Lipids
-
Upload
university-of-tripoli-food-science-dept-tripoli-libya -
Category
Education
-
view
2.946 -
download
3
description
Transcript of Lipids

Lipids

• Lipids are a broad group of naturally occurring molecules which includes fats, waxes, sterols, fat-soluble vitamins (such as vitamins A, D, E and K),monoglycerides, diglycerides, phospholipids, and others.
• The main biological functions of lipids include energy storage, as structural components of cell membrane, and as important signaling molecules (substances synthesized by cells for purposes of extracellular communication between cells).

• Fats consist of a wide group of compounds that are generally soluble in organic solvents and largely insoluble in water.
• Chemically fats are generally triesters of glycerol and fatty acids.
• Fats may be either solids or liquid at room temperatures , depending on their structure and composition.
• Although the words "oils", "fats", and “lipids" are all used to refer to fats, "oils" is usually used to refer to fats that are liquids at normal room temperature
ester

• while "fats" is usually used to refer to fats that are solids at normal room temperature.
• "Lipids" is used to refer to both liquid and solid fats, along with other related substances.
• The word “oile" is also used for any substance that does not mix with water and has a greasy feel, such as petroleum (or crude oil), heating oil, and essential oils, regardless of its chemical structure

• Lipids may be broadly defined as hydrophilic (having a strong affinity for water).
• or amphiphilic( Amphiphile from the Greek, amphis: both and,philia : love, friendship) is a term describing a chemicals compound possessing both hydrophilic (water-loving) and lipophilic (fat-loving) properties. Such a compound is called amphiphilic or amphipathic) .


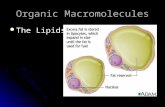
• amphiphilic small molecules; the amphiphilic nature of some lipids allows them to form structures such as vesicles( is a relatively small and enclosed compartment, separated from the cytosol by at least one lipid bilayer. Vesicles store, transport, or digest cellular products digest and wastes) , liposomes (membrane-bound digestive vesicles) can digest macromolecules (break them down to small compounds) that were taken in from the outside of the cell by an endocytic vesicle. This is the basic way for a cell to feed (except for photosynthesis in plants, which don't have lysosomes)., or membranes in an aqueous environment.

• Biological lipids originate entirely or in part from two distinct types of biochemical subunits or "building blocks": ketoacyl and isoprene groups.

(membrane-bound digestive vesicles)

• Using this approach, lipids may be divided into eight categories:
1. fatty acyls,2. glycerlipids,3. glycerophospholipidsd,4. sphingolipids,5. saccharolipids and 6. polyketides (derived from condensation of
ketoacyl subunits); 7. and sterol lipids and8. prenol lipids (derived from condensation of
isoprene subunits

• Although the term lipid is sometimes used as a synonym for fats, fats are a subgroup of lipids called triglycerides.
• Lipids also encompass molecules such as fatty acids and their derivatives (including tri- di-, and monoglycerides and phospolidis), as well as other sterols- containing metabolites such as cholesterol.
• Although humans and other mammals use various biosynthetic pathway to both break down and synthesize lipids, some essential lipids cannot be made this way and must be obtained from the diet.

• Sterols are a subgroup of the steroids and an important class of organic molecules.
• They occur naturally in plants, animals, and fungi, with the most familiar type of animal sterol being cholesterol.
• Cholesterol is vital to cellular function, and a precursor to fat-soluble vitamins and steroid hormones.

• steroid Any of numerous naturally occurring or synthetic fat-soluble organic compounds having as a basis 17 carbon atoms arranged in four rings
• and including the sterols and bile acids, adrenal and sex hormones, certain natural drugs such as digitalis compounds, and the precursors of certain vitamins.

• A monoglyceride, more correctly known as a monoacylglycerol, is a glyceride consisting of one fatty acid chain covalently bonded to a glycerol molecule through an ester linkage.
• Monoacylglycerol can be broadly divided into two groups;
• 1-monoacylglycerols and• 2-monoacylglycerols, • depending on the position of
the ester bond on the glycerol moiety.
Monoglyceride

• Mono- and diglycerides are commonly added to commercial food products in small quantities.
• They act as emulsifiers, helping to mix ingredients such as oil and water that would not otherwise blend well.

• The commercial source may be either animal (cow) or vegetable, and they may be synthetically made as well.
• They are often found in bakery products, beverages , ice cream, chewing gum, shortening, whipped toppings, margarines, and confections. When used in bakery products, monoglycerides improve loaf volume, and create a smooth, soft crumb.

