Keratocystic odontogenic tumor: A retrospective analysis ......Keratocystic odontogenic tumors...
Transcript of Keratocystic odontogenic tumor: A retrospective analysis ......Keratocystic odontogenic tumors...

Vol. 116 No. 1 July 2013
Keratocystic odontogenic tumor: A retrospective analysis ofgenetic, immunohistochemical and therapeutic features. Proposalof a multicenter clinical survey toolMichael W. Finkelstein, DDS, MS,a John W. Hellstein, DDS, MS,a Kimberly S. Lake, BA,b andSteven D. Vincent, DDS, MSc
University of Iowa, College of Dentistry, Iowa City, IA, USA
Objective. In 2005, the World Health Organization reclassified the parakeratinizing odontogenic keratocyst as a neoplasm.
This article reviews the research leading to this reclassification, and validates a new survey tool that can be easily used to pool
surgical and recurrence data from multiple offices.
Study design. All odontogenic lesions accessioned in the Iowa Surgical Oral Pathology Laboratory between 1949 and 2010
were identified from the database. A survey tool to assess treatment and follow-up was created. A total of 46 surgeons agreed
to participate.
Results. A total of 70 keratocystic odontogenic tumors (KOTs) had documented recurrences at follow-up intervals ranging
from 6 months to 5 years. Primary tumors that recurred ranged in size as measured by greatest radiographic diameter from 0.7
to 6 cm.
Conclusions. This survey tool is recommended as standard allowing treatment of cases by multiple practitioners to be
compared retrospectively or prospectively. (Oral Surg Oral Med Oral Pathol Oral Radiol 2013;116:75-83)
Statement of Clinical Relevance
Genetic and immunohistochemical features of kera-tocystic odontogenic tumors have resulted in this
Keratocystic odontogenic tumors (KOTs) were firstdescribed by Philipsen in 1956. Over 50 years laterthe debate continues as to their proper classification, be-havior, and recommended therapeutic management.1-4
Until 2005, the lesion was classified as a develop-mental odontogenic cyst that had an unusually highrecurrence rate following simple enucleation whencompared to other odontogenic cysts and most odon-togenic neoplasms. In 2005, following a series ofpublications highlighting specific genetic mutations andnoting the immunohistochemical similarity of thislesion to a variety of odontogenic neoplasms, the WorldHealth Organization (WHO) reclassified the lesion asa benign cystic neoplasm.5,6
This article reviews the research leading to thisreclassification, and introduces a new, standardizedsurvey tool that can be easily used to pool surgical andrecurrence data from multiple offices. Because a majorityof these tumors are managed in private oral and maxil-lofacial surgery offices, rather than in large teachinghospitals, prospective multicenter studies regardingtherapeutic management and long-term follow-up oflarge numbers of cases cannot be easily accomplished atsingle treatment centers. Based on the number of officesthat chose to participate in this initial study, this survey
aProfessor, University of Iowa, College of Dentistry.bLaboratory Manager, University of Iowa, College of Dentistry.cProfessor and Head, University of Iowa, College of Dentistry.Received for publication Jan 21, 2013; returned for revision Mar 18,2013; accepted for publication Mar 21, 2013.� 2013 Elsevier Inc. All rights reserved.2212-4403/$ - see front matterhttp://dx.doi.org/10.1016/j.oooo.2013.03.018
tool seems to be an acceptable tool that can be used byresearchers wishing to collect treatment and follow-updata over time.
CLINICAL FEATURESA majority of KOTs occur in males, with many studiessuggesting a ratio of about 2:1. Most tumors are firstdiagnosed in the second, third, and fourth decade oflife.7 As with all odontogenic lesions, KOTs can occurin any tooth-bearing site. However, the mandible is thesite of occurence of most KOTs with approximately75% occurring in the posterior body. Tumors originatingin the maxilla can expand superiorly to involve thesinuses, nasal antrum, and even the floor of the orbit.
