Laboratory Diagnosis of Radicular and Pseudoradicular ...
Transcript of Laboratory Diagnosis of Radicular and Pseudoradicular ...

Kleine et al.: Workshop rcport: CSF diagnosis of radicular and pseudoradicular syndromes 45
Eur. J. Clin. Chem. Clin. Biochem.Vol. 32, 1994, pp. 45-52© 1994 Walter de Gruyter & Co.
Berlin · New York
Laboratory Diagnosis of Radicular and Pseudoradicular Syndromesin Cerebrospinal Fluid (CSF):Reliability of Methods in Consideration of Pathogenetic AspectsReport on Section "CSF Diagnosis" of 4th Klagenfurter Neurology Workshop Conferenceon Radicular and Pseudoradicular Syndromes1, Klagenfurt, Austria, August 28—29, 1992
By T. O. Kleine \ R. Hackler \ A. Lütcke 2, P. Zöfel3, J. Albrecht4, E. Rumpls and H. J. Meyer-Rienecker 6
1 Med. Zentrum für Nervenheilkunde, Funktionsbereich Neurochemie der Universität Marburg2 Med. Zentrum für Nemenheilkunde, Abteilung für Neuroradiologie der Universität Marburg3 Abteilung Anwendung im Rechenzentrum der Universität Marburg4 Becton Dickinson GmbH, Heidelberg5 Abteilung für Neurologie, Landeskrankenhaus Klagenfurt6 Abteilung Neurologie, Nervenklinikt Universität Rostock
(Received August 5/November 5, 1993)
Dedicated to Prof. Dr. K. Felgenhauer on the occasion ofhis 60th birthday
Participants
J. Albrecht, Heidelberg H. J. Meyer-Rienecker, Rostock H. Scholz, VillachU. Baumhackl, St. Polten /. Mohsenipour, Innsbruck G. Stanek, WienW. Grisold, Wien E. Plank, Villach L. Weber, MarburgR. Hackler, Marburg P. Pohl, Innsbruck H. Ziervogel, VillachT. O. Kleine, Marburg E. Rumpl, Klagenfurt P. Zöfel, MarburgA. Lütcke, Marburg E. Schmutzhard, Innsbruck
Summary: Laboratory tests may be used to confirm the clinical differentiation of pseudoradicular syndromes andradicular syndromes. In the presence of pseudoradicular syndromes, CSF and blood samples yield no positiveresults with either non-specific or specific methods. Radicular syndromes give rise to positive findings; using non-specific methods they can be subdivided into inflammatory and non-inflammatory forms, with and without blood-nefve barrier impairment. Non-specific quantities of CSF routine diagnosis are total protein, albumin, leukocytecounts and differential cell courit, i-läctäte, intrathecal -IgG, -IgA, -IgM and immunoglobulin-class oligoclonalbands. Oligoclonal bands enable the highly sensitive differentiation of non-inflammatory firom subacute-chronicallyinflammatory forms of radicular syndromes. Most of the specific quantities are the subject of current research, e. g.bacterial antigens, Z)-lactate, cultivation tests, polymerase chain reaction tests and pathogen-specific oligoclonalbands. Pathomechanisms affecting the permeability of the blood-^nerve barrier to increasing concentrations of proteinand to leukocyte subsets possibly explain the CSF fmdings in radicular and pseudoradicular syndromes.
*) Organization: Univ.-Prof. Dr. E. Rumpl, Abteilung für Neurologie, Landeskrankenhaus Klagenfurtj 2) Enzyme:
Creatine kinase EC 2.7.3.2
Eur. J. Clin. Chem. Clin. Biochena. / Vol. 32, 1994 / No. l

46 Kleine et al.: Workshop report: CSF diagnosis of radicular and pseudoradicular syndromes.
Definition and Pathogencsis of Radicularand Pseudoradicular Syndromes
Radicular and pseudoradicular syndromes frequently be-come symptomalic äs sensations and pain localized indermatomes.
Clinically, radicular syndromes usually include sensi-tivity and motor deficits of characteristic distribution (1).They are of different causality. Thus, trauma of the spineand compression of nerve roots or spinal cord by discherniation may produce similar clinical Symptoms, re-sembling those sometimes found in patients with in·*flammatoiy diseases of the peripheral nervous System,caused e. g. by viral infections (2, 3), especially by tick-bome polyneuritis (l, 4). Varioüs neuropathies of in-flammatory and non-inflammatory genesis may alsoshow radicular Symptoms. As the blood-nerve-barrierappears to be involved in inflammatory diseases, humo-ral and cellular quantities of blood-nerve barrier ilinctionare important components of laboratory diagnosis.
With pseudoradicular syndromes, pain and sensationsare not restricted to dermatoma and, by definition, thereare no lesions of nerve roots, leading to sensitivity ormotor deficits (5). Pseudoradicular syndromes may becaused by torsional or compression injuries of the discanulus fibrosus (6), or by spondylopathic pain in inter-vertebral joints, leading to tendinosis and myogelosiswith rupture of muscle fibres (cf. 1. c. (7)). The lattermay also be of rheumatic genesis e. g. with collagenosisor immune vasculitis (cf. 1. c. (7)).
