2C03 (2.3
equiv.) and 1-bromobutene {1.0 equiv.) in CH3CN gave monomer 7c in 73.2% yield.
Similarly 7d was obtained in 68% yield. 7c-d thus obtained showed the molecular ion
peaks at m/z corresponding to their molecular weights (7c, m/z 144; 7d, m/z 158) in
their MS spectra. The IR spectra of 7c and 7d showed the characteristic absorption at
1740 and 1739 cm-1 respectively due to ester carbonyl whereas the 1H-NMR spectra of
7c showed characteristic peaks at o 5.8 (m, 1H), o 5.09 (m, 2H), o 3.74 (s, 3H) and o 3.44
(s, 2H) due to CH2=CH, CH2=CH, COOCH3 and NHCH2C02CH3 respectively (Fig. 3).
178
"' U>
"' _../""" 0
1.06 ---.. U> u.
_../"""
lilQ.._ ~
:!I !"-IQ' U•
c:: ~ ~
!"-0
.... =t: m:::: < w
1-' s:: I][: v.
-..J :::0 lO ~ w
0 r, 0
~ 1.90
0 '" u. t: :::, Q.. 1.97
~ !" 0
liil_ u.
0
I';
]
~ :i z ;;:: ?J w 0
~ IZ 0
) ;;::
() if 0 () 0 0 () ()
;I- ..
im 5.8758 5.8642 5.8539 5.8417
~5.8191 5.8070 5.7967 5.7849 5.7628
~5.0n9 5.0670
,5.0157 5.0098 4.9979 4.9639
--3.7482
--3.4286
"6558 _.........- 2:6321 ~2.6077
_/2.1589 ~2.1352 ~2.1107
2.0870
./ 1.6617 ::::::;;=======~---------- :::::-- 1.6253
~1.5882 "-1.5640
"' :r: + ;f. 0
~t.l:~Ji~'H'!~l ~~;.!~
~ g -~
" ., ~~] i:<>;<>ie:~~
:::!! IQ c:: ~ ~
.... :r::
I
~ :::0
~ r, 0 3
1:::! 0 t: :::, Q..
~
"' 0 __r 1.00 ~
U> u.
__/ 2.03 Vl
~0
... u.
.. 0
Til(
bill._~
__, 1Q:!.__
__, 2.08 ---..
w 0
'" u.
"' 0
u.
0
c u.
]
:£ z ;;:: ? ~ 0
;;::
!f () 0
£l
~ (:t J:Z
) ()
8 ()
;I-
r::;~~;~~::~ ~~~B i I
"" ~ ~) !; .?; g
'i " '
;,.,~ .. -~! m.r
15.8584 5.8472 5.8358 5.8243
,5.8014 5.7901 5.7786 5.7673 5.7447
.L5.1565 5.1507
~5.0934 5.0593 5.0563
--3.7466
--3.4422
_.........- 2.7210 ........____ 2.6981
2.6755
L2.3166 ~2.2940
~2.2713 2.2486
)::. ,... Q
t§ ~ -· Q :::, '""' ~ -· :::, ~ Q
3 0 -(1) r, t: -Q ~
< II
Q r-
~ II Q
r,
II '""' o· :::,
9 .g ~ .l::lo,
Aza variant of intramolecular NCAL reaction Chapter4
Similar pattern was also observed in the 1H spectrum of compound 7d (Fig. 4). The
amino group of amino ester 7e-d was then protected by Cbz group using Cbz-CI and TEA
to give N-Cbz protected derivative which were taken up directly for the hydrolysis step.
Basic hydrolysis of methyl ester with LiOH.H20 afforded acids 7e-d in 90% and 79%
isolated yields respectively.
Acids Se-d thus obtained were fully characterized by spectroscopic methods
using NMR, IR and MS. Compound Se and Sd showed M+1 peak at m/z 264 and 278
respectively corresponding to their molecular weight. The IR spectrum of Se showed
characteristic absorptions at 1696 cm-1 due to acid functionality whereas it appeared at
1699 cm-1 for acid Sd. The terminal olefinic bond of Se-d was then oxidised to aldehydes
9e-d by ozonylsis. All the aldehyde acid 9a-d thus obtained were characterized by their
IR and MS spectra and were utilized immediately for NCAL reaction without any further
purification.
4.4. Racemic NCAL reactions
The scope of intramolecular aza NCAL reaction was studied using substrates 9a-d and
mukaiyama's reagent lOa under conditions as described by Romo et a/. The reactions
were performed by slow syringe pump addition of aldehyde-acids 9a-d over a period of
10 h to the mixture of Mukaiyama reagent lOa and TEA in acetonitrile at room
temperature (Scheme 5, condition A). The results are summarized in Table 1.
G:l N X re R y
n=m=l,2,3
9a-d
10a-c (3.0 equiv) TEA (4.0 equiv)
CH3CN, rt, 12 h
condition A-C
lOa: R=Me; X=CI; Y=l lOb: R=Me; X=Br; Y=OTf 10c: R=Et;X=Br;Y=BF4
i:"r? Cbz-N~
0
(±)-lla-d
condition A: lOa, addn. time (10 h) condition 8: lOb, addn. time (3 h) condition C: lOc, addn. time (3 h)
Scheme 5. Racemic NCAL reaction
180
Aza variant of intramolecular NCAL reaction Chapter4
Table 1. Optimization of racemic aza-NCAL reactions
entry aldehyde-acid (±) bicyclic-,8-lactone condition %yielda
A 53
1-CHO Cbz-N~ Cbz-N B 76 1 ~
9a COzH 0 lla
c 69
A 51 Cbz, /"-.... Cb''()::.t N CHO N 0
2 ~C02H B 71 6 0
9b llb c 63
b
rCHO rn A
3 N~ B 55 1 C02H I 0 Cbz Cbz
9c llc c 51
eCHO CA 4 N~CO H B 52 I 2 I Q Cbz Cbz
9d lld
•Isolated yield
bN-benzyloxycarbonyl-4,5-dihydro-lH-Pyrrole-2-carboxylic acid (12) was isolated albeit in low yields.
It was found that the reactions with 9a and 9b afforded racemic beta lactones, lla and
llb in 53% and 51% yield, respectively (Table 1, entries 1 and 2). However reaction with
c~ 8 , o ___ cbz,,~-{co12 (Et ~
llc ( 8 I
~-elim.
12
Scheme 6. Undesired decomposition pathway
181
Aza variant of intramolecular NCAL reaction Chapter4
aldehyde-acid 9c gave only the decomposed 12 product presumably produced by the
attack of iodide ion at the ,8-carbon of the resulting ,8-lactone followed by ,8-elimination
(Scheme 6).
Since Mukaiyama reagents with non-nucleophilic counter ions such as triflate
and tetrafluoroborate reduces this undesired ring opening, the NCAL reactions with
pyridinium salts lOb and lOc were studied next. The pyridinium salt lOb was
synthesized in a sealed tube by N-methylation of 2-bromopyridine with the
corresponding methyl triflate in nearly quantitative yield as colorless crystals by
literature procedures (Scheme 7). A singlet of three protons corresponding to N-CH3
apper at o 4.48 in the 1H-NMR spectrum of lOb (Fig. 4).
u N Br sealed tube, CH2CI2
-78 to 25 °C, 12 h
u 0 N Br
1 e 1ob OTf
Scheme 7. Synthesis of Mukaiyamas reagent {lOb}
1H-NMR, 300 MHz, CDCI3+C0300
C1 ®7 eBr
OTt 10b
~V'"'~ ~ "''"
1~)..'1! Hl:!3
,,;xi~ t'\J:!l~
'" ' g:~~;!g ~~ ~--·,.~:l~~; ~"'"'·
;~.nen
G.CC zn.~
:. ur~·Jccc~
5.0 4.5 4.0 3.5 3.0 2.5 2.0 1.5 1.0 0.5 0.0 ppm
I~(
Figure 4. 1H-NMR of compound lOb 182
Aza variant of intramolecular NCAL reaction Chapter4
Use of N-Methyl-2-bromopyridinium triflate (lOb) and N-Ethyl-2-
bromopyridinium tetrafluoroborate (lOc) greatly improved efficiency of the reaction
and better yields of the cyclised products were obtained (Scheme 5, Table 1). These
results were in conformity with the observations of Romo et al.ret All the bicyclic fJ
lactone fused pyrrolidine and piperizines lla-b were characterized by their IR, HRMS,
NMR and HSQC spectra.
4.5. Catalytic, asymmetric intramolecular NCAL reaction
Having optimized reaction conditions, we next studied the asymmetric NCAL reactions
of substrate 9a-c by employing chiral amine catalysts (Scheme 8). It was observed that
slow addition of aldehyde acid 9a-c to a mixture of 0-Acetyl quinidine,31 10b and hunig's
base in CH3CN gave the enantiomeric bicyclic lactones, (+)-lla, (+}-llb and (-)-11c with
an ee of 92, 97 and 91% respectively as determined by chiral HPLC (Table 2, entries 1, 3
and 5). Likewise the enantiomeric bicyclic lactones, (-)-lla, (-)-11b and (+)-llc were
synthesized using 0-acetyl quinine32 with an ee of 83, 88 and 95% respectively (Table 2,
entries 2, 4 and 6).