Similar to other benign tumors, KOTs seldom causesymptoms unless secondarily inflamed. Clinical signsusually include a uniform expansion of the buccal corticalplate of the mandible, or the buccal or palatal alveolus ofthe maxilla. Crepitus may be evident on palpation.
lesion being reclassified by the World HealthOrganization in 2005, and may hold the key to therelatively high recurrence rates following conserva-tive excision when compared to most other odon-togenic tumors. This article reviews those featuresand attempts to retrospectively analyze therapeuticmanagement and follow-up at multiple independentoral surgery offices using a new standardized tool.
75

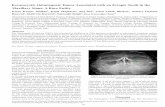
Fig. 1. Panoramic radiograph of a KOT. A well-demarcated,corticated radiolucency in the right posterior mandible showsresorption of overlying tooth roots and the crown of animpacted canine displaced to the inferior border.
ORAL AND MAXILLOFACIAL PATHOLOGY OOOO
76 Finkelstein et al. July 2013
If multiple odontogenic cysts are present or developsequentially the consideration of basal cell nevus (Gorlin,GorlineGoltz) syndrome must be considered.
RADIOGRAPHIC FINDINGSKOTs appear radiographically as well-demarcatedradiolucencies with thin, well-defined, corticated bor-ders (Figure 1). Despite its reported aggressive nature,the tumor is slowly growing and usually displacesnormal anatomy, including teeth. Resorption of toothstructure can happen but does not seem to be morefrequent than with other odontogenic tumors or cysts.The radiolucency may appear multilocular dependingprimarily on the size of the lesion. In a recent study,KOTs larger than 31 mm diameter were more likelyto appear as multilocular lesions radiographically.7
Computed tomography (CT) scans are often valuable inassessing these lesions in 3 dimensions prior to surgicalman-agement.8
GROSS FINDINGSKOTs at the time of surgery and on the grossing tablepresent as tissue-paper thin strips and sheets of softtissue. The lining epithelium can separate from theconnective tissue and will often appear as a translucentmembrane. The fixative will often contain varyingamounts of amorphous “cheese-like” material, which ifprocessed can be identified as parakeratin.
MICROSCOPIC FINDINGSClassic microscopic features include a relatively uniformlayer of stratified squamous epithelium 6-10 cells in
thickness without rete ridge formation (Figures 2 and 3).With hematoxylin-eosin staining, the parakeratinizedepithelium will appear pink and is often corrugated orwavy. Focal variations can include lack of corrugation orminimal keratinization (Figure 4). The histopathologicfeature that is most important for the diagnosis isthe uniform, picket-fence-like basal cell layer withovoid, hyperchromatic nuclei often polarized awayfrom the basement membrane. The underlying fibro-vascular connective tissue can contain varying numberof epithelial rests or small microcysts (Figure 5).
Secondary inflammation, which can occur with manyodontogenic tumors and cysts, including KOTs, willsubstantially alter the microscopic features (Figure 5).Acute or chronic inflammation in the connective tissuesdirectly subjacent to the KOT lining will often result inloss of keratinization, increases and decreases in thick-ness of the epithelium, and loss of the uniform basal celllayer. Additionally, tumor tissue removed followingmarsupialization can be substantially altered, showingincreased thickness and the formation of epithelial tuftsand whorls within the stratum spinosum (Figure 6).
TUMOR SUPPRESSOR GENE STUDIES ANDIMMUNOHISTOCHEMISTRYParakeratinizing odontogenic keratocysts were reclas-sified by the WHO in 2005 as KOTs, in recognition ofgenetic and downstream immunohistochemical featureswhich were similar to those noted in other benignneoplasms.
In 2004, Agaram et al. examined 10 odontogenickeratocysts for loss of heterozygosity of 10 commontumor suppressor genes as well as the PTCH gene.5
Loss of heterozygosity was seen in 7 of 10 cases, witha frequency between 11% and 80% of genes studied.The genes that exhibited the most frequent allelic losseswere p16, PTCH, and MCC. The finding of clonaldeletion mutations of genomic DNA supported thehypothesis that these cystic lesions were neoplastic.