The significance and reliability of laboratory methods inthe differential diagnosis of radicular and pseudoradicu-lar syndromes were discussed at the CSF laboratory sec-tion of the 4th Klagenfürter Neurology Workshop Con-ference on radicular and pseudoradicular syndromes, re-cently published in German in Berichte der ÖGKC. Inview of the important aspects of the laboratory diagnosisand pathogenesis of radicular and pseudoradicular syn-dromes reported at this Conference, a summary report ispresented here in English.
Laboratory Diagnosis of Radicular andPseudoradicular Syndromes
Pseudoradicular syndromes generally do not give rise topositive results for CSF analysis: but slightly elevatedprotein Contents were found in some cases of lumbardisc protrusions (tab. 1), obviously caused by protrusionof the degenerated disc into the spinal space. A discprotrusion can lead to back pain by Irritation of nerveendings in the compromised anulus fibrosus (6). Inblood serum, occasionally elevated creatine kinase (cre-
atine kinase-MB < 6%) activities from affected skeletalmuscles were detected, äs well äs rheumatic factors (8).
Radicular syndromes are indicated by non-specificquantities in CSF; they can be verified by specific quan-tities (8).
Non-specific Laboratory Quantities of RadicularSyndromes
A: Quantities ofthe blood-nerve barrier. As in the caseof the blood-brain barrier (9, 10), increased total protein(and albumin) contents in CSF and decreased blood/CSFprotein (albumin) gradients are signs of blood-nerve bar-rier disturbance in the CSF (see tab. 1). To explain thepathogenetic aspects, blood-nerve barrier is definedhere briefly:
Blood-nerve barrier is present in the perineurium of ev-ery nerve consisting of concentric sleeves of flättenedpolygonal cells (11), and in endothelial cells from thevasa nervorum (12), both linked by "tight jumctions",similar to the blood-brain barrier (cf. I.e. (10)). In thearea of nerve roots, however, the perineurium barrierand endothelial barrier are partially permeable to albu-min, immunoglobulins and their complexes, to medic-aments and other compounds äs well äs to leukocytes orpathogens (11, 12). Therefore, the CSF space becomesaccessible to systemic influences in the area of nerveroots. This may explain the pathomechanisms of neuro-pathies associated with metabolic or immune System^·diseases or intoxications (see tab. l and 1. c. (13, 14)).
Moreover, constituents of the endoneurinal space ofnerves may enter retrogradely the CSF in the root area,so that they are detected in CSF samples. Thus, blood-nerve barrier breakdown with protein^rich vasogenicoedema of the endoneurinal space, äs found e. g. in in-flamed or mechanically damaged nerve roots, results inthe elevation of protein in lumbar CSF. Examples ofthese pathomechanisms with inflammatory neuropathiesand non-inflämrnatory neuropathies or compressionneuropathies are presented in table 1. Note that thesteepness of serum/CSF protein gradients correlates withthe magnitude of the nerve damage produced by differ- ·ent forms of disc herniation with foraminal or spinalStenosis (causing nerve compression). To discrirninatebetween blood-nerve barrier and blood-brain barrierfunctions barrier quantities in cisternal and lumbar CSFsamples from the same patient have to be analysed sim-ultaneously. However, it is inconvenient and inappropri-ate to perform cisternal and lumbar punctures simul-taneously on patients for daily routine analysis.
As patients with viral meningitis may show radieularSymptoms (7), the extent of blood-nerve barrier disturb-
Bur. J. Clin. Chem. Clin. Biochem. / Vol. 32, 1994 / No, l

Kleine et a!.: Workshop rcport: CSF diagnosis of radicular and pscudoradicular syndromes 47
Tab. 1 Lcukocytc counts using a Fuclts-Rosenthal chamber (9)und total protcin (biurct incthod (10)) in ccrcbrospinalsainptcs (Vom controls and various paticnts
Patient Group(number of cases)
Controls of lumbar CSF (72)Controls of cisternal CSF (43)
Traumatic and comprcssion nettropathiesContusio spinalis (21)Lumbar disc protrusion (156)Lumbar disc herniation medial (36)
latcro-medial (65)lateral (331)
Massive disc herniation lumbar (28)Cervical disc hcrniation (37)
Cervical syndromc (22)
Inflammatory diseases ofthe nenvus systemMeningitis
bacterial (25)viral (30)
Meningeosis neoplastica (15)Tick-borne polyncuritis (30)
Garin-Bujadoux-BannwarthFacial paresis peripheral (33)Mono-, di-, tctra-plegia (38)
Inflammaton' neitropathyNeuritis (12)Landiy-Gwllain-Barre Syndrorne (31)Multiple sclerosis (175)
relapsing (36)Optic neuritis (25)
Neuropathy associated with systemic diseaseDiabetic neuropathy (14)Polyneuropathy due to alcoholism (9)Polyneuropathy of unknown causality (64)
Neuro-muscular diseases (19)
fluid (CSF)with respect to thc
CSF
LC
LLLLLLCC
LLLL
LL
LLLLL
LLL
L
Lcukocyte
Mediän10r'/l
11
2
01
635A
40A
277
2Ö
1
4A
5A
6A
7A
2B
211
1
differcntial diagnosis of radicular and pseudoradicularDiagnosislaboratory
counts
was ascertatncdtcchniques.