8 "'0 Cbz-N I
Ill~
0
(-)-lla, (-)-llb, (+)-llc
DIPEA (4.0 equiv), lOb (3.0 equiv)
10 mol% 0-Ac Quinine
CH3CN, rt, 30 h condition B (Table 1)
OAc D-Ac quinine
DIPEA (4.0 equiv), Cbz lOb (3.0 equiv) I 10 mol% 0-Ac Quinidine
~Nrr,;C02H -------0 m n CH3CN, rt, 30 h
3a-c condition B (Table 1)
n = m =1, 2,3
OAc
0-Ac quinidine
Cbz-N&_
0
(+)-lla, (+)-llb, (-)-llc
Scheme 8. Catalytic, asymmetric intramolecular NCAL reaction
183
l
Aza variant of intramolecular NCAL reaction Chapter4
Table 2. Ccatalytic, asymmetric intramolecular NCAL reactions
entry bicyclic-,8-lactone %eea %yieldb
55
1 Cbz-N~
92 65 15 0
(+)-lla
SR
2 Cbz-N:J'''t
83 ····~ 66 1R O
(-)-lla
Cb,,~ N 0
3 65 0 97 63
(+)-llb
Cbz, ~1R N '''0
4 v. .... ~ 88 67 6R O
(-)-llb
5 ffi 1N 1s O
Cbz 91 51
(-)-llc
6
df .. o ""~
1N 1R O 95 53
Cbz (+)-llc
a enantiomeric excess was determined by chiral HPLC
bisolated yields
The (15,55) configuration of bicyclic lactone (+)-lla was confirmed by its
reduction with DIBAL-H to the known Cbz protected (35,4R) 4-
(hydroxymethyl)pyrrolidin-3-ol (13a) in 43% yield which was compared with the
authentic sample ([a]290 +2.01 (c 0.71, MeOH) [lit.33 [af5
0 +2.6 (c 0.835, MeOH)].
184
l
Aza variant of intramolecular NCAL reaction Chapter4
Likewise, (-)-13a could also be obtained from lactone (-)-lla with an overall yield of 45
%which has not been reported in literature so far ([a] 290 -5.00° (c 0.69, MeOH) (Scheme
9}.
4.6. Conclusion
Cbz-NA
15 0 (+)-lla
~OH Cbz-N~
4R OH
{+)-13a
Cbz-N/j~~'? 0·~~·~
lR 0 {-)-lla
~
8~~'0H Cbz-N
""" 45 OH
(-)-13a
Scheme 9. Synthesis of diols
Aza variant of intramolecular catalytic, asymmetric nucleophile-catalyzed, aldol
lactonization (NCAL} reaction has been explored to synthesize ,8-lactone fused nitrogen
hetrocyclics as aza-sugars precursors by employing achiral amino acids. The utility of
these bicyclic fJ -lactones is presented by the formal synthesis of aza-sugars, (35,4R} and
(3R,45} 4-(hydroxymethyl)pyrrolidin-3-ol.
185
Aza variant of intramolecular NCAL reaction Chapter4
4. 7. Experimental
4.7.1. General Methods
All NCAL reactions were done using flame-dried glassware under nitrogen atmosphere.
Acetonitrile (CH3CN), Triethylamine (TEA), N,N-diisopropylethylamine (DIPEA) and
dichloromethane (CH2Cb) were distilled over calcium hydride. Reactions were
monitored by thin layer chromatography (TLC) using 0.25 mm E. Merck pre-coated
(Merch 60 F254) silica gel plates and using ninhydrine or KMn04 as visualizing agent.
Purification was performed by flash chromatography using silica gel (230-400 mesh).
NMR spectra were recorded on a Bruker Advance-300 spectrometer. Chemical shifts are
reported as ppm (delta) relative to TMS as internal standard. Mass spectra were
recorded on LCQ Advantage MAX (ESI) and JOEL JMS-600H (EI/HRMS) mass
spectrometers. IR spectra were recorded on a Perkin-Elmer FT-IR RXI spectrometer.
Microanalytical data were obtained using Vario-EL-111 elemental analyzer. Optical
rotations were determined on an Autopol Ill polarimeter. Chiral HPLC analysis were
performed using Daicel Chiralpak lA column. 7a/9 7b/9 Methyl glycinate.HCI/0 O
Acetylquinidine31 and O-Acetylquinine32 were prepared according to the literature
procedures.
4.7.2. Experimental details
General procedure for N-allylation (Sa-b)
N-allyi-N-benzyloxycarbonyl-f3-alanine (Sa)
To a cooled (0 oq suspended stirred solution of NaH (4.48 g, 112.1 mmol, 60% NaH in
mineral oil) in anhydrous DMF (25 ml) was added a solution of 7a (5.0 g, 22.4 mmol) in
DMF (25 ml) dropwise under nitrogen. Once the hydrogen evolution ceased, allyl
bromide (2.9 ml, 33.6 mmol) was added dropwise into the above heterogenous solution
and stirring was continued for additional 2 h at 0 oc. The reaction mixture was quenched
at same temperature by addition of 1N HCI to become acidic (pH 2); diluted with water
(250 ml) and extracted with ethyl acetate (3 x 80 ml). The combined organic extracts
186
Aza variant of intramolecular NCAL reaction Chapter4
were washed with brine (2 x 40 ml), dried (Na2S04) and concentrated in vacuo. The
resulting crude oil was purified by flash chromatography over silica gel using ethyl
acetate-hexane (3:7) to afford Sa as clear oil (4. 76 g, 80.81%).
Cbz I
~N~C02H Sa
R1 (1:1, EtOAc/Hexane) 0.25; Anal. Calcd: [Found: C, 63.58; H,
6.39; N, 5.12. C13H15N04 requires C, 63.87; H, 6.51; N, 5.32%];
IR (neat, cm-1) 3069, 2928, 1688, 1474, 1419, 1249, 1135,
1104, 1032, 990, 926; 1H NMR (300 MHz, CDCI3) 7.33 (br s, 5H),
5.75-5.77 (br, 1H), 5.13 (br s, 4H), 3.93 (br, 2H), 3.51-3.56 (t, 2H, J 6.9 Hz), 2.59 (br, 2H);
13C NMR (75 MHz, CDCI3) 176.7, 156.1, 136.6, 133.5, 128.6, 128.1, 127.9, 117.0, 67.4,
50.5, 43.4, 36.8; MS (ESI) m/z 263, found 264 [M+Ht.
N-allyi-N-benzyloxycarbonyi-GABA {Sb)
The acid was prepared from 7b (6.0 g, 25.2 mmol), NaH (5.05 g, 126.4 mmol, 60% NaH in
mineral oil) and allyl bromide (3.28 ml, 37.9 mmol) at room temperature in 10 h.
Purification by flash chromatography over silica gel using ethyl acetate-hexane (1:4)
afforded Sb as clear oil (4.8 g, 68.57%).
R1 (3:2, EtOAc/Hexane) 0.3; Anal. Calcd: [Found: C, 64.73; H, Cbz I
~ N~co2H 6.96; N, 5.00. C1sH1gN04 requires C, 64.97; H, 6.91; N,
sb 5.05%]; IR (neat, cm-1) 3020, 2360, 1692, 1597, 1472, 1422,
1216, 1045; 1H NMR (300 MHz, CDCI3) 7.32-7.34 (br m, 5H), 5.76 (br, 1H), 5.13 (br s, 4H),
3.87 (br, 2H), 3.31 (br, 2H), 2.34 (br, 2H), 1.86 (br, 2H); 13C NMR (75 MHz, CDCI3) 178.2,
156.4, 136.7, 133.6, 128.5, 128.1, 127.9, 117.0, 67.4, 49.7, 46.3, 31.2, 23.3; MS (ESI) m/z
277, found 278 [M+Ht
General procedure for mono N-alkenylation {7c-d)
Methyl N-{but-3-enyl)glycinate {7c)
K2C03 (9.39 g, 67.9 mmol) was added to a solution of methyl glycinate.HCI (3.15 g, 35.4
mmol) in CH3CN (150 ml) and the mixture was stirred for 1 h. 4-Bromo-1-butene (3.0 ml,
29.5 mmol) was added to the mixture and the reaction mixture was stirred for 48 h. The 187
Aza variant of intramolecular NCAL reaction Chapter4
insoluble materials were filtered off and the filtrate was concentrated under reduced
pressure. To the residue was added water (150 ml) and extracted with ethyl acetate {3 x
60 ml). The combined organic extracts were washed with brine (2 x 25 miL dried
(Na2S04) and concentrated in vacuo to afford a clear liquid. Purification by flash
chromatography on silica gel using ethyl acetate-hexane (4:6 -710:0) furnished 7c as
clear oil {3.1 g, 73.28%).