As early as 1989, Scharffetter et al. documentedincreased mitotic activity within the epithelial lining ofKOTs, the initial building-block for its eventualreclassification by the WHO as a cystic neoplasm.9
During the following 15 years, a variety of immuno-histochemical studies suggested that the cystic epithe-lium of KOTs had many similarities with other benignodontogenic neoplasms and suggested possible reasonsfor the observation that these tumors exhibit a relativelyhigh recurrence rate.4,10
The epithelial proliferation and apoptotic index ofboth sporadic and syndromic KOTs in basal cell nevussyndrome have been shown to be similar to amelo-blastomas and higher than dentigerous cyst epithe-lium.12,13 Survivin, an inhibitor of apoptosis, hasbeen shown to be expressed in KOTs but not in

Fig. 2. KOT (low power). The specimen consists of a thinfibrovascular connective tissue surrounding a lumen lined bya uniformly thin epithelium with no evidence of rete ridgeformation. Keratin partially fills the lumen (hematoxylin-eosinstain, original magnification �100).
Fig. 3. KOT (high power). The epithelial lining is of uniformthickness with evidence of parakeratin formation, anda prominent, uniform basal cell layer (hematoxylin-eosinstain, original magnification �400).
Fig. 4. KOT variations. The epithelium within the same tumorcan show marked corrugation (upper left) of the parakerati-nized surface, minimal corrugation (middle), or little evidenceof parakeratin (lower right). All variants exhibit the prominentbasal cell layer, which is necessary for the diagnosis (hema-toxylin-eosin stain, original magnification �400).
OOOO ORIGINAL ARTICLE
Volume 116, Number 1 Finkelstein et al. 77
nonkeratinizing periapical cysts.11 Keratinocytegrowth factor and receptor expression has been iden-tified more often in KOT epithelium compared todentigerous cysts, and the intensity of growthfactor staining is significantly reduced followingmarsupialization.14
Bax and bcl-2 are two important anti-apoptotic andpro-apoptotic factors of the bcl-2 family. Immunore-activity for bcl-2 is detected in the basal layer of KOTswhile orthokeratinizing cysts are negative.15 KOTshave shown decreased expression of cell adhesionproteins b-catenin and E-cadherin and differences ofWnt-1 and Wnt-10A signaling when compared todentigerous cysts.16
Aberrant sonic hedgehog signaling proteins, whichare critical to tissue development, have been identifiedin KOTs.4,17,18 Epithelial expression of the hedgehogtranscriptional effector Gli2 has also been identified inthe development of laboratory KOTs from rests ofMalassez in transgenic mice.19
An evaluation of common tumor suppressor geneshas concluded that clonal deletion mutations ofgenomic DNA suggest a neoplastic rather than cysticorigin.5 Proliferating cell nuclear antigen (PCNA), cell
proliferation markers (Ki-67), and tumor suppressorprotein (p53) occur more frequently and more intenselyin KOTs, compared with the other odontogenic tumorsand cysts.20-25 Gadbail et al. have recently suggestedthat Ki-67 and p53 protein quantitative and qualitativeexpression can be used as a prognostic marker ofaggressive behavior in odontogenic lesions includingKOTs.26 High expression of Ki-67 and expression ofp53 have also been identified more often in tumors thatrecurred.27,28
Vascular endothelial growth factor (VEGF) has beenshown to be more prominent in KOTs compared to

Fig. 5. KOT with satellite epithelial islands and inflamma-tion. Inflammation of the connective tissue can render theepithelial lining focally nondiagnostic. Microcysts are oftennoted in the connective tissues and have not been asso-ciated with differences in recurrence rates (hematoxylin-eosinstain, original magnification �200).
Fig. 6. Marsupialized KOT. For tumors that have undergonea marsupialization procedure, the epithelial lining of the tumoroften undergoes acanthosis, with formation of tufts within thestratum spinosum. However, the diagnostic basal cell layerremains focally evident (hematoxylin-eosin stain, originalmagnification �400).