clinically and
Total protcin
Upper ränge Mediän106/1
53
517669
14510
30773608
1571553
20718
42270323913
46
18
6
mg/1
310220
420A
440A
385A
460A
470A
625A
280A
250°
2150A
480A
670A
960A
41 0A
490A
810A
1030A
440A
420A
330
710A
490A
455A
430A
Rangemg/1
170-450120-280
220-1000180-535140-860230-1160150-2200300-1440170-530140-515
390-24640250-3550300-10720330-4250
180-910250-6150
320-1690330-13070210-1325290-830220-590
350-1670300-1490220-3190
210-660
verified bysyndromes.imaging or
Serum proteinCSF protein
X
217313
162A
164A
194A
158A
147A
108A
254A
272»
31A
143A
101A
70A
162A
140A
74A
68A
158A
161A
221
94A
156A
153A
158A
Range
140-404225-550
75-32765-34378-51465-32230-46057-250
123-433120-450
3-24120-2366-240
16-239
81-3949-288
40-2286-188
25-33686-248
119-332
42-19746-19723-272
100-314
Abbrevations used: C: cisternal CSF; L: lumbar CSF: S: bloodserum. Significance between median value of controls and medianvalue of patients was calculated by the Mann-Whitney U-test (51):
A) p < 0.001; B) p < 0.05. The values given for controls agree withreference values (9,10).
ances appeared to be useful in discriminating betweenviral and bacterial genesis of radicular syndromes (15),but it was useless in the differential diagnosis of radicu-lar syndromes (16). Diseases in the »peripheral nerve(e. g. peripheral facial paresis, optic neuritis) exterior tothe CSF space rarely show signs of blood-nerve barrierdisturbance (tab. 1).
B: Leukocytosis and its differentiation into granulo-cytosis (with acute inflammation), lymphocytosis (withsubacute inflammation), or monocytosis, äs well äsmixed pleocytosis in CSF indicate the cellular stage ofinflammation in the nervous system (cf. I.e. (9, 1!0)).With radicular syndromes caused by inflammatioiis (e. g.
inflammatory neuropathy), all stages were found, de-pending on the disease process (l, 15, 17). No typicalpattern of cellular differentiation was associated with in-flammatory radicular syndromes.
Lymphocyte subsets were therefore investigated in CSFand venous blood by flow cytometry (18, 19), to detecta blood-CSF barrier: a blood/CSF ratio of « 2,000 to lwas found for total lymphocytes and activated T orheiper/inducer T cells in controls (tab. 2); higher ratioswere observed in the order cytotoxic/suppressor T cells>CD3+16+56+ cells (a subset of cytotoxic T cells)> NK cells > B lymphocytes. The ratio was lower forCD8+4+ cells (premature T lymphocytes (cf. 1. c. (20))
Eur. J. Clin. Chem. Clin. Bioohem. / Vol. 32,1994 / No. l

48 Kleine et al.: Workshop report: CSF diagnosis of radicular and pseudoradicular syndromes.
Tab. 2 Lymphocyte subsets determined by flow cytometry (18, controjs and patients with inflammatory diseases of the nervous sy-19) in cerebrospinal fluid (CSF) and venous blood samples from stem.
Lymphocyte Subseiand Diseases [n]
T-Lymphocytes CD3+)Controls (cf. I.e. (18, 19)) [22]Inflammation of nervous System
acute, bacterial [6]subacute [9]
Inflammatory neuropathy [5]
Activated T-lymphocytes (CD3+ HLA-DR+)Controls (cf. I.e. (18, 19)) [22]Inflammation of nervous System
acute, bacterial [6]subacute [9]
Inflammatory neuropathy [5]
Helper-linducer T-lymphocytes (CD3+4+)Controls (cf. I.e. (18, 19)) [22]Inflammation of nervous System
acute, bacterial [6]subacute [9]
Inflammatory neuropathy [5]
Cytotoxic/suppressor T-lymphocytes (CD3+8"*")Controls (cf. I.e. (18, 19)) [22]Inflammation of nervous System
acute, bacterial [6]subacute [9]
Inflammatory neuropathy [5]
CD3+ 1 6+56+ T-lymphocytesControls (cf. I.e. (18, 19)) [22]Inflammation of nervous System
acute. bacterial [6]subacute [9]
Inflammatory neuropathy [5]
CD$+4+-LymphocytesControls (cf. I.e. (18, 19)) [22]Inflammation of nervous System
acute, bacterial [6]subacute [9]
Inflammatory neuropathy [5]
Natitral killet- (NK) cells (CD16+56+3~)Controls (cf. I.e. (18, 19)) [22]Inflammation of nervous System
acute, bacterial [6]subacute [9]
Inflammatory neuropathy [5]
B-Lymphocyies (CDI9"1")Controls (cf. 1. c. (18, 19)) [22]Inflammation of nervous System
