More on Electron Configurations
description
Transcript of More on Electron Configurations

More on Electron Configurations


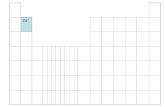
Identify the blocks on your periodic table. Each block is named after its characteristic orbital; thus, the blocks are:
Shell number minus 1.
Shell number minus 2.
He is in the S Block.


2. Write the electron and orbital diagram for Z = 20.

2. Write the electron configuration and orbital diagram for Z = 20 Ca.
Ca
Valence Electrons (Lewis Dot Structure) – are all e’ in the outermost e’ shell (in the shell with the highest shell number.

3. Write the condensed electron configuration for Z = 20 Ca.
Last Noble Gas
[Ar]

4. Write the electron Configuration and orbital diagram for Z = 26 Fe.
3d6

5. Write the condensed electron configuration for Z = 26 Fe.
Last Noble GasSd
3d6[Ar]
-1
-2

6. Write the electron and orbital diagram for Z = 38 Sr.
Valence Electrons

7. Write the condensed electron configuration for Z = 38 Sr.
Last Noble GasS pd

7. Write the condensed electron configuration for Z = 50 Sn.
Last Noble GasS pd
Sn

8. Write the condensed electron configuration for Z = 6 C.
Last Noble Gas
S p
[He] 2s2 2p2 CValence Electrons

9. Write the condensed electron configuration for Z = 31 Ga.
Last Noble GasS p
[Ar] 4s2 3d10 4p1
dGa
Valence Electrons

10. Write the condensed electron configuration for Z = 98 Cf Californium.
Last Noble Gas
S
[Rn] 7s2 5f10
f -2

11. What element has the condensed electron configuration [Ar] 4s1?
Last Noble Gas
KValence Electrons

12. What element has the condensed electron configuration [Xe] 6s2 4f14 5d4?
Last Noble Gas
S
fd
4f

13. What element has the condensed electron configuration [Ne] 3s2 3p2 ?
Last Noble GasS P
SiValence Electrons

14. What element has the condensed electron configuration [Ne] 3s2 3p6 ?
Last Noble GasS P
ArValence Electrons

15. What element has the condensed electron configuration [Xe] 6s2 4f14 5d10?
Last Noble Gas
Sd
f

Valence ElectronsDraw the condensed electron configuration and electron dot structures for:
1.Na 6. F2.B 7. S3.N 8. Ba4.Cl 9. Mg5.Kr 10. C

[Ne] 3s11. Na: Na

[He] 2s22. B: B2p1

[He] 2s23. N: N2p3

[Ne] 3s24. Cl: Cl3p5

[Ar] 4s25. Kr: Kr3d10 4p6

[He] 2s26. F: F2p5

[Ne] 3s27. S: S3p4

[Xe] 6s28. Ba: Ba

[Ne] 3s29. Mg: Mg

[He] 2s210. C: C2p2