ORAL AND MAXILLOFACIAL PATHOLOGY OOOO
78 Finkelstein et al. July 2013
dentigerous cysts.21 Use of CD34 to evaluate angio-genesis has shown microvessel density to be signifi-cantly higher in KOTs compared to dentigerouscysts, although the densities of both were lower thanameloblastoma.29 Seifi et al. showed a statisticallysignificant difference in microvessel density in ame-loblastomas and KOTs when compared to follicularcysts.30
Podoplanin, a lymphatic endothelial marker, ishighly expressed in ameloblastomas and also in thecytoplasm of most of the basal and parabasal cells andperipheral cells of daughter cysts in the stromalconnective tissues of KOTs.31 Podoplanin expression isabsent or weakly positive in orthokeratinizing cysts,suggesting a relationship to proliferative activity andrecurrence rates.32
Osteopontin, an extracellular protein important inbone remodeling, and often found in malignant epithe-lial tumors, is important in bone metastasis througha process of osteoclast activation. Cytoplasmic osteo-pontin was identified in the epithelial cells of 8 of 20KOTs, whereas no evidence of staining was identified indentigerous or radicular cysts.33
Tsuneki et al. evaluated for the presence of keratins10 and 17, perlecan, PCNA, and UEA-I lectin ina variety of cystic jaw lesions. Keratin 10 was positivein KOTs and dentigerous cysts. Perlecan was found inunicystic ameloblastomas, KOTs, and lateral peri-odontal cysts. PCNAþ cells were found frequently inunicystic ameloblastomas and KOTs.34
Senguven and Oygur have suggested that theexpression rates of cytokines interleukin 1a (IL-1a) andIL-6, which showed a positive correlation with tumorsize in ameloblastomas, and wall thickness in KOTs
may play a role in the aggressive behavior of thesetumors by facilitating bone resorption.35 By polymerasechain reaction, Wang and Li suggested that increasedCOX-2 and VEGF expression may be responsiblefor the increased osteoclastogenic effects of KOTfibroblasts.36
Collagen IV, matrix metalloproteinase (MMP) 9, andtissue inhibitor of MMP 2 may be important factors forthe establishment of differences in the biologic behaviorof dentigerous cysts, radicular cysts, KOTs, and ame-loblastomas.37 Most dentigerous and radicular cystsshowed a predominance of continuous staining forcollagen IV in the basement membrane of the epithe-lium, whereas discontinuous staining was observedmore frequently in KOTs and ameloblastomas. MMP-9was identified in epithelial and mesenchymal cells of allthe lesions analyzed, but the staining percentage washigher in the epithelium and mesenchyme of KOTs andameloblastomas.
REVIEW OF TREATMENT FOR KOT’SInitial attempts to explain the recurrence rates, esti-mated to be as high as 60% following simple enucle-ation, included a variety of microscopic and clinicfeatures including the presence of dental lamina rests orsatellite cyst formation in the cyst walls,38 surgicaldifficulty in identification and removal of the paper-thinepithelial lining, collagenase activity in the fibrovas-cular connective tissue subjacent to the epithelium,39 orprostaglandin-induced bone resorption.40
In a recent series of 120 cases of KOTs treated withsimple enucleation, 28 tumors (26%) recurred.41
Average follow-up was 86 months with a rangeof 18-151. There was no correlation between tumor

Fig. 7. The survey tool. The patient and surgeon information was auto-filled from the accession database, and a single formrepresenting each odontogenic tumor was mailed to each surgeon’s office.
OOOO ORIGINAL ARTICLE
Volume 116, Number 1 Finkelstein et al. 79
site, cortical perforation or radiographic features. Inanother series of 32 KOTs treated with enucleation,4 recurred during follow-up ranging from 1 to 114months.27
Because simple enucleation is often deemed inade-quate, some reports have claimed success with decom-pression and subsequent enucleation, whereas othersadvocate enucleation and excision of overlying mucosa,peripheral osteotomy, and chemical curettage.42-47
Carnoy’s solution is a volatile pharmacologic com-pound that varies in formulation somewhat from insti-tution to institution. The solution usually consists of 1 gof ferric chloride (FeCl3) dissolved in 24 mL of abso-lute ethanol, with 12 mL of chloroform and 4 mL ofglacial acetic acid.