acute, bacterial [6]subacute [9]
Inflammatory neuropathy [5]
Cerebrospinal fluidcell count [103/1J*
818
1094656712
1088
83
16765578
44
600
764742128
651
153
32129514312
21
286594
27
32
39968038
37
4812173
160
9
2831095
59
Peripheral bloodcell count [1Ö6/I]*
1516 t f
79966
1413
180
112180129
1071
473677926
507
326372452
127
646884
27
121733
387
193296623
266
162204248
Blood /
1900:
70:20:
1300:
2200:
70:30:
2900:
1800:
60:20:
1400:
3300:
100:40:
1500 :
6100 :
220:120:
3100:
840:
30:24:
860:
10500 :
400 :140 :
3900 :
30000 :
600 :190:
4200:
CSF
1
111
1
11l
1
111
1
111
1
ttl
l
111
1
111
1
11l
* Medians are given
(tab. 2). The preliminary findings indicate the existence eases of the nervous System (tab. 2). Thus, during acuteof a blood-CSF barrier with different permeability rates inflammation, barrier permeability increased by a factorto lymphocyte subsets, and this permeability appeared of.- 30 to all subsets, except for B lymphocytes (per-to be altered to different extents in inflammatory dis- meability increased by a factor of * 50). During the
Eur. J. Clin. Chem. Clin. Biochem. / Vol. 32,1994 / No, l

Kleine et al.: Workshop report: CSF diagnosis of radicular and pseudoradicular syndromes 49
subacute stage of inflammation (e. g. six days after thefirst clmical signs) permeability further increased,especially to helper/inducer T cells (responsible for anti-gen recognition and B cell activation) and NK cells, butnot to CD8"I"4+ T cells. With inflammatory neuropathies(e.g. multiple sclerosis, tick-borne polyneuritis), per-meability was particularly increased to B lymphocytes(responsible for the humoral immune response), s wells to cytotoxic/suppressor T cells, CO3+16+56+ T cells
mediating non MHC restricted cytotoxicity (cf. I.e.(18)), and NK cells (tab. 2). Our preliminary data indi-cate that blood-nerve barrier (and blood-brain barrier)may be involved in the regulation of the cellular immuneresponse in the nervous System by controlling per-meability to lymphocyte subsets. Further experimentsare needed e. g. to explain increased suppressor activityin Lyme disease. The latter involves retarded antibodyformation (21, 22), which may be explained by normalIL-6 activity (23) to B and T lymphocytes in CSF, incontrast to higher IL-6 concentrations in bacterial andnonbacterial meningitis (24).
C: Metabo Utes. The increased L-lactate concentration inCSF is a sign of acute ischaemia in the nervous System(10). Cytotoxic and intramyelinic oedema may cause el-evated concentrations of ί,-lactate in lumbar CSF withintact blood-nerve barrier in acute spinal ischaemia, butthey were rarely found with acute compressed nerveroots (16); in contrast inflamed nerve roots resulted inelevated concentrations of I-lactate and protein. Glu-cose concentrations (compared with blood) were withoutany diagnostic relevance for different radicular syn-dromes (1), although they were found to be diminisheddistal from a spinal blockage and in the presence of thecauda-equina syndrome of progressive HIV-1 polyradic-uloneuritis (17).
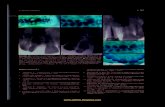
D: Intrathecally produced immunoglobulins (Ig) weredetermined by calculation or by detection of oligoclonalbands in CSF (15, 25-27). Verification of oligoclonalIg bands by isoelectric focusing (IEF), using the PhastSystem™ with self-made gels and immunofixation, pro-ved to be more sensitive and specific than IEF with n n-specific silver staining (fig. 1) (25-27). Moreover, com-pared with calculation procedures, the method was alsomore sensitive and specific for the determination of in-trathecal IgG production with an intact or defectiveblood-brain barrier (28, 29). Therefore, IEF with Ig-specific oligoclonal band detection also provides a non-specific routine quantity for differentiating between non-inflammatory and infiammatory forms of radicular syn-dromes, especially in multiple sclerosis, with intact ordefective blood-nerve barrier.