R1 (EtOAc) 0.3; Anal. Calcd: [Found: C, 58.82; H, 9.10; N, 9.81. H
~N'-../C02Me C7H13N02 requires C, 58.72; H, 9.15; N, 9.78%]; IR (neat, cm-1)
3020, 1743, 1634, 1437, 1215; 1H NMR (300 MHz, CDCI3)
5.74-5.88 (m, 1H), 5.05-5.15 (m, 2H), 3.74 (s, 3H), 3.44 (s, 3H), 2.67-2.72 (t, J = 6.9 Hz,
7c
2H), 2.24-2.31 (q , J = 6.9, 13.8 Hz, 2H); 13C NMR (75 MHz, CDCb) 172.9, 136.1, 166.6,
51.8, 50. 7, 48.5, 34.3; MS (ESI) m/z 143, found 144 [M+Ht
Methyl N-(pent-3-enyl)glycinate (7d)
7d was prepared from methyl glycinate.HCI (3.15 g, 35.4 mmol), K2C03 {9.39 g, 67.9
mmol) and 5-Bromo-1-pentene {3.5 ml, 29.5 mmol). Purification by flash column
chromatography on silica gel using chloroform as eluant isolated 7d as clear oil {3.16 g,
68%);
Rt (CHCI3) 0.3; Anal. Calcd: [Found: C, 61.01; H, 9.28; N,
8.93. C8H15N02 requires C, 61.12; H, 9.62; N, 8.91%]; IR
(neat, cm-1) 3020, 2933, 1740, 1639, 1439, 1217; 1H NMR
{300 MHz, CDCI3) 5.76-5.89 (m, 1H), 4.96-5.07 (m, 2H), 3.74 (s, 3H), 3.42 (s, 3H), 2.60-
H ~N'-../C02Me
7d
2.65 (t, J = 7.1 Hz, 2H), 2.08-2.15 (q, J = 7.1, 14.4 Hz, 2H), 1.56-1.66 (m, 2H); 13C NMR (75
MHz, CDCI3) 173.1, 138.4, 114.8, 51.8, 50.8, 49.1, 31.4, 29.2; MS (ESI) m/z 157, found
158 [M+Ht
General procedure for one pot Cbz protection and hydrolysis of ester (Se-d)
N-(benzyloxycarbonyi)-N-(but-3-enyl)glycine (8c)
To a cooled (0 oq stirred solution of 7c (2.63 g, 18.3 mmol) and TEA (5.63 ml, 40.4
mmol) in anhydrous CH2Ch (45 ml) was added Cbz-CI (2.97 ml, 21.2 mmol) dropwise 188
Aza variant of intramolecular NCAL reaction Chapter4
under nitrogen. The clear yellow solution was warmed to room temperature and stirred
for 2 h, at which point the volatiles were removed under reduced pressure to afford
yellow oil. The crude oil was then dissolved in THF/H 20 (4:1, 50 ml) with stirring
followed by the addition of liOH.H20 (2.31 g, 55.1 mmol) at room temperature. After
stirring for 2 h, the reaction mixture was diluted with water (30 ml) and extracted with
ether (2 x 35 ml). The pH of aqueous layer was adjusted to 2-3 by the addition of dilute
hydrochloric acid (1:1) at 0 oc and extracted the liberated oil with ethyl acetate (4 x 50
ml). The combine organic extracts were washed with brine (2 x 45 ml) and dried
(Na2S04). Removal of the solvent in vacuo gave the analytically pure product Sc as clear
oil (4.38 g, 90.6%).
R1 (7:3, EtOAc/Hexane) 0.35; Anal. Calcd: [Found: C, 63.76; H, Cbz I
~ N~co2H 6.40; N, 5.12. C14H11N04 requires C, 63.87; H, 6.51; N, 5.32%];
8c IR (neat, cm-1) 3020, 2361, 1696, 1475, 1431, 1367, 1217, 1149;
1H NMR (300 MHz, CDCI3) 7.27-7.37 (br m, 5H), 5.72-5.78 (m, 1H), 5.03-5.19 (m, 4H),
4.06 (br, 2H), 3.43 (br, 2H), 2.32 (br, 2H); 13C NMR (75 MHz, CDCI3) 174.5/174.3
(rotamers), 156.8/156.0 (rotamers), 136.4/136.3 (rotamers), 135.0/134.8 (rotamers),
128.57/128.51 (rotamers), 128.1/128.0 (rotamers), 127.8/127.7 (rotamers), 117.2/117.0
(rotamers), 67.7/67.5 (rotamers), 49.4/48.9 (rotamers), 48.4/48.0 (rotamers), 32.9/32.4
(rotamers); MS (ESI) m/z 263, found 264 [M+Ht
Cbz N-(benzyloxycarbonyi)-N-(pent-3-enyl)glycine (Sd) I
~N~C02H Clear oil (78.8%); Anal. Calcd: [Found: C, 64.73; H, 6.77; N,
8d 4.99. C15Hi9N04 requires C, 64.97; H, 6.91; N, 5.05%]; R1
(1:9, MeOH/CHCI3) 0.3; IR (neat, cm-1) 2935, 2363, 1699, 1470, 1433, 1221; 1H NMR
(300 MHz, CDCI3) 7.28-7.37 {m, 5H), 5.71-5.83 (m, 1H), 4.96-5.19 (m, 4H), 4.02-4.07 (d, J
= 12.6 Hz, 2H), 3.33-3.40 {m, 2H), 2.01-2.12 (m, 2H), 1.30-1.68 (m, 2H); 13C NMR (75
MHz, CDCI3) 175.2/174.8 (rotamers), 156.9/156.0 (rotamers), 137.8/137.6 (rotamers),
136.4, 128.6, 128.2/128.1 (rotamers), 127.9/127.8 (rotamers), 115.3/115.2 (rotamers),
189
Aza variant of intramolecular NCAL reaction Chapter4
67.8/67.6 (rotamers), 49.1/48.7 (rotamers), 48.5/48.0 (rotamers), 30.9/30.8 (rotamers),
27.5/27.1 (rotamers); MS (ESI) m/z 277, found 278 [M+Ht
General procedure for ozonolysis as described for 9a
Ozone was bubbled through a cooled (-78 oq solution of 8a (3.2 g, 11.0 mmol) in 75 ml
of anhydrous CH2Cb for 1 h. After that, a stream of argon was passed through the
cooled solution for 30 min to eliminate the excess of ozone. The cooled reaction mixture
was then quenched with excess of Me2S (3.57 ml, 48.6 mmol), allowed to warm up to
room temperature and treated with cold water (40 ml), extracted with CH2CI2 (3 x 45
ml), dried (Na2504) and concentrated in vacuo to obtain the aldehyde 9a (2.59 g, 80.4%}.
· Similarly 9b-d were obtained from 8b-d in 81-82% and were used immediately for NCAL
reaction without any further purification
G1 N G Br I -
OTf
N-methyl-2-bromopyridinium triflate (lOb)
Pyridinium salt lOb was prepared from 2-bromopyridine (2.0 ml, 20.9
mmol) and methyl trifluoromethanesulfonate (2.3 ml, 20.9 mmol) in
lOb CH2Cb (10 ml) according to the literature method5 as white crystalline
solid (6.46 g, 96%}; mp 158-160 oc; IR (KBr, cm-1) 3087, 1616, 1491, 1443, 1267, 1156,
1032; 1H NMR (300 MHz, CDCI3+CD30D) 9.10-9.12 (d, J = 5.2 Hz, 1H), 8.27-8.40 (m, 2H),
8.0-8.05 (m, 1H), 4.48 (s, 3H); 13C NMR (75 MHz, CDCI3) 149.0, 146.3, 133.9, 127.1,
122.5, 118.2, 50.8.
General procedure for the Racemic Aza-NCAL reaction as described for ,8-lactone -(±)
lla
benzyl 7-oxo-6-oxa-3-azabicyclo[3.2.0]heptane-3-carboxylate[(±)-lla]
Condition B: To a stirred solution of Mukaiyama's reagent lOb {1.65 g, 5.13 mmol, 3.0
equiv) and TEA {0.95 ml, 6.85 mmol, 4.0 equiv) in CH3CN (30 ml) at 25 oc was added via
syringe pump a solution of aldehyde-acid 9a {0.45 g, 1.71 mmol) in CH3CN (20 ml) over a
190
Aza variant of intramolecular NCAL reaction Chapter4
period of 3 h. Stirring of the resulting dark red solution was continued for another 12 h.
The volatiles were removed under reduced pressure and to the crude reaction mixture
was added ethyl acetate (150 ml) and saturated aqueous NH4CI (150 ml). The phases
were separated, and the aqueous layer was extracted with ethyl acetate {2 x 50 ml). The
combined organic phases were washed with brine {100 ml), dried (NazS04), filtered, and
concentrated to afford a brown residue. Purification of the crude residue by flash
chromatography on Si02 using ethyl acetate-hexane (13:7) afforded the ,B-Iactone lla
as light yellow oil {0. 7 g, 76.07%).
