Lipids
Transcript of Lipids

Lipids
Contain the elements C, H and O. But the ratio of H:O is higher than that found in carbohydrates.
These are produced by condensation reaction of a glycerol (propan-1,2,3-triol) and 3 fatty acids. Triglycerides are the commonest lipids in nature and are classified in to fats or oils (solid, liquid at RT).
Primary importance of triglycerides is as energy stores. They can release twice as much as energy as the same mass of carbohydrate. They are non-polar and insoluble in water; they can be stored in high concentrations without affecting the water potential of cells.
STRUCTURE OF TRIGLYCERIDES
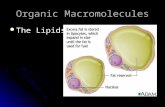
Phospholipids: these are made of glycerol, 2 fatty acids and a phosphate group. Phospholipids have a polar head (the phosphate group) and 2 non-polar tails (hydrocarbon fatty acid chains). They are important components of cell membrane and also form the myelin sheath around neurones.
STRUCTURE OF PHOSPHOLIPID
Condensation reaction: triglycerides are formed when each fatty acid joins with one of the OH group of glycerol with the removal of a water molecule, resulting in an ester bond/link.
DIAGRAM OF REACTION

Variation in triglycerides:
All glycerol molecules are the same. The fatty acids vary in:
The length of the hydrocarbon chain, from 8 to 20 carbons The absence or presence and number of double bonds The mix of fatty acids (i.e. all three the same, all different or some other
combination).
Saturated and unsaturated lipids
Carbon compounds that contain double carbon-carbon bonds are known to chemists as unsaturated compounds.
Carbon compounds that contain single carbon-carbon bonds are known to chemists as saturated compounds.
Lipids made of saturated fatty acids are known as saturated fats. Examples include palmitic acid, C15H31COOH and stearic acid (butter).Lipids made of unsaturated fatty acids are known as unsaturated fats. Example is oleic acid C17H33COOH.
Saturated and unsaturated fatty acids differ in the amount of hydrogen. The saturation refers to the amount of hydrogen in the molecule.
The difference in saturation has important effects on triglycerides.
Structure/physical properties/biological significance
Saturated (fats) Monounsaturated (one double bond) (oils)
Polyunsaturated (more than one double bond) (oils)
Strong intermolecular bonds Intermolecular bonds weaker because of kinked shape
Intermolecular bonds are weaker because of kinked shape
Solid at RT Liquid at RT Liquid at RTStraight chain molecules Molecule with one kink in
chain Molecule with x kink in chain (x is the no. of double bonds).

CHOLESTEROL
Cholesterol is also a class of lipid, even though it does not form from fatty acids and glycerol
like triglycerides and phospholipids do. It is a small molecule made from four carbon-based
rings. Cholesterol is a small structure which is very hydrophobic. It is found in all biological
membranes, and these features allow it to sit nicely between the fatty acid tails.
This molecule is vital to living organisms, so many cells (especially those in the
liver) can manufacture it. Excess cholesterol can cause health problems. A
condition known as familial hypercholesterolemia (FHC: high blood
cholesterol levels that run in families) is a genetic disorder, where cells
manufacture and secrete cholesterol even though there is already sufficient
in the blood to provide for the organism’s requirements. This happens because
the cells do not obey the signals to stop cholesterol production, as they lack a
particular cell surface receptor.