Complementary treatment with Carnoy’s solutionand peripheral ostectomy has been advocated as an
effective treatment for KOTs. An evaluation of 22cases treated in this manner and followed for 12-78months reported a recurrence rate of only 4.5%.Complications included wound dehiscence (22.7%),infection (4.5%), and paresthesia (18.2%). These com-plications were less frequent and less serious thancomplications associated with cryotherapy.48 Use ofCarnoy’s solution on the inferior alveolar vascularenervous plexus can be utilized as a complementarytreatment for the KOTs with low and transient neuralmorbidity.49
In an analysis of 257 KOTs, 7.4% recurred within 6years after initial treatment with either enucleation ora combination of enucleation and Carnoy’s solution.The recurrent KOTs were more likely to be multilocularor multifocal than the primary cases and often involvedthe alveolar bone around remaining teeth.50

Table I. Summary of total number of central bonyodontogenic neoplasmsAdenomatoid odontogenic tumor 21Ameloblastic fibroma 18Ameloblastic fibro-odontoma 43Ameloblastoma 106Calcifying epithelial odontogenic tumor 02Calcifying cystic odontogenic tumor 15Central odontogenic fibroma 23KOT (OKC) 896Odontogenic myxoma 07Squamous odontogenic tumor 02Total 1133
ORAL AND MAXILLOFACIAL PATHOLOGY OOOO
80 Finkelstein et al. July 2013
In a review of cases published between 1999 and2010, Johnson et al. found that cases treated withenucleation and enucleation with adjunctive measuresother than Carnoy’s solution had recurrence rates of25.6% and 30.3%, respectively. Marsupialization withadjunctive measures produced a recurrence rate of15.8%, whereas enucleation with Carnoy’s solutionpresented a recurrence rate of only 7.9%.51
Marsupialization of large lesions has been shown todecrease tumor size by 47% making follow-upenucleation much simpler.52 Using CT 3-dimensionalreconstruction data from 15 patients, the volume ofmarsupialized KOTs was reduced by half in an averageof about 8 months.8 This approach allows for a lessinvasive approach with excellent results, avoidingextensive disfiguring procedures.53 Treatment of 3patients with GorlineGoltz syndrome, using marsupi-alization for 10 months, followed by enucleation withperipheral ostectomy resulted in no recurrences duringa 5 year follow-up.
In a systematic review of the literature regardingtreatment of KOTs from 1999 to 2010, Johnson et al.found only 8 of 206 published manuscripts that met 4inclusion criteria: (1) tumors were diagnosed by histo-pathologic evaluation; (2) the patient selection processwas described and consisted of consecutive patients; (3)cases had an adequate period of follow-up; (4) treat-ment was rendered in specific detail to repeat theprocedure and each treatment was correlated withrecurrence rate.51 This report brings into focus thesubstantial lack of uniformity in reporting treatmentmodalities for KOTs and other odontogenic lesions.
The literature has abundant case reports and shortseries describing both peripheral and central odonto-genic tumor recurrences. Due to the fact that many ofthese lesions are treated in the private clinics of oral andmaxillofacial surgeons, or in relatively small, regionalhospitals, data regarding treatment and follow-up forodontogenic tumors in general are lacking whencompared to prospective, multicenter hospital-basedclinical trials for many other tumors, both benign and
malignant. Too often, as noted by Johnson et al., a lackof standardization makes meta-analyses difficult.51
MATERIALS AND METHODSOur study was designed to evaluate treatment of KOTsat multiple clinical settings using a standard reportingtool (Figure 7). The clinical trial was approved by theUniversity of Iowa IRB # 201105716. Letters weremailed to 15 oral surgery offices asking for participa-tion. Eleven oral surgery clinics responded and receivedInternal Review Board approval. The laboratory data-base was searched for odontogenic tumors and devel-opmental cysts submitted by practitioners in theseclinics and individual forms were printed for eachaccession included in the study.
The individual patient information section, whichincluded the patient name, practitioner, and diagnosissection of the clinical data information form (Figure 7),was auto-filled directly from the database. This madeinformation gathering at each office simple, andprovided uniformity of information retrieved. Thecontributors’ name was necessary because many oralsurgery offices had multiple practitioners. A total of 11offices with 46 oral surgeons participated in the study.
RESULTSThe Surgical Oral and Maxillofacial Pathology Labo-ratory at the University of Iowa has been in continuousoperation since 1949 and through December 2010recorded 100,804 accessions. If odontomas areexcluded, the total number of central bony odontogenicneoplasms, as defined by the 2005 WHO classification,system totaled 1133. These are outlined in Table I.