Cbz-N:q 1 0
{±)-lla
R1 (7:3, EtOAc/Hexane) 0.35; Anal. Calcd: [Found: C, 63.23; H,
5.41; N, 5.65. C13H13N04 requires C, 63.15; H, 5.30; N, 5.67%]; IR
(neat, cm-1) 1836, 1705, 1423, 1356, 1261, 1227, 1187, 1114; 1H
NMR (300 MHz, CDCI3) 7.32-7.40 (SH, m, ArH), 5.15 (2H, br s,
PhCH2), 5.09-5.12 (1H, dd, J 4.0, 5.9 Hz, H-5), 4.23-4.28 {2H, br m, H-2+H-4), 4.08-4.12
{1H, m, H-1), 3.29-3.35 {2H, m, H-2'+H-4'); 13C NMR (75 MHz, CDCI3) 168.6 (C=O,
lactone), 155.0 (C=O, carbamate), 136.1 (qC), 128.5, 128.2, 128.0 (ArC), 74.9/74.3 (C-5,
rotamers), 67.5 (PhCHz), 56.1/55.4 (C-1, rotamers), 49.6 {C-4), 45.6 (C-2); HRMS (ESI}:
calcd for C13H14N04 [M+Ht 248.0922, found 248.0919.
benzyl 7 -oxo-8-oxa-3-azabicyclo[ 4.2.0]octane-3-carboxylate[(±}-11b]
This lactone was prepared from oxo-acid 9b (0.286 g, 1.02 mmol), pyridinium salt lOb
{0.989 g, 3.07 mmol), TEA {0.49 ml, 3.55 mmol). Purified by flash column
chromatography using ethyl acetate-CH2CI 2 (7:93) as eluant isolated llb as clear viscous
oil (0.19 g, 71.16%).
cbz,~r9 ~ 6 0
{±)-llb
Rt {1:9, Et0Ac/CH2CI2) 0.5; Anal. Calcd: [Found: C, 64.24; H, 5.81;
N, 5.34. C14H1sN04 requires C, 64.36; H, 5.79; N, 5.36%]; IR (neat,
cm-1) 1826, 1698, 1421, 1353, 1290, 1224, 1117, 1052; 1H NMR
{300 MHz, CDCI3) 7.34 (SH, br s, ArH), 5.08-5.21 (2H, m, PhCH2),
4.74-4.80 (1H, br d, J 15.6 Hz, H-1), 4.42-4.47 {0.5 H, d, J 15.6 Hz, H-2, rotamers), 4.32-
191
Aza variant of intramolecular NCAL reaction Chapter4
4.37 {0.5 H, d, J 15.6 Hz, H-2, rotamers), 3.82-3.87 (1H, m, H-6), 3.67-3.69 {1H, br, H-4),
3.46-3.56 (1H, td, J 4.5, 12.6 Hz, H-4'), 3.36-3.41 {1H, d, J 15.6 Hz, H-2'), 2.09-2.18 {1H,
app t, J 15.5 Hz, H-5), 1.89-2.02 (1H, m, H-5'); 13C NMR (75 MHz, CDCI3) 169.6 (C=O,
lactone), 155.8 (C=O, carbamate), 136.4 (qC}, 128.6, 128.2, 127.9 (ArC}, 69.1/68.7 {C-1,
rotamers), 67.5 (PhCH2), 47.6 (C-6), 42.0/41.5 (C-2, rotamers), 39.9 (C-4), 19.7 (C-5);
HRMS (ESI): calcd for C14H16N04 [M+Ht 262.1079, found 262.1080.
benzyl 7-oxo-8-oxa-2-azabicyclo[4.2.0]octane-2-carboxylate[(±}-11c]
This lactone was prepared from oxo-acid 9c (0.225 g, 0.84 mmol), pyridinium salt lOb
(0.819 g, 2.54 mmol), TEA (0.47 ml, 3.39 mmol). Purified by flash column
chromatography using ethyl acetate-CH2CI2 {1:49) as eluant isolated llc as viscous oil
(0.114 g, 55%).
Rt (1:9, EtOAc/CH2Cb) 0.6; Anal. Calcd: [Found: C, 63.05; H, 5.39; N,
5.61. C13H 13N04 requires C, 63.15; H, 5.30; N, 5.67%]; IR (neat, cm-1)
1837, 1708, 1422, 1347, 1306, 1266, 1216, 1108, 1059; 1H NMR {300
MHz, CDCI3) 7.32-7.36 (5H, m, ArH), 5.53-5.65 (1H, br, H-1), 5.15-5.17
(3H, m, PhCH2+H-5), 4.12-4.19 (1H, app t, J 9.7 Hz, H-3), 3.37-3.46 (1H, td, J 6.2, 11.5 Hz,
H-3'), 2.29-2.36 {1H, dd, J 6.2, 14.8 Hz, H-4), 1.87-2.00 (1H, m, H-4'); 13C NMR (75 MHz,
CDCI3) 166.7 (C=O, lactone), 153.3 (C=O, carbamate), 135.9 (qC}, 128.7, 128.4, 128.2
(ArC}, 77.8 (C-5), 70.2/69.8 (C-1, rotamers), 67.9 (PhCH2), 44.0 (C-3), 29.1 (C-4); HRMS
(ESI): calcd for C13H13NNa04 [M+Nat 270.0737, found 270.0741.
benzyl 8-oxo-7-oxa-2-azabicyclo[4.2.0]octane-2-carboxylate[(±}-11d]
This lactone was prepared from oxo-acid 9d {0.41 g, 1.46 mmol), pyridinium salt lOb
{1.418 g, 4.4 mmol), TEA {0.81 ml, 5.87 mmol). Purified by flash column chromatography
using ethyl acetate-hexane (3:7) as eluant isolated lld as viscous oil {0.202 g, 53%). Rt
(1:1, EtOAc/Hexane) 0.45; Anal. Calcd: [Found: C, 64.29; H, 5.65; N, 5.39. C14H15N04
requires C, 64.36; H, 5.79; N, 5.36]; IR(neat, cm-1) 1832, 1703, 1418, 1310, 1216, 1114,
1037; 1H NMR {300 MHz, CDCI3) 7.35 {5H, br s, ArH), 5.80-5.82 {0.5H, d, J 6.6 Hz, H-1,
192
Aza variant of intramolecular NCAL reaction Chapter4
()to I Cbz
(±)-lld
rotamersL 5.58-5.60 (O.SH, d, J 6.6 Hz, H-1, rotamersL 5.12-5.22 (2H,
m, PhCH2), 4.97 {1H, br s, H-6), 3.68-3.72 (1H, dd, J 3.6, 11.8 Hz, H-3),
3.36-3.41 (1H, m, H-3'}, 2.26-2.30 {1H, d, J 12.6 Hz, H-4), 1.80-1.99
(3H, m, H-5+H-5'+H-4'); 13C NMR (75 MHz, CDCI3) 170.5/169.9 (C=O,
lactone, rotamers), 155.7/154.6 (C=O, carbamate, rotamers), 136.0 (qC), 128.6, 128.4,
128.1 (ArC), 72.1/71.9 (C-6, rotamers), 68.1/68.0 (PhCH2, rotamers), 59.7/59.5 (C-1,
rotamers), 43.0/42.9 (C-3, rotamers), 25.9 (C-4), 16.1/15.8 (C-5, rotamers); HRMS (ESI):
calcd for C14H1GN04 [M+Ht 262.1079, found 262.1094.
General procedure for asymmetric Aza-NCAL reaction as described for 13-lactones-(+)
lla
Cbz-N~ 15 0
(+}-lla
(lS,SS)-benzyl 7-oxo-6-oxa-3-azabicyclo[3.2.0]heptane-3-
carboxylate[(+)-11a]
To a stirred solution of 0-Acetylquinidine {71 mg, 0.196 mmoiL
Mukaiyama's reagent lOb {1.895 g, 5.88 mmol) and DIPEA (1.36
ml, 7.84 mmol) in CH3CN {35 ml) was added a solution of aldehyde-acid 9a {0.52 g, 1.96
mmol) in CH3CN {30 ml} via syringe pump over 3 h. After the addition was complete, the
reaction was stirred for additional 30 h at 25 oc. The solvent was removed in vacuo, and
the residue was partitioned between ethyl acetate {150 ml) and saturated NH4CI (100
ml). The phases were separated, and the aqueous layer was extracted with ethyl acetate
(2 x SO ml). The combined organic phases were washed with brine (100 ml), dried
(Na2S04), and concentrated in vacuo. Purification of the crude residue by flash
chromatography on Si02 using ethyl acetate-hexane {13:7) afforded the beta lactone
(+)-lla as light yellow oil {0.315 g, 65%). [af9o +111.4° (c 0.20, CHCI3}. Enantiomeric
excess (ee) was determined to be 92% by chiral stationary phase HPLC analysis using
Daicel Chiralpak lA column (MtBE/EtOH = 99:1, flow rate 0.6 ml/min, Amax 213.9 nm),
t1s,ss = 20.74 min (major), t1R,sR = 22.66 min (minor). All other spectroscopic data
matched that displayed by (±)-lla.
