Separating Mixtures Grade 7 Science: Pure Substances and Mixtures S. Willis.
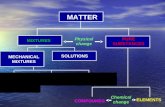
Mixtures. Mixtures A physical blend of more than TWO SUBSTANCES A physical blend of more than TWO...
-
Upload
myles-jones -
Category
Documents
-
view
216 -
download
0
Transcript of Mixtures. Mixtures A physical blend of more than TWO SUBSTANCES A physical blend of more than TWO...

MixturesMixtures

MixturesMixtures
A physical blend of more than TWO A physical blend of more than TWO SUBSTANCESSUBSTANCES
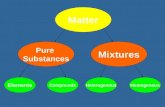
There are TWO classifications of There are TWO classifications of mixturesmixtures
HOMOGENEOUSHOMOGENEOUS HETEROGENEOUSHETEROGENEOUS

HOMOGENEOUS MIXTURESHOMOGENEOUS MIXTURES
HOMO HOMO – From the Greek word – From the Greek word meaning THE SAMEmeaning THE SAME
Completely uniform distributionCompletely uniform distribution Each sample will contain the same Each sample will contain the same
proportions of the mixtureproportions of the mixture Solutions are examples of Solutions are examples of
HOMOGENEOUS MIXTURESHOMOGENEOUS MIXTURES

SOLUTIONSSOLUTIONS
Place salt in waterPlace salt in water Solid phase added to liquid phaseSolid phase added to liquid phase Homogeneous?Homogeneous? YES! YES! The salt dissolves in the water leading The salt dissolves in the water leading
to a to a UNIFORM DISTRIBUTIONUNIFORM DISTRIBUTION Solutions can be gas/gas, liquid/gas, Solutions can be gas/gas, liquid/gas,
gas/liquid, liquid/liquid, solid/liquid and gas/liquid, liquid/liquid, solid/liquid and solid/solidsolid/solid

EXAMPLESEXAMPLES
G/G ? AIRG/G ? AIR L/G ? Water vapor in air (clouds - L/G ? Water vapor in air (clouds -
localized)localized) G/L? SodaG/L? Soda L/L? Coffee/TeaL/L? Coffee/Tea S/L? anything that dissolves in waterS/L? anything that dissolves in water S/S? SteelS/S? Steel

HETEROGENEOUS MIXTURESHETEROGENEOUS MIXTURES
HETEROHETERO – From the Greek word – From the Greek word meaning differentmeaning different
Not uniform in compositionNot uniform in composition Oil and waterOil and water Heterogeneous?Heterogeneous? YES!YES! The oil will not mix with the water The oil will not mix with the water
creating two phasescreating two phases

A Phase?A Phase?
Any part of a system with uniform Any part of a system with uniform propertiesproperties
Homogeneous Mixtures consist of a Homogeneous Mixtures consist of a single phasesingle phase
Heterogeneous mixtures consist of Heterogeneous mixtures consist of two or more phasestwo or more phases

ExamplesExamples
Lava Lamp – Water Phase/Wax PhaseLava Lamp – Water Phase/Wax Phase Soup – Liquid/Chunky BitsSoup – Liquid/Chunky Bits Burgers – Meat/Bread/Salad/Special Burgers – Meat/Bread/Salad/Special
SauceSauce Your turn – Think of 3 more examples Your turn – Think of 3 more examples
of heterogeneous mixturesof heterogeneous mixtures

Separating MixturesSeparating Mixtures
Some mixtures are easily separated Some mixtures are easily separated by simple physical meansby simple physical means
Burger with no pickle – Just take out Burger with no pickle – Just take out the picklethe pickle
Some mixtures may rely on physical Some mixtures may rely on physical properties to separateproperties to separate

Lets think about a complex Lets think about a complex mixturemixture
Salt and waterSalt and water HomogeneousHomogeneous Salt dissolved in the waterSalt dissolved in the water What can we do to separate this What can we do to separate this
mixture mixture Are there any physical properties we Are there any physical properties we
can make use ofcan make use of

Salt and WaterSalt and Water
Water evaporates at much lower Water evaporates at much lower temperatures than salttemperatures than salt
Heat the mixtureHeat the mixture The water will evaporate, leaving the The water will evaporate, leaving the
saltsalt How could we recover the water?How could we recover the water?

DistillationDistillation
Salt + Water
Heat
Liebig Condenser
Water
•Water is evaporated by the heat•Cooled and condensed in the condenser•Collected in the beaker•The salt will remain in the conical flask as a crystallized solid

More DistillationMore Distillation
What else can we separate with distillation What else can we separate with distillation apparatus?apparatus?
Mixtures of LiquidsMixtures of Liquids
How?How?
We use the physical property of BOILING POINT We use the physical property of BOILING POINT to separate different liquidsto separate different liquids
This type of distillation is called FRACTIONAL This type of distillation is called FRACTIONAL DISTILLATIONDISTILLATION

DistillationDistillationThermometer
Salt + Water
Condenser
CollectionVessel
Heat

ReviewReview
Give five examples of the following:Give five examples of the following:
1.1. Pure substancePure substance
2.2. Homogeneous MixtureHomogeneous Mixture
3.3. Heterogeneous MixtureHeterogeneous Mixture
4.4. SolutionSolution Outline a method for separating Outline a method for separating
components from of your examples for components from of your examples for 2, 3 and 42, 3 and 4

Other Separation MethodsOther Separation Methods
ChromatographyChromatography LiquidLiquid IonIon Size ExclusionSize Exclusion
PrecipitationPrecipitation Solvent Extraction (filtration)Solvent Extraction (filtration)

DEMODEMO
Set up a CHROMATOGRAPHY Experiment Set up a CHROMATOGRAPHY Experiment from the handoutfrom the handout
Refer to the instructions for writing up a labRefer to the instructions for writing up a lab PURPOSE – To see if ink is a mixture or a pure PURPOSE – To see if ink is a mixture or a pure
substancesubstance Analysis and conclusions questionsAnalysis and conclusions questions
What did you observe?What did you observe? How do we classify the ink? Is it a pure substance?How do we classify the ink? Is it a pure substance?