E: Nerve Constituents. The presence of different myelinproteins (e. g. P0, PI, ?2) in CSF and blood samples is
* —
=t
-
ι*
;-j "'Λ
!ji4JM
*CSF S
Protein
DD
« .̂
-
^~^
--
'
*.·
rl.ί ί
CSF S
γ-chain
*—^^
' >
¥r
|;:
1)J
CSF S
κ-chain
DQ
3
rfc
»
^
• j
K
W
^i
i jτ r
1 i
CSF S
λ-chain
Fig. l IEF pattern of native cerebrospinal fluid (CSF) and serum(S) samples from a patient with tick-borne polyneuritis. Isoelectricfocusing (IEF) was done s described (25-27) followed by silverstaining after different precipitation methods:"protein": non-specific precipitation with trichloroacetic acid;immunoglobulins of "γ-chain" type: immunofixation with mono-specific antiserum against human IgG (γ-chain);immunoglobulins of "κ-chain" type: immunofixation with mono-specific antiserum against human immunoglobulin kappa-chains(free and bound);immunoglobulin of "λ-chain" type: immunofixation with mono-specific antiserum against human immunoglobulin lambda-chains(free and bound).Samples were diluted to 20 mg/1 IgG (protein, κ-chain, λ-chain) orto 8 mg/1 (γ-chain) with saline and 4 μΐ per lane were applied 4 mmbefore the an de (D) using a selfmade applicator and selfmadepolyacrylamide gels in the pH r nge 3 — 10.Note: The sum of additional bands in CSF (some are marked byarrows) after fixation with antisera against free and bound lightchains of kappa chain type and lambda chain type was higher thanafter fixation with antiserum against IgG (γ-chain) alone or non-specific fixation with trichloroacetic acid yielding two additionalbands (marked by *).
indicative of demyelinating processes due to autoim-mune, inflammatory, or degenerative diseases, s well sto anoxia in the nervous System (cf. 1. c. (30)). The inci-dence of creatine kinase-MB values higher than 20% ofthe total serum creatine kinase value was small (indicat-ing creatine kinase-BB) (16). These quantities were notdisease-specific.
Conclusion
Non-specific CSF quantities are helpful in discriminat-ing between pseudoradicular syndromes and radicularones. Nearly all pseudoradicular syndromes caused bydisc protrusion or cervical syndromes s well s neuro-muscular diseases, exhibited normal leukocyte and pro-tein values (tab. 1). By combining leukocyte counts andthe values for non-specific quantities of blood-nerve bar-
Eur. J. Clin. Chem. Clin. Biochem. / Vol. 32, 19941 No. l

50 Kleine et al.: Workshop report: CSF diagnosis of radicular and .pseudoradicular syndrornes
rier ftmction, some radicular syndronies can be dis-tinguished: disc herniation (with nearly normal leuko-cyte counts and blood-nerve barrier quantities) fromother blood-nerve barrier disturbances with or withoutelevated leukocyte counts; but neuropathies associatedwith systemic diseases cannot be excluded (tab. 1). Rad-icular syndromes with high pleocytosis and blood-nervebarrier defects were found in a few cases of bacterialinflammations with ,-lactate concentration 2* 3.5mmol/1 (9, 10), whereas viral inflammations showed lowpleocytosis combined with normal or slightly elevatedprotein and I-lactate values (tab. 1). Tick-borne poly-neuritis can show similar findings (4, 32, 33). Most in-flammatory neuropathies showed normal or slightly el-evated leukocyte counts (and Z-lactate concentrations)together with normal or high protein Contents (see tab.1). High protein Contents with meningeosis neoplasticamay be specified by detection of tumour-like cells.
Therefore, it is useful to look for specific quantities inthe CSF to determine the cause of a radicular syndrome.
Specific Laboratory Quantities ofRadicular Syndronies
The following disease specific quantities were investi-gated at different stages of radicular syndromes:
1. Quant i t ies of the acute state
Detection of bacteria in CSF by testing for bacterial anti-gens or by culturing any bacteria present may be helpfulin some cases. Detection of free antigens was restrictedby the antibodies available and the test sensitivity. Sometherapeutic measures (34, 35) also affect the ability toproduce bacteria cultures. Borrelia cultures were foundto be positive only in < 20% of the cases showing theclinical signs of tick borne polyneuritis (36).
D-Lactate, a bacterial metabolite (37, 38), is of low rel-evance in the diagnosis of neuropathies or radicular syn-dromes.
meated into CSF äs a consequence of radicular syn-dromes or CNS diseases. The calculation was performed
a) with the aid of the barrier indicator albumin (39, 40)
b) by comparing the IgG contents of CSF and serumsamples (36), or assaying a specific antibody in CSFand serum (41, 42). A comparative evaluatiqn of theseprqcedures is still lackin&
Detection of oligocloncal bands specific to baicteria andviruses in CSF, by applying different blotting procedures(43 5 44) ? eliminates the effect of specific serum anti-bodies permeated throügh the blood-brain barrier intoCSF. Using quantitative methods, this effect cannot bereliably extrapolated by arithmetical procedures. More-over, the diagnostic sensitivity of Borrelia burgdorferidetection proved to be highly dependent on tested anti-gen (41).
3. Bacterial or viral DNA or RNA may be detectedby the polymerase chain reaction (PCR) in CSF duringall sta.ges of inflammation. The PCR test of Borreliaburgdorferi was more sensitive in CSF samples than inblood (45, 46) (and still more sensitive in urine (47)).Borrelia burgdorferi and Treponema pallidum infectionsmay be reliably discriminated by the PCR test, but notwith ELISA tests, because of cross-reacting antibodies(48, 49). Further investigations are needed to determinewhether PCR tests for viral and bacterial infections (cf.1. c. (50)) are more sensitive and give a positive earlierresponse than the procedures discussed above.