Central bony odontogenic neoplasms comprised1.12% of the total accessions. Not included in thisnumber are 483 complex and compound odontomas,which are considered by many to be hamartomas. Alsonot included in this total were a variety of peripheralodontogenic neoplasms including 165 peripheralodontogenic fibromas, 9 peripheral KOTs, and 3peripheral ameloblastomas. A total of 439 peripheralgiant cell granulomas, and 792 peripheral ossifyingfibromas, both of which are considered by most to bereactive lesions, were also identified.
RETROSPECTIVE ANALYSIS OF TREATMENTFOR KOTSKOT comprised 896 central bony tumors, far out-distancing all other odontogenic neoplasms combined.A total of 763 individual KOT case forms were mailedto participating oral and maxillofacial surgery offices.Of these, 446 had surgical management and follow-upinformation.
The tumors were identified in 474 males and 390females, a ratio of 1.2/1. In 32 accessions, the sex was

OOOO ORIGINAL ARTICLE
Volume 116, Number 1 Finkelstein et al. 81
not identified. A total of 539 were from the mandibleand 222 from the maxilla, a ratio of 2.4/1. In 135 cases,the location was not stated. A total of 8 tumors wereremoved from patients with documented GorlineGoltzsyndrome.
A total of 70 KOTs had documented recurrences atfollow-up intervals ranging from 6 months to 5 years. Atotal of 253 had follow up with no recurrence, and 123were unknown.
Recurrence by location of the primary tumor, whenreported, was posterior mandible 29, posterior maxilla6, anterior maxilla 5, and anterior mandible 5. Primarytumors that recurred ranged in size as measured bygreatest radiographic diameter from 0.7 to 6 cm.Excluding known syndromic KOTs, the average age ofthe patients for primary tumors that recurred was 41years with a range from 7 to 74 years.
Most of the documented recurrences (73%) wereidentified during the first 24 months following initialtreatment. The recurrence rate for cases treated withsimple curettage was 32%. For tumors treated withexcision followed by mechanical or chemical curettage,the recurrence rate was 15%. For tumors that recurred,the primary tumor size, measured by greatest diameteras reported by the clinician, was on average 3.3 cm,whereas overall primary tumor size for all KOTs was2.9 cm.
DISCUSSIONAll benign odontogenic neoplasms have been reportedto recur, but risk of recurrence alone should not be usedas a justification for overaggressive treatment. Recur-rences can be related to tumor characteristics at thecellular level, or difficulty in surgical management. Themorbidity of the treatment should not exceed the ex-pected morbidity of the tumor. Following initialsurgical removal, based on the best scientific evidenceavailable, the most important aspect to assurea successful patient outcome is regular extended follow-up to assure that any recurrent lesions are identified andtreated early.
Because tumors that subsequently recurred werelarger than those that did not and because larger tumorsare more often multiloculated, difficulty accessing theentire tumor may have some effect on recurrence rates.
Regardless of the initial treatment provided, patientstreated for KOTs need to be followed radiographicallyfor a minimum of 10 years. Clinical work-up to assessfor basal cell nevus syndrome is always prudent whenKOTs are diagnosed in patients under 20 years of age,or multiple concurrent or sequential OKCs are identi-fied. In addition, clinicians need to be reminded thata variety of jaw cysts including radicular, dentigerous,lateral periodontal, and glandular odontogenic cysts areclinically and radiographically indistinguishable from
cystic neoplasms including KOTs and unicystic ame-loblastomas. These can only be diagnosed microscopi-cally, making it imperative that all jaw cysts besubmitted for microscopic examination.
The current survey tool is promoted as an attempt tostandardize reporting of surgical management fortumors of the jaws so that prospective and retrospectiveanalyses from multiple surgical offices and institutionscan be compared, thereby giving rise to more reliabledata. Based on responses from 11 offices and 40 prac-titioners, the form is easy to understand and takes verylittle office time to complete.
REFERENCES1. Macdonald-Jankowski DS, Li TK. Keratocystic odontogenic
tumour in a Hong Kong community: the clinical and radiologicalfeatures. Dentomaxillofac Radiol. 2010;39:167-175.