• A diglyceride, or a diacylglycerol (DAG), is a glyceride consisting of two fatty acid chains covalently bonded to a glycerol molecule through ester linkages.
• One example, is 1-palmitoyl-2-oleoyl-glycerol, which contains side-chains derived from palmitic acid and oleic acid.
• Diacylglycerols can also have many different combinations of fatty acids attached at both the C-1 and C-2 positions.

• Phospholipids are a class of lipids and are a major component of all cell membranes as they can form lipid bilayers.
• Most phospholipids contain a diglyceride, a phosphate group, and a simple organic molecule such as choline; one exception to this rule is sphingomyeline, which is derived from sphingosine instead of glycerol.
• The first phospholipids identified as such in biological tissues was lecithin, or phosphatidylcholine, in the egg yolk


1. What are the main types of lipids?• The main types of lipids are triglycerides
(fats and oils), phospholipids, waxes and steroids.
2. What is the structural formula of glycerol? To which organic function do these molecules belong?
• Glycerol is a linear chain of three carbons; the central carbon is bound to one hydroxyl radical and to one hydrogen and the two other carbons in the extremities are bound to a hydroxyl radical and to two hydrogens.
• Spatial sides of the hydroxyls are the same

• 3. How are triglycerides made?
• Triglycerides, fats or oils, are made of three molecules of fatty acids bound to one molecule of glycerol.
• Hydroxyls of each one of the three fatty acids and each hydrogen of the hydroxyls of the glycerol bind to form three molecules of water that are liberated

•4. What are phospholipids?
• Phospholipids are molecules made of glycerol bound to two long molecules of fatty acids and to one phosphate group.
• Therefore, phospholipids are amphipathic molecules, i.e., they have a non-polar portion, due to the long fatty acid chains, and a polar portion, due to the group phosphate.
• Phospholipids are the main component of cell membranes. Sphingomyelin, the substance that forms the myelin sheath of axons in the nervous system, is a phospholipid too.

5. What are steroids? What are some examples of steroids with a biological function?
• Steroids are lipids based in an angular combination of four carbon rings, three of them made of six carbons and one ring made of five carbons in the extremity.
• The union of each ring to the adjacent ring is made by the sharing of two adjacent carbons belonging to both rings.
• Bile salts, cholesterol, the sexual hormones estrogen, progesterone and testosterone, the corticosteroids and the pro-vitamin D are examples of steroids.

6. What are hydrophobic molecules (or hydrophobic molecular regions)? What are hydrophilic molecules? How can they be characterized in relation to their polarity?
• Hydrophobic molecules are those that have little or no propensity to dissolve in water (hydro = water, phobia = fear). Hydrophilic molecules are those that have great propensity to dissolve in water (philia = friendship).
• Water is a polar substance. Remembering the rule that “equal dissolves equal” one can conclude that hydrophobic substances are non-polar molecules while hydrophilic molecules are polar molecules.

• 7. Are organic solvents like benzene and ether polar or non-polar substances?
• Benzene and the ethers are molecules without electrically charged portions and thus they are non-polar substances.

• 8. Regarding solubility, how are lipids classified? • Fats and oils are hydrophobic molecules, i.e.,
they are non polar and insoluble in water. Lipids in general are molecules with a large non-polar extension and so they are soluble in non polar solvents, like benzene, ether and chloroform.
• There are some amphipathic lipids, i.e., lipids whose molecules have a hydrophilic portion, like the phospholipids, giving them the property of being dragged by water, and a hydrophobic portion (non polar).

• 9. What is meant by saturation or unsaturation of oils and fats?
• When it is said that a triglyceride is saturated it means that in its molecule the carbon chain is bound in its maximum capacity to hydrogens, i.e., there are no double or triple bonds between carbons. These saturated molecules are generally solid fats at normal temperature.
• Unsaturated triglyceride molecules are those in which there are double or triple bonds between carbons and so they do not accomplish their maximum capacity of hydrogenation. These unsaturated molecules in general are oils, liquid at normal temperature.
• The terms saturated or unsaturated refer then to the saturation of the carbonic chain by hydrogen atoms.


• 10. Why do fats have thermal isolation properties? • Triglycerides are weak heat conductors and in
addition they form thick layers of fat tissue when accumulated by the organism.
• That is why they are good thermal isolators.• In animals that live in cold climates, like polar bears,
seals and whales, the adipose tissue helps the maintenance of the internal body temperature.

• 11. How are lipids used as an energy source by the organism?
• Carbohydrates are the main energy source for aerobic cell respiration.
• In the absence or deficiency of such substances the organism can use lipid stores since fats (like proteins) can be degraded into acetyl-CoA to feed the Krebs cycle (a stage of the aerobic cellular respiration).