In summary, an efficient laboratory diagnosis is a valu-able aid for confinning the clinical discrimination be-tween pseudoradicular and radicular syndromes. Normalfindings with non-specific and specific methods in CSFand blood indicate the presence of a pseudoradicularsyndrome not accompanied by a nerve root disorder.Laboratory findings may also classify radicular syn-dromes into inflammatory and non-inflammatory formswith or without blood-nerve barrier disturbances.Mainly non-specific methods are used in the daily rou-tine because most of the specific methods are still underinvestigation.
2. Quant i t ies of subacute and chronic state
Antibodies specific to bacteria and viruses were deter-mined in CSF using quantified ELISA, and subtractingthe portion of specific serum antibody that had per-
AcknowledgementWe are grateful to Prof. Dr. R. Puiz, München, for bis advice andsuggestions. We thank Dr. L. Weber, Marburg, collecting some ofthe data. The investigations were supported in part by the P. E.Kempkes Stiftung, the Manfred and Ursula Kulemann Stiftung, andBecton Dickinson GmbH, Heidelberg.
Eur. J. Clin. Chem. Clin. Biochem. / Vol. 32, 1994 / No. l

Kleine et al.: Workshop report: CSF diagnosis of radicular and pseudoradicular syndromes 51
References1. Baumhackl, U. (1992) Radikulitis und die Beziehung zur Bor-
relia-biirgdorferi-lnfQktion. Berichte der ÖGKC 15, 117-118.2. Jensen, R K., Andersen, E. B., Boesen, F., Dissing, I. & Vester-
gaard, B. F. (1989) The incidence of herniated disc and var-icella zoster virus infection in lurnboradicular syndrome. ActaNeurol. Scand. 80, 142-144.
3. Weiss, S., Streifler, M. & Weiser, H. J. (1975) Motor lesionsin herpes zoster. Eur. Neurol. 13, 332-338.
4. Schmutzhard, E., Pohl, R, Mohsenipour, L & Stanek, G.(1992) Inzidcnz von intrathekal gebildeten Borrelia burgdor-feri Antikörpern bei 103 konsekutiven Patienten mit lumbalemradikulären Syndrom. Berichte der ÖGKC 75, 119-120.
5. Brüggcr, A. (1962) Über vertebrale, radikuläre und pseudora-dikuläre Syndrome. Acta Rheumatica (Documenta Geigy) INr. 18 und II Nr. 19.
6. Bogduk, N. (1990) Pathology of lumbar disc pain. J. Man.Med. 5, 72-79.
7. Berlit, R (1992) Klinische Neurologie. Eine Einßihrung.VCH, Weinheim.
8. Kleine, T. O., Hackler, R. & Rumpl, E. (1992) Laboratori-umsdiagnostik von radikulären und pseudoradikulären Syn-dromen im Liquor cerebrospinalis: Überblick über Methodenund ihre Zuverlässigkeit. Berichte der ÖGKC 75, 110-112.
9. Kleine, T. O. (1984) Diagnostische Untersuchungen im Liquorcerebrospinalis. In: Labor und Diagnose (Thomas, L., ed.) pp.937—965, Medizinische Verlagsgesellschaft, Marburg.
10. Kleine, T. O. (1989) Nervensystem. In: Lehrbuch der Klini-schen Chemie und Pathobiochemie, 2nd edn. (Greiling, H. &Gressner, A. M., eds.) pp. 859-893, Schattauer, Stuttgart.
11. Thomas, R K. & Olsson, Y. (1984) Microscopic anatomy andfunction of the connective tissue components of peripheralnerve. In: Peripheral Neuropathy (Dyck, R J., Thomas, R K.,Lambert, E. H. & Bunge, R., eds.) vol. l, pp. 97-120, W. B.Saunders, Philadelphia-London.
12. Olsson, Y. (1984) Vascular permeability in the peripheral ner-vous System. In: Peripheral Neuropalhy (Dyck, R J., Thomas,P. K., Lambert, E. H. & Bunge, R., eds.) vol. l, pp. 579-598,W. B. Saunders, Philadelphia—London.
13. Dyck, P. J., Thomas, R K., Lambert, E. H. & Bunge, R. (eds.)(1984) Peripheral Neuropathy, vol. l, 2, W. B. Saunders, Phil-adelphia— London.
14. Adams, D., Kuntzer, T., Burger, D., Chofflon, M., Magistris,M. R., Regli, F. & Steck, A. J. (1991) Predictive value of anti-GMj ganglioside antibodies in neuromuscular diseases: Astudy of 180 sera. J. Neuroimmunol. 32, 223-230.
15. Plank, E., Scholz, H. & Ziervogel, H. (1992) Radikuläre Syn-drome mit entzündlichem Liquorbefund. Berichte der ÖGKC75, 116-117.
16. Kleine, T. O., Hackler, R., Weber, L., Lütcke, A., Zöfel, R &Meyer-Rienecker, H. (1992) Betrachtungen zur Blut-Nerven-Schranke bei entzündliehen und nicht-entzündlichen Erkran-kungen. Berichte der ÖGKC 75, 113-116.
17. Pohl, P. (1992) Liquordiagnostische Aspekte peripher-neurolo-gischer Manifestationen der %14 ^ , Berichte derÖGKG75, 126-127.