193
Aza variant of intramolecular NCAL reaction Chapter4
cbz,~r9 ( 15,65)-benzyl 7-oxo-8-oxa-3-azabicyclo[ 4.2.0]octane-
3-carboxylate[( +)-Sb]
This lactone was prepared from oxo-acid 9b (0.25 g, 0.89 mmol),
pyridinium salt lOb {0.864 g, 2.68 mmol), DIPEA (0.62 ml, 3.58
mmol) and 0-Acetylquinidine (32 mg, 0.089 mmol). Purification by flash column
~ 65 0
(+)-llb
chromatography on silica gel using ethyl acetate-CH2Cb (7:93) as eluant gave (+}-llb as
viscous oil (0.147 g, 63%). [a]290 +118.r (c 1.09, MeOH). Enantiomeric excess (ee) was
determined to be 97% by chiral stationary phase HPLC analysis using Daicel Chiralpak lA
column (MtBE/EtOH = 99:1, flow rate 0.6 ml/min, Amax 209 nm), t15,65 = 20.98 min
(major), t 1R,GR = 32.31 min (minor). All other spectroscopic data matched that displayed
by (±)-llb.
!T-9 }~o
Cbz {-}-llc
(15,SR)-benzyl 7-oxo-8-oxa-2-azabicyclo[4.2.0]octane-2-
carboxylate[(-)-llc]
This lactone was prepared from oxo-acid 9c {0.29 g, 1.09 mmol),
pyridinium salt lOb {1.05 g, 3.27 mmol), DIPEA (0.76 ml, 4.37 mmol)
and 0-Acetylquinidine (40 mg, 0.109 mmol). Purification by flash column
chromatography on silica using ethyl acetate-CH2CI2 (1:49) as eluant gave (-)-llc as
viscous oil (0.137 g, 51%). [a]27 0 -132.4° (c 0.16, MeOH). ). Enantiomeric excess (ee) was
determined to be 91% by chiral stationary phase HPLC analysis using Daicel Chiralpak lA
column (MtBE/EtOH = 98:2, flow rate 0.8 ml/min, Amax 213 nm), t1R,ss = 8.82 min (minor),
t 1s,sR = 11.64 min (major). All other spectroscopic data matched that of the racemic
compound (±)-llc
Cbz- N /---?· , ? 0 .... ~
lR Q
(-}-lla
(lR,SR)-benzyl 7-oxo-6-oxa-3-azabicyclo[3.2.0]heptane-3-
carboxylate[(-)-lla]
The lactone was prepared from aldehyde-acid 9a (0.348 g, 1.31
mmol), pyridinium salt lOb {1.268 g, 3.93 mmol), DIPEA (0.91 ml,
5.25 mmol) and 0-Acetylquinine (48 mg, 0.13 mmol) following the procedure as
described for (+)-lla. Purification by flash chromatography on Si02 using ethyl acetate-
194
Aza variant of intramolecular NCAL reaction Chapter4
hexane (13:7) gave the (-)-lla as colorless oil {0.214 g, 66% yield). [a] 29 0 -39.1 o (c 0.44,
CHCI3). Enantiomeric excess (ee) was determined to be 83% by chiral stationary phase
HPLC analysis using Daicel Chiralpak lA column (MtBE/EtOH = 99:1, flow rate 0.6 ml/min,
"-max 213.9 nm), t 15,55 = 20.56 min (minor), t1R,SR = 22.42 min (major). All other
spectroscopic data matched with (±)-lla.
Cbz,OlR N '"0
""~ 6R 0
(-)-llb
( 1R,6R)-benzyl 7 -oxo-8-oxa-3-azabicyclo[ 4.2.0]octane-3-
carboxylate[(-)-llb]
This lactone was prepared from oxo-acid 9b {0.195 g, 0.698
mmol), pyridinium salt lOb {0.674 g, 2.09 mmol), DIPEA {0.48 ml,
2.79 mmol) and 0-Acetylquinine {25 mg, 0.069 mmol). Purification by flash column
chromatography on silica gel using ethyl acetate-CH2Cb {7:93) as eluant gave (-)-llb as
viscous oil (0.12 g, 67%). [a] 290 -63.3° (c 1.11, MeOH). Enantiomeric excess (ee) was
determined to be 88% by chiral stationary phase HPLC analysis using Daicel Chiralpak lA
column (MtBE/EtOH = 99:1, flow rate 0.6 ml/min, "-max 209 nm), t15,65 = 20.05 min
(minor), t1R,GR = 33.36 min (major). All other spectroscopic data matched with the
racemic compound (±)-llb
(j~"O
""~ N lR 0 cb.f
(+)-llc
( lR,SS)-benzyl 7-oxo-8-oxa-2-azabicyclo[ 4.2.0]octane-2-
carboxylate[(+)-11c]
This lactone was prepared from oxo-acid 9c (0.2 g, 0.753 mmol),
pyridinium salt lOb {0.728 g, 2.26 mmol), DIPEA {0.52 ml, 3.01 mmol)
and 0-Acetylquinine (27 mg, 0.075 mmol). Purification by flash column chromatography
on silica using ethyl acetate-CH2Cb (1:49) as eluant gave (+)-llc as viscous oil {0.098 g,
53%). [a]280 +67.r (c 0.37, MeOH). Enantiomeric excess (ee) was determined to be 95%
by chiral stationary phase HPLC analysis using Daicel Chiralpak lA column (MtBE/EtOH =
98:2, flow rate 0.8 ml/min, A-max 213 nm), t1R,ss = 8. 76 min (major), t1s,sR = 11.63 min
(minor). All other spectroscopic data matched with the,racemic compound (±)-llc
195
Aza variant of intramolecular NCAL reaction Chapter4
(35,4R) N-benzyloxycarbonyl-4-( hydroxymethyl)pyrrolidi n-3-ol [ ( + )-13a]
To a magnetically stirred solution of (+)-lla {0.17 g, 0.687 mmol) in CH2CI2 (5.3 ml) was
added dropwise a solution of DIBAI-H {1.0 M in toluene, 0.738 ml, 0.742 mmol) at 0 °C.
After 5 min, the solution was warmed to room temperature and stirred overnight. The
reaction mixture was then cooled to 0 oc, diluted with ethyl acetate (2.4 ml) and
quenched with acetone {1.5 ml) and Rochelle's salt (4.0 ml). The mixture was vigorously
stirred at 25 oc for 10 h. The layers were separated and the aqueous layer was extracted
with ethyl acetate (2 x 4 ml). The combined organic layers were washed with brine (8
ml), dried over Na2S04, filtered, and concentrated in vacuo. Purification by flash
chromatography on Si02 using MeOH-ethyl acetate (1:9) gave the dial (+)-13a as
colorless oil (74 mg, 43.19% yield).
Cbz-N~ 4R OH
(+)-13a
Rt {1:9, MeOH/EtOAc) 0.3; Anal. Calcd: [Found: C, 62.23; H, 6.75;
N, 5.52. C13H11N04 requires C, 62.14; H, 6.82; N, 5.57%]; IR (neat,
cm-1) 3389, 2920, 1682, 1433, 1358, 1216, 1136, 1097; [a] 29
0
+2.01 (c 0.71, MeOH) [lit.6 [a] 250 +2.6 (c 0.835, MeOH)]; 1H NMR
(300 MHz, CDCI3) 7.28-7.32 (SH, m, ArH), 5.04-5.14 (2H, m, PhCH2), 4.41 {1H, br s, H-3),
3.78-3.83 {2H, t, J 7.8 Hz, CH20H), 3.44-3.56 (3H, m, H-2+H-2'+H-5), 3.32-3.39 (1H, t, J
10.5, H-5'), 2.28-2.29 (1H, br, H-4); 13C NMR {75 MHz, CDCI3) 155.5 (C=O), 136.8 (qC),
128.6, 128.1, 127.9 (ArC), 72.3/71.5 (C-3, rotamers), 67.1 (PhCH2), 60.3 (CH20H),
55.2/54.9 (C-2, rotamers), 45.9/45.6 (C-5, rotamers), 45.1/44.3 (C-4, rotamers); MS (ESI)
m/z 251, found 252 [M+Ht; HRMS (ESI): calcd for C13H18N04 [M+Ht 252.1235, found
252.1216.
{3R,4S) N-benzyloxycarbonyl-4-(hydroxymethyl)pyrrolidin-3-ol [(-)-13a]
Cbz-N~ 4s OH
(-)-13a
The dial was prepared from lactone (-)-lla (0.142 g, 0.574 mmol)
and DIBAI-H {1.0 M in toluene, 0.618 ml, 0.62 mmol). Purification
by flash chromatography on Si02 using MeOH-ethyl acetate (1:9)
gave the dial (-)-13a as colorless oil {58 mg, 44.16% yield). [a]290 -
5.0° (c 0.69, MeOH). All other spectroscopic data matched with dial (+)-13a.