18. Kleine, T. O. & Albrecht, J. (1991) Vereinfachte Durchfluß-zytometrie von Liquorzellen mit FACScan. Lab. Med. 75,73-78.
19. Kleine, T. O., Hackler, R. & Albrecht, J. (1992) Monitoring ofcellular immunoreactivity in human central nervous System:Adaptation of flow cytoraetry to lymphocyte subpopulationsin cerebrospinal fluid (CSF). In: Recent Advances in Cellularand Moleculqr Biology (Wegmann, R. J. & Wegmann, M. A.,eds.) vol. 3, pp. 59-67, Peeters Press, Leuven, Belgium.
20. Munschauer, F., Stewart, C., Jacobs, L., Kaba, S., Ghorishi,Z., Greenberg, S. J. & Cookfair, D. (1993) Circulating CD3 +CD4+ CD8+ T lymphocytes in multiple sclerosis. J. Clin,Immunol. 7J, 113-118.
21. Moffat, C. M., Sigal, L. H., Steere, A. C., Freeman, D. H. &Dwyer, J. M. (3984) Cellular immune Undings in Lyrne dis-
ease. Correlation with serum IgM and disease activity. Am. J.Med. 77, 625-632.
22. Dattwyler, R. J., Volkman, D. J., Luft, B. J., Halperin, J. J.,Thomas, J. & Golightly, M. G. (1988) Dissociation of specificT- and B-lyrnphocyte responses to Borrelia burgdorferi. N.Engl. J. Med. 319, 1441-1446.
23. Meyer-Rienecker, H. J., Köhler, H., Hornig, K. & Werner, H.(1993) Interleukin 6 (IL 6) and soluble interleukin 2 receptors(s-IL 2R) in ccrebrospinal fluid of patients with various ncuro-logical diseases. In: CNS Barriers and Modern CSF Diagnosis(Felgenhauer, K., Holzgraefe, M. & Prange, H. W., eds.) VCHWeinheim-New York, pp. 423-426.
24. Chavanet, R, Bonnotte, B., Guiguet, M., Zeller, V., Solary, E.,Maurice, L., Casasnovas, O., Caillot, D., Waldner, A., KJster-man, J. P. & Portier, H. (1992) High concentrations of intrathe-cal interleukin-6 in human bacterial and nonbacterial menin-gitis. J. Infect. Dis. 166, 428-431.
25. Hackler, R. & Kleine, T. O. (1992) Der spezifische Nachweisoligoklonaler Immunglobulin-Banden: Ein sicherer Indikatorfür die intrathekale Produktion von IgG und anderen Immun-globulinen. Berichte de ÖGKC 75, 120-123.
26. Hackler, R. & Kleine, T. O. (1991) Modifikation des PhastSys-tem™ zum automatisierten Nachweis oligoklonaler Bandenim nativen Liquor cerebrospinalis durch IEF mit Immundetek-tion. Lab. Med. 75, 185-192.
27. Hackler, R. & Kleine, T. O. (1992) Specific and non-specificdetection of oligoclonal baiids in cerebrospinal fluid (CSF)frorn patients with inflammatory diseases of central nervousSystem (CNS) utilizing PhastSystem™ (Pharmacia-LKB). J.Neurol. 239, Suppl. 3, 86.
28. Kleine, T. O., Hackler, R., Schlenska, G. K., Hase, H. L. &Rytlewski, D. (1991) Zur Evaluierung der intrathekalen Im-munglobulin-Produktion. Lab. Med. 75, 193-203.
29. Hackler, R. & Kleine, T. O. (1992) Evaluation of immunoreac-tivity of human central nervous system: Automated determi-nation of oligoclonal IgG bands versus calculation of intrathe-cal IgG production. In: Recent Advances in Cellular and Mol-ecular Biology (Wegmann, R. J. & Wegmann, M. A., eds.)vol. 3, pp. 119—128., Peeters Press, Leuven, Belgium.
30. Cohen, S. R., Brooks, B. R., Jubelt, B., Herndon, R. M. &McKhann, G. M. (1980) Myelin basic protein in cerebrospinalfluid. In: Neurobiology of Cerebrospinal Fluid (Wood, J. H.,ed.) Plenum Press, New York, pp. 487-494.
31. Heller, J., Holzer, G. & Schimrigk, K. (1990) Immunologicaldifferentiation between neuroborreliosis and multiple sclerosis.J. Neurol. 237, 465-470.
32. Schmutzhard, E., Stanek, G., Pletschette, M., Hirschl, A. M.,Pallua, A., Schmitzberger, R. & Schlögl, R. (1988) Infectionsfollowing tickbites. Tick-borne encephalitis and Lyme borre-liosis — A prospective epidemiological study frorn Tyrol. In-fection 16, 269-272.
33. Mautner, V. F., Gittermann, M., Freitag, V. & Schneider, E.(1990) Zur Epidemiologie der Borrelia burgdorferi-lnfekuon.Nervenarzt 61, 94-97.
34. Kleine, T. 0. (1989) Valuation of bacterial and viral antigendetection against lactate determination and leukocyte counts inCSF meningitis diagnosis. J. Clin. Chem. Clin. Chem. Bio-chem. 27, 901-903.
35. Kleine, T. O. & Cambiaso, C. L. (1991) Grenzen des manu-ellen und voll-mechanisierten Nachweises von Erreger-spezi-fischen Antigenen im Liquor cerebrospinalis. Lab. Med. 75,109-113.
36. Stanek, G., Baumhackl, U. & Schmutzhard, E. (1992) Labora-toriumsdiagnose der von Schildzecken übertragenen Borrelia-Infektion des Zentralnervensystems. Berichte der ÖGKC 75,124-125.
37. Kleine, T. O. (1991) D-Lactat und L-Lactat im Liquor cerebro-spinalis bei akuten entzündlichen Erkrankungen des Zentral-nervensystems (ZNS). Lab, Med. 75, 114-116.
38. Kleine, T. O., van der Meij, R. A., Dauch, W. A. £ Lütcke,A. (1992) Laktatdiffcrenzierimg im Liquor und Blutplasma bei
Eur. J. Clin. Chem. Clin. Bioehem. / Vol. 32, 1994 / No. l

52 Kleine et al.: Workshop report: CSF diagnosis of radicular and pseudoradicular syndromes
der Akut-Diagnostik zerebraler Notfälle. Intensiv- und Not-fallbehandlung 17, 134-137.
39. Hofstad, H., Matre, R., Nyland, H. & Ulvestad, E. (1987)Bannwarth's syndroine: Serum and CSF IgG antibodiesagainst Borrelia burgdorferi examined by ELISA. Acta Neu-rol. Scand. 75, 37-45.
40. Reiber, H. & Lange, P. (1991) Virus-spezifische Antikörper inLiquor und Serum. ELI SA-Analytik und Auswertung mittelsAntikörper-Index und Quotientendiagramm. Lab. Med. 15,204-207.
41. Wilske, B., Schierz, G., Preac-Mursic, V., von Busch, K.,Kühbeck, R., Pfisler, H. W. & Einhäupl, K. (1986) Intrathecalproduction of specific antibodies against Borrelia burgdorferiin patients with lymphocytic meningoradiculitis (Bahnwarth'sSyndroine). J. Infect. Dis. 153, 304-314.
42. Hansen, K. & Lebech, A. M. (1992) Lyme neuroborreliosis:A new sensitive diagnostic assay for inlrathecal synthesis ofBorrelia burgdorferi-speciRc immunoglobulin G, A, and M.Ann. Neurol. 30, 197-205.
43. Martin, R., Martens, U., Sticht-Groh, V., Dörfies, R. & Krüger,H. (1988) Persistent intrathecal secretion of oligoclonal, Borre^lia burgdorferi-spQcific IgG in chronic meningoradiculomyel-itis. J. Neurol. 235, 229-233.
44. Hansen, K., Cruz, M. & Link, H. (1990) Oligoclonal Borreliaburgdorferi-specific IgG antibodies in cerebrospinal fluid inLyme neuroborreliosis. J. Infect. Dis. 767, 1194-1202.
45. Guy, E. C. & Stanek, G. (1991) Detection of Borrelia burgdor-feri in patients with Lyme disease by the polymerase chainreaction. J. Clin. Pathol. 44, 610-611.
46. Keller, T. L., Halperin, J. J. & Whitman, M. (1992) PCR detec*tion of Borrelia burgdorferi DNA in cerebrospinal fluid ofLyme neuroborreliosis1 patients. Neurology 42, 32-42.
47. Lebech, A. M. & Hansen, K. (1992) Detection of Borreliaburgdorferi DNA in urine samples and eerebfospinal fluidsamples from patients with early and late Lyme meuröborre-liosis by polymerase chain reaetion. J. Clin. Microbiol. 30,1646-1653. ; f
48. Noordhoek, G. T., Wolters, E. C, de Jonge, M. E. J. & vanEmbden, J. D. A. (1991) Detection of polymerase chain reae^tion of Treponema pallidwn DNA in cerebrospinal fluid fromneurosyphilis patients before and after antibiotic treatment. J.Clin. Microbiol. 29, 1976-1984.
49. Magnarelli, L. A., Anderson, J. F. & Johnson, R. C. (1987)Cross-reactivity in serological tests for Lyrne diseases andother spirochetal infections. J. Infeet. Dis. 156, 183-188.
50. Jaton, K., Sahli, R. & Bille, J. (1992) Development of poly-merase chain reaction assays for detection of Listeria monocy-togenes in clinical cerebrospinal fluid sarnples. J. Clin. Micro^·biol. 30, 1931-1936.
51. Zöfel, R (1985) Statistik und Praxis. Fischer, Stuttgart.
Prof. Dr. T. O. KleineMed. Zentrum für NervenheitkundeFunktionsbereich NeurochemieRudolf-Bultmann-Straße 8D-35033 MarburgGermany
Eur. J. Clin. Chem. Clin. Biochem. / Vol. 32, 1994/No. l