196
Aza variant of intramolecular NCAL reaction
4.8. Spectral data
7.5 7.0 6.5 6.0 5 . .1 5.0 4.5 4.0
1~1 1~( ~slisl 3.5 3.0
!5(
1H NMR, 300 MHz, CDC!,
;--.,:... Cbz-N~
' "o (.:!)-11a
2.5 2.0 1..1
Figure 5. 1H-NMR of lactone (t}-lla
J). x. Dibb.it .bl'MPY-91
"""'' i-4-01
C.ta Collect...S oct eckUOD·i.Don.~OO
kehiw 4inct.ozyl
j:~i --~ ,1
---:<:::::::=J- '
5
J 6
8
9
10
- 0
180 160 140 120 100 80 60
Fl (ppml
Figure 6. 1H-13C-HSQC of lactone (t)-lla 197
Chapter4
1.0 0.5 ppm
- 0 0
1H-13C-HSQC, 600 MHz, CDCI3
Cbz-N:f:J 1 "-·o
(±)-11a
40 20 0
Aza variant of intramolecular NCAL reaction Chapter4
1 H NMR, 300 Mz. CDCI3
Cbz, ~
tJ.J:o (±)-11b
7.5 7.0 6.5 M ~ ~ ~ U ~ ~ 2.5 2.0 1.5 1.0 0.5 ppm
I~( I~( i§( ~s( \§~§~~~~ 1sns(
Figure 7. 1H-NMR of lactone (:!)-11b
0
20
40
60
80
100
1H-13C, HSOC, 300 Mz, CDCI3 120
Cbz, /"....' N ro ~~'
0 140 (±)·11b
8.0 7.5 7.0 6.5 6.0 5.5 5.0 4.5 4.0 3.5 3.0 2.5 2.0 1.5 1.0 0.5 ppm
Figure B. 1 H-13 C-HSQC of lactone (:!)-11b
198
Aza variant of intramolecular NCAL reaction
f""'-C\ '.0~QCV'lC'.t"-N V'lM t-\Otr. \CV'l---..n.r. or) or;..,.;
\1 \V 1H NMR, 300 Mz, CDCia
CON'"' I
1 Q
Cbz (±)-11c
8.0 7.5 7.0 6.5 6.0 5.5 5.0
)~( I~( I~( ~.5 ~.0
~3( 2.5 1.0 .1.5 1.0 0.5 0.0
ls(~s(
Figure 9. 1H-NMR of lactone (t)-llc
0
1 H-13C HSQC. 300 Mz. CDC!3
rt N:q, I Q
Cbz (±)-11c
r r··-~~ , .....,~·······T··--···...,---~~ ·"·····-T·~~....,.,..,---.,-.~r···~·~r~-~..,...~
Chapter4
ppm
8.0 7.5 7.0 6.5 6.0 5.5 5.0 4.5 4.0 3.5 3.0 2.5 2.0 1.5 1.0 0.5 ppm
Figure 10. 1H-13C-HSQC of lactone (t)-11c
199
Aza variant of intramolecular NCAL reaction
--
-
--
-
~ '"' I
I 1H NMR, 300 Mz, CDC~
cu N , ~ ' 0
Cbz
(±)-11d
g~~~~~~~~~ ~<:"I ""'~ 0\ 0\ ~ :-.e te ::A; r-i ri...:.....;,..;:-'-' _;-'...:
75 7J) tl.5 (1.0 5.5 5.0 4.5 4.0 3.5 3.1! 2.5 2.0 1.5
I~(
J
.. I
1~1 l~nsl lsi Is( !~( ~~(
Figure 11. 1 H-NMR of lactone (t)-11d
A A ~ A.A JL.I\ J
-- -.. -
•
Figure 12. 1H-13C-HSQC of lactone (t)-11d 200
Chapter4
1.0 0.5 ppm
1H-13C HSQC, 300 Mz,
c;q'o 0P" Cbz
(+)-11d
'.:;.v
' ::n
'
Aza variant of intramolecular NCAL reaction
7.5 7.0
I~(
"l .,., ~
160 150
1 H-NMR, 300 MHz, CDCI,
6.5 6.0
N .,-,oo
"' \0 -a-. ._; oOoOt' ::) ~s.!~
\V
140 130
5.5 5.0 4.5 4.0 3.5 3.0 2.5 2.0
Is( i§( l~rl~('l~( Is( Figure 13. 1H-NMR of diol (+}-13a
120
1H-NMR, 75 MHz, CDC~
3S
Cbz-N:t.:',H
4R OH
(+)-13a
1l0 HXl 90 80 70 60
Figure 14. 13C-NMR of diol (+}-13a
201
Chapter4
cuu'>r•t r:"~c ru"-"""'t.<"'" Nl\.l'e .:h..o:py H~·-!H
f:Xlf:r) 34:': i'lloct<O !
i:'1 · A::q•>iution P<>r<>r.>->t.e:·:. !Jl!l!~- ~t\lCCt~l
h""' ~c.tn l~IS':i't!N "P"C~ i'HO!l"O S ""' Ot-'l" 1!1!1, Pu~.;.·xtx; t.<:3:1 T'' <;.S.>H-so;.,;,·:rNr ~c.lJ
~ ;~ ~lf~. f.~~~-"" llr. l"fi,J<J:'$ C. 09~~/.:1 II<
~ ~-~~5~;~~ ,e;r: :;>;It ij(l,~~:l I'U"::
~~ 2:~ ~~ ~"~" ~G l.Cco:woo•,i-.c
~--····· c;u\.'l:<lr.:, n -···-·· ~EJCl ~H
rJ lL~O "~'"' ?t.l ··L!lO ,..,; srv! !0·~-nH~J-: ¥.H~
~-~ - P~"<.<.:<:,.<lir.<; p~n"""';;e!( !;.l 3~'/11.~
Sf ~00. ~>CCC!! :<iff;:
~~~ ll~ Lll C.C!J)lz cs !: i'C 1.1)!)
1.5 1.0 0.5 ppm
50 40 30 20 ppm
Aza variant of intramolecular NCAL reaction
(I
......
1H-13C-HSQC, 300 MHz, CDCI3
Cbz-N~,H "" OH
(+)-13a
Figure 15. 1H-13C-HSQC of diol (+}-13a
202
Chapter4
30
40
50
60
70
80
90
100
110
120
130
140
150
Aza variant of intramolecular NCAL reaction
4.9. Chiral HPLC data
CDRI SAIF GLC & HPLC LAB Lucknow
SAMPLE INFORMATION
Safll)leNarrc: SanpleType: Yiat kljectX)t;lt hleet!onVOk.!rre: ~nTi:Te:
OMJY·4C2 lklld'.,OW'r. 14 1 3.00ul 30.0Mnu!es
·············
Acq>.nred 8"/. RK_AII'Shottam SarrpteNarre £lM""f -4C 2
Acq. tl.etl'lod Set a<D_fwElHOD P."oeessina l~ttlod: Lifter A Ol'.tnnel ~ 213.9nm
Date Acqu;rea: 612412010 3:57:12 FM 1ST Date A'ocessed: 512-4i2010 4'34:13 PfA 1ST
0.050·!
o.o.~oj I
' oooo)
<. 0.0'20
o.o;o
Peak Results ........... , •••••••••••• y ............. ,
... :~~ .. ~=-~-~.~-~+ ~ .. ~.,' 1913409:569:.\.4: S0.2'J'
DAICEL CHIRAL PAK lA COLUMN, MtBE'EtOH = 99: I, FLOW RATE 0.6 mL/min, A...x 213.9 nm
Figure 16. HPLC of lactone-(!:}-11a
CDRI SAIF GLC & HPLC LAB Lucknow
SAMPLE INFORMATION
.. ,.."""'. Sarmlelype: Vial: njectiOnii: tJie<:tionVoturre Runfr.oo:
DIM'Y-58 lk'lkr,owo
" 1 .'iOOu! 30.0Mnutes
O:lte Acqurred; 5124rlQ10 5:04:34 PM 1ST Date Rocessed: 512412010 5:~:47 Ftf. 1ST
,.,,.,,,
AcqulredBy: RK_F\Jishoftam
Safl1)ieNarre DM'Y-58
Acq. Method Set: 0<0_ ~ Processi'lp tkttloc1 LMer A
21:3.9nm
Auto-5cated Chromatogram
-~-~~~Etts R '!" I Ar.l ~ l<•:gM ~ "4 Ar•a"1
DAICEL CHIRAL PAK lA COLUMN, MtBE'EtOH = 99: I, FLOW RATE 0.6 rnUmin, 1.,., 213.9 nm
Figure 17. HPLC of lactone-(+}-11a
203
Chapter4
Aza variant of intramolecular NCAL reaction
SarrpeNarre: 1 Sarllie Type:
Vial
CDRI SAIF GLC & HPLC LAB Luck now
SAMPLE INFORMATION
IJIW(.68 ~own 12
Ac:p!redBy: Sarrpie:i'e!Tl!'C».f'Y-68
RK_A.Jrshottum
tlje(:tion# nieclionVo!urre:
1 A.cq, Method Set: O<.D_~.£n-oo
3.00ul Aocessirv Method Uter A Rmrrne. 30.0 tMli!es 0\anr.e! Narre: 213 Snm
Ootle Acquired: 612412010 4:29.51 PM 1ST O!teA'"ocessed. 6l24o'20105:1t30PMIST
Auto-Scaled Chromatogram
~- ··~· """¥
2.0!1 400 soo a.OO 1o·oo 12.00
Arn : H~illht!%Arta! t···s;;.;;;-r·25ii(i;4~ "-5<'' ~-a_~:;:oo;·t:znii!/ 9145!
DAICEL CHIRAL PAK lA COLUMN, MtBE/EtOH = 99: I, FLOW RATE 0.6 mL/min, Am, 213.9 nm
Figure 18. HPLC of lactone (-)-11a
CDRI SAIF GLC & HPLC LAB Lucknow
SAMPLE INFORMATION
Sa!TJiei'G:Te: Df..p( ... 78 A:quired By: RK_F\:fsbottam Sarrp!e Type: lklknoWn Viat 15
SiltTpleN<ure Ot..PY -78
tljeeboo# 1 ~!eCticn Vobre: 3.00 lJI
Acq. ~.-lethod Set O<D_NETI-00 A"~esshQ Metnod: Uter A
Run rrne: 45.0 Mnl.lte$ Olannei Nan-e: 209 0nm
OateAcquired: S125120102.i4:59PMIST Date Processe-d: &'25'2010 3:C-5:53 RA 1ST
Aut<>-Sca1ed Chromatogram
Cbz ... /"-.....'
~ 0
(±~11b
DAICEL CHJRAL PAK lA COLUMN, MtBE!EtOH = 99: I, FLOW RATE 0.6 mUmin, '-m, 209 nm
Figure 19. HPLC of lactone (t}-11b
204
Chapter4
Aza variant of intramolecular NCAL reaction
&urp!flf'.brre SarrpleType: v:a1 ~;j.ection::::. lnieclionVoltJ:ne· RunT1rre
CDRI SAIF GLC & HPLC LAB Lucknow
SAMPLE INFORMATION
flt-.PY-9 Unknov1n 1T
' 3.00ul
Acquired By: RK_Purshcttam Sa~1N3rre Ot-.Pf -9
Acq. f/ett:od Set: CKO_fE:'t-ro Ftocessir!Q !>.-\11hod: Utef A
45.0 Mm.rtes O'lannel Narre: 2G9 Onm
euteAcquired 6!25/20101:11:19tlvl1ST DateA'~sed: 6J25..'20102:·1841 FMIST
Auto-Scaled Chromatogfam
DAICEL CHIRAL PAK lA COLUMN, MtBEIEtOH = 99: I, FLOW RATE 0.6 mL/min, J..""" 209 nm
Figure 20. HPLC of lactone (+)-11b
-t·t ..
& ..
Sarrplef'.arre $arrpleType: V13l: hjection#: hieCIIOnVofurre: RunTrne.
CDRI SAIF GLC & HPLC LAB Lucknow
SAMPLE INFORMATION
01.\!py'-8 !Jfl.kncWn
16
' 3.00<11 4~.0Mnutea
Acquiri:1d By: RK_P.Jr5;hottam Sarrple~.Brre!l.\'P!'-8
~cq. Method Set EKD _ :\t:1!-00 FtocesSir:~ f.1ethod: Litter J.. Ct'.armel Narre: 209.01'\m
Da+.e Acq:Jil'ed. 6l25120~0 12.2£55 Ft.l! 1ST Dlrte Rocessed· 6/25.'2010 2:40.47 PM 1ST
Auto-Seated Chromatogram
Cbz~Ovt
···~ 6R. Q
(-)-11b
2:ioo· 2~.co so.oo '<>.oo
DAICEL CHIRAL PAK lA COLUMN, MtBEIEtOH = 99: I, FLOW RATE 0.6 mUm in,~ 209 nm
Figure 21. HPLC of lactone (-}-11b
205
Chapter4
Aza variant of intramolecular NCAL reaction
Sa!TpleNarre: Sa:rple Type Via!: ~je:::t!o!"#. tliect:onVO~Jme RunTI('I"e:
CDRI SAIF GLC & HPLC LAB Lucknow
SAMPLE INFORMATION
Dif.JPY-01~A Unlc'la.vn 8 1 4.00u! 300/..tnutes
k:r:;uired By R!<_F\.tmhOiiam San-pleNa!reOM"'¥·C1.A
Aeq ~hod Set O<..O_~,aroo
A"OCe$SinQ ~/elhod Lilt~ A
Olar:nel Narre. 213.5nm
!l:iieAcq!.:ted: 5123/2010112.9:33AM1Si !)ate M"ocessed: 5.'2312010 12:02:41 A.-liST
Auto-Seated Chrom.rtogram
u.ns-·
/1-o \N~ ' 0 Cbz (±~11c
i i
,.,._, ,! ,,
aoo·!·-··· ···-· ...... fh'-.. .. A -,,'···'tc····{···-~'o:,-················-···············································- __ ··---· __ 1
1<1.00 1'!..00 ' '.,. ~-~ ~ : ~ .. i
22.00 24.00 16.00 2S.OO 30.00
DAICEL CHIRAL PAK lA COLUMN, MtBE!EtOH ~ 98:2, FLOW RATE 0.8 mUmin, \,x 213 nm
Figure 22. HPLC of lactone (t)-11c
Satfl'loe Name: SafrPeType: Vial: r~ieclbn# hjeclionVoJurre: RunTime·
COR! SAIF GLC & HPLC LAB Lucknow
SAMPLE INFORMATION
""""·02 t.r.ia'lown 9 1 •tOOut 30.0Mnutes
Acqu;redB"f RK_A.rrshottam
SarrpleNiirreOWr'-02
Acq. fkt'tod Set O<D_fvETt-00 Rocessin!l f.-P-fhOCI: Uter A
2~3.5nm
DaleAcquired: 6J23120'1012:10:43RAIST t::ate ftocessed €/2"",j/)010 12:56.27 FM 1ST
"' o.oof"··~--
;:,.00 2.0;)
Auto-Scaled Chromatogram
8.GO 1000 12.00
DAICEL CHIRAL PAK lA COLUMN, MtBE!EtOH ~ 98:2, FLOW RATE 0.8 mUmin, \,."' 213 nm
Figure 23. HPLC of lactone (-}-11c
206
Chapter4
Aza variant of intramolecular NCAL reaction
CDRI SAIF GLC & HPLC lAB Lucknow
SAMPLE INFORMATION
Salrole Nlrre: DM'Y' -03 Sarri:lleType: ll":IO'lO'Ivn Vial: 10 Fl}<.">C""Jon#. 1 fliectlonVct"Jm;o: -1.00UI ~r. TCTe: 30.0 Mnvtes
Date Acqul"ed: GIT.d2010 12:43:53 PM 1ST ()ate Ro=essect 6/2312010 1·19:22 PM 1ST
.·· ................. ' y··· ·················-------·----
Acquired By: RK_A.:rsnottam Sar.'pleNa:'re [)1,PY -03
Acq. Wett1od Set D<O_r.ErtOO Ftocessma ~ Utter A
Qlanr.el Nan-e: 213.5nm
Auto-Seated Chromatogram , ..................•.................................................
n II
e.oo 10.00 ~$.00 1!J:.OO 20~00 22:00 N:o0
~•m.•tes
DAICEL CHIRAL PAKIA COLUMN. MtBE/EtOH = 98:2, FLOW RAlE 0.8 mUmin, \m 213 nm
Figure 24. HPLC of lactone (+}-11c
207
Chapter4
Aza variant of intramolecular NCAL reaction Chapter4
4.10. References
1. (a) Winchester, B.; Fleet, G. W. J. Glycobiology 1992, 2, 199. {b) O'Hagan, D. Nat.
Prod. Rep. 1997, 14, 637. (c) Stu .. tz, A. E. lminosugars as Glycosidase Inhibitors;
Wiley: Weinheim, 1999. (d) Asano, N.; Nash, R. J.; Molyneux, R. J.; Fleet, G. W. J.
Tetrahedron: Asymmetry 2000, 11, 1645.
2. (a) Karpas, A.; Fleet, G. W. J.; Dwek, R. A.; Petursson, S.; Namgoong, S. K.;
Ramsden, N. G.; Jacob, G. S.; Rademacher, T. W. Proc. Nat/. Acad. Sci., U.S.A.
1988, 85, 9229. (b) Block, T. M.; Xu, X. L.; Platt, F. M.; Foster, G. R.; Gerlich, W. H.;
Blumberg, B. S.;. Dwek, R. A. Proc. Nat/. Acad. Sci., U.S.A. 1994, 91, 2235.
3. Goss, P. E.; Baker, M.A.; Carver J. P.; Dennis, J. W. Clin. Cancer Res. 1995, 1, 935.
4. Watson, K. A.; Mitchell, E. P.; Johnson, L. N.; Son, J. C.; Bichard, C. J. F.; Orchard,
M. G.; Fleet, G. W. J.; Oikonomakos, N. G.; Leonidas, D. D.; Kontou, M.;
Papageorgiou, A. C. Biochemistry 1994, 33, 5745.
5. (a) Davis, B. G.; Brandstetter, T. W.; Hackett, L.; Winchester, B. G.; Nash, R. J.;
Watson, A. A.; Griffiths, R. C.; Smith, C.; Fleet, G. W. J.; Tetrahedron 1999, 55,
4489. (b) Shilvock, J. P.; Wheatley, J. R.; Nash, R. J.; Watson, A. A.; Griffiths, R. C.;
Butters, T. D.; Muller, M.; Watkin, D. J.; Winkler, D. A.; Fleet, G. W. J. J. Chern.
Soc., Perkin Trans. 11999, 2735.
6. Fan, J. Q.; Ishii, S.; Asano N.; Suzuki, Y. Nat. Med. 1999, 5, 112.
7. (a) Li, C. M.; Tyler, P. C.; Furneaux, R. H.;. Kicska, G.; Xu, Y. M.; Grubmeyer, C.;
Girvin, M. E.; Schramm, V. L. Nat. Struct. Bioi. 1999, 6, 582. {b) Miles, R. W.; Tyler,
P. C.; Evans, G. B.; Furneaux, R. H.; Parkin, D. W.; Schramm, V. L. Biochemistry
1999, 38, 13147.
8. (a) Scott, J. D.; Tipple, T. N.;Williams, R. M. Tetrahedron Lett. 1998, 39, 3659; (b)
Scott, J. D.; Williams, R. M. Tetrahedron Lett. 2000, 41, 8413.
9. Asano, N.; Ishii, S.; Kizu, H.; Ikeda, K.; Yasuda, K.; Kato, A.; Martin, 0. R.; Fan, J. Q.
Eur. J. Biochem. 2000, 267,4179-4186.
10. (a) Kuehl, F. A., Jr.; Spencer, C. F.; Folkers, K. J. Am. Chern. Soc. 1948, 70, 2091. (b)
Kobayashi, Sh.; Ueno, M.; Suzuki, R. Tetrahedron Lett. 1999,40, 2175. 208
Aza variant of intramolecular NCAL reaction Chapter4
11. (a) Watanabe, J.; Nakada, N.; Sawairi, S.; Shimada, H.; Ohshima, S.; Kamiyama, T.;
Arisawa, M. J. Antibiot. 1994, 47, 32. (b) Lewis, R. J.; Singh, 0. M. P.; Smith, C. V.;
Maxwell, A.; Skarzynski, T.; Wonacott, A. J.; Wigley, D. B. J. Mol. Bioi. 1994, 241,
128.
12. Gardiner, R. A.; Rinehart, K. L.; Snyder, J. J.; Broquist, H. P. J. Am. Chern. Soc. 1968,
90, 5639-5640.
13. Hohenschutz, L. D.; Bell, E. A.; Jewess, P. J.; leworthy, D. P.; Pryce, R. J.; Arnold, E.;
Clardy, J. Phytochemistry 1981, 20, 811-814.
14. Kakinuma, K.; Otake, N.; Yonehara, H. Tetrahedron Lett. 1972, 2509-2512.
15. Linda M. Mascavage, Qing Lu, Jessica Vey, David R. Dalton, Patrick J. Carroll J. Org.
Chern. 2001, 66, 3621-3626.
16. Evans, G. B.; Furneaux, R. H.; Tyler, P. C.; Schramm, V. L. Org. Lett. 2003, 5, 3639-
3640.
17. Evans, G. B.; Furneaux, R. H.; Lewandowicz, A.; Schramm, V. L.; Tyler, P. C. J. Med.
Chern. 2003,46,5271-5276.
18. Kotian, P. L.; Chand, P. Tetrahedron Lett. 2005, 46, 3327-3330.
19. (a) For a review describing routes to optically active ~-lactones, see: Yang, H. W.;
Romo, D. Tetrahedron 1999, 51, 6403-6434. (b) For a recent excellent advance in
this area, see: Nelson, S. G.; Peelen, T. J.; Wan, Z. J. Am. Chern. Soc. 1999, 121,
9742-9743.
20. (a) Pommier, A.; Pons, J. M. Synthesis 1993, 441-459. (b) For more recent
transformations, see: Yang, H. W.; Romo, D. J. Org. Chern. 1999, 64, 7657-7660
and references cited therein.
21. Wynberg, H.; Staring, E. G. J. Am. Chern. Soc. 1982, 104, 166.
22. (a) In early studies by Wynberg, it was determined that at least two R-halogen
atoms were required, see: Wynberg, H.; Staring, E. G. J. J. Org. Chern. 1985, 50,
1977-1979. (b) For other activated carbonyl compounds that participate in this
reaction, see: Ramiandrasoa, P.; Guerin, P.; Girault, J. P.; Bascou, P.; Hammouda,
A.; Cammas, S.; Vert, M. Polym. Bull. 1993, 30, 501-508.
209
Aza variant of intramolecular NCAL reaction Chapter4
23. (a) Cortez, G. S.; Tennyson, R. L.; Romo, D. J. Am. Chern. Soc. 2001, 123, 7945. (b)
Purohit, V. C.; Richardson, R. D.; Smith, J. W.; Romo, D. J. Org. Chern. 2006, 71,
4549. (c) Cortez, G. 5.; Oh, S. H.; Romo, D. Synthesis 2001, 1731.
24. Oh, S. H.; Cortez, G. S.; Romo, D. J. Org. Chern. 2005, 70, 2835.
25. (a) Scott, J. D.; Tippie, N. R.; Williams, R. M. Tetrahedron Lett. 1998, 39, 3659-
3662. (b) Knight, D.W.; Lewis, N.; Share, A. C.; Haigh, D. Tetrahedron Asymmetry
1993, 4, 625-628. (c) Ohara, C.; Takahashi, R.; Miyagawa, T,; Yoshimura, Y.; Kato,
A.; Adachib, 1.; Takahataa, H. Bioorg. Med. Chern. Lett. 2008, 18, 181Q-1813.(d)
Quibell, M.; Benn, A.; Flinn, N.; Monk, T.; Ramjee, M.; Wang, Y.; Watts, J. Bioorg.
Med. Chern. 2004, 12, 5689; (e) Sugisaki, C: H.; Carroll, P. J.; Correia, C. R. D.
Tetrahedron Lett. 1998, 39, 3413; (f) Shibasaki, T.; Sakurai, W.; Hasegawa, A.;
Uosaki, Y.; Mori, H.; Yoshida, M.; Ozaki, A. Tetrahedron Lett. 1999, 40, 5227-5230.
(g) Gotschi, E.; Jenny, C. J.; Reindl, P.; Ricklin, F. Helvitica Chimica Acta. 1996, 79,
2219.
26. (a) Jourdant A.; Zhu, J. Tetrahedron Lett. 2000, 41, 7033-7036 (b) Takahata, H.;
Banba, Y.; Ouchi, H.; Nemoto, H.; Kato, A.; Adachi, I. J. Org. Chern. 2003, 68,
3603-3607 (c) Liang, N.; Datta, A. J. Org. Chern. 2005, 70, 10182-10185 (d)
Karjalainen, 0. K.; Passiniemi, M.; Koskinen, A. M. P. Org. Lett. 2010, 12, 1145 (e)
Gryko, D.; Prokopowicza, P.; Jurczaka, J. Tetrahedron: Asymmetry 2002, 13,1103-
1113
27. (a) Kalamkar, N. B:; Kasture, V. M.; Dhavale, D. D. J. Org. Chern. 2008, 73, 3619-
3622 (b) Patil, N. T.; John, 5.; Sabharwalb, S. G.; Dhavalea, D. D. Bioorg. Med.
Chem. 2002, 10, 2155-2160 (c) Steiner, A. J.; $chitter, G.; StOtz, A. E.; Wrodnigg, T.
M.; Tarling, C. A.; Withers, S. G.; Fantur, K.; Mahuran, D.; Paschke, E.; Tropak, M.
Bioorg. Med. Chern. 2008, 16, 10216-10220 (d) Boucheron, C.; Compain, P.;
Martin, 0. R. Tetrahedron Lett. 2006, 47, 3081-3084
28. For a review see: Kacprzak, K.; Gawronski, J. Synthesis 2001, 961-998.
29. Schmuck, C.; Rehm, T.; Geiger, L.; Schfer, M. J. Org. Chern. 2007, 72, 6162-6170.
210
Aza variant of intramolecular NCAL reaction Chapter 4
30. Almeida, J. F.; Anaya, J.; Martin, N.; Grande, M.; Moran, J. R.; Caballero, Ma. C.
Tetrahedron: Asymmetry, 1992, 3, 1431-1440.
31. Waddell, T. G.; Woods, L. A.; Harrison, W.; Meyer, G. M. J. Tennessee Acad. Sci.
1984,59,48-50.
32. Pracejus, H.; Matje, H. J. Prak. Chern. 1964, 195-205.
33. Asahina, Y.; Takei, M.; Kimura, T.; Fukuda, Y. J. Med. Chern. 2008, 51, 3238-3249.
211