2. Shear M. The aggressive nature of the odontogenic keratocyst: isit a benign cystic neoplasm? Part 1. Clinical and early experi-mental evidence of aggressive behaviour. Oral Oncol. 2002;38:219-226.
3. Shear M. The aggressive nature of the odontogenic keratocyst: isit a benign cystic neoplasm? Part 2. Proliferation and geneticstudies. Oral Oncol. 2002;38:323-331.
4. Shear M. The aggressive nature of the odontogenic keratocyst: isit a benign cystic neoplasm? Part 3. Immunocytochemistry ofcytokeratin and other epithelial cell markers. Oral Oncol.2002;38:407-415.
5. Agaram NP, Collins BM, Barnes L, et al. Molecular analysis todemonstrate that odontogenic keratocysts are neoplastic. ArchPathol Lab Med. 2004;128:313-317.
6. Barnes L, Eveson JW, Reichart P, Sidransky D, eds.World HealthOrganization Classification of Tumours. Pathology and Geneticsof Head and Neck Tumours. Lyon: IARC Press; 2005:283-327.
7. Boffano P, Ruga E, Gallesio C. Keratocystic odontogenic tumor(odontogenic keratocyst): preliminary retrospective review ofepidemiologic, clinical, and radiologic features of 261 lesions fromUniversity of Turin. J Oral Maxillofac Surg. 2010;68:2994-2999.
8. Shudou H, Sasaki M, Yamashiro T, et al. Marsupialisation forkeratocystic odontogenic tumours in the mandible: longitudinalimage analysis of tumour size using 3D visualised CT scans. IntJ Oral Maxillofac Surg. 2012;41:290-296.
9. Scharffetter K, Balz-Herrmann C, Lagrange W, Koberg W,Mittermayer C. Proliferation kinetics-study of the growth ofkeratocysts. Morpho-functional explanation for recurrences.J Craniomaxillofac Surg. 1989;17:226-233.
10. Shear M. Odontogenic keratocysts: natural history and immuno-histochemistry. Oral Maxillofac Surg Clin North Am. 2003;15:347-362.
11. Andric M, Dozic B, Popovic B, et al. Survivin expression inodontogenic keratocysts and correlation with cytomegalovirusinfection. Oral Dis. 2010;16:156-159.
12. Thosaporn W, Iamaroon A, Pongsiriwet S, Ng KH.A comparative study of epithelial cell proliferation between theodontogenic keratocyst, orthokeratinized odontogenic cyst, den-tigerous cyst, and ameloblastoma. Oral Dis. 2004;10:22-26.
13. Mateus GC, Lanza GH, de Moura PH, Marigo Hde A, Horta MC.Cell proliferation and apoptosis in keratocystic odontogenictumors. Med Oral Patol Oral Cir Bucal. 2008;13:E697-E702.
14. Suyama Y, Kubota Y, Yamashiro T, Ninomiya T, Koji T,Shirasuna K. Expression of keratinocyte growth factor and itsreceptor in odontogenic keratocysts. J Oral Pathol Med. 2009;38:476-480.

ORAL AND MAXILLOFACIAL PATHOLOGY OOOO
82 Finkelstein et al. July 2013
15. Rangiani A, Motahhary P. Evaluation of bax and bcl-2 expres-sion in odontogenic keratocysts and orthokeratinized odonto-genic cysts: a comparison of two cysts. Oral Oncol. 2009;45:e41-e44.
16. Hakim SG, Kosmehl H, Sieg P, et al. Altered expression ofcellecell adhesion molecules beta-catenin/E-cadherin and relatedWnt-signaling pathway in sporadic and syndromal keratocysticodontogenic tumors. Clin Oral Investig. 2011;15:321-328.
17. Vered M, Peleg O, Taicher S, Buchner A. The immunoprofile ofodontogenic keratocyst (keratocystic odontogenic tumor) thatincludes expression of PTCH, SMO, Gli-1 and bcl-2 is similar toameloblastoma but different from odontogenic cysts. J OralPathol Med. 2009;38:597-604.
18. Freier K, Pungs S, Flechtenmacher C, Hofele C. Activation ofsonic hedgehog signaling in keratocystic odontogenic tumors.HNO. 2009;57:345-350.
19. Grachtchouk M, Liu J, Wang A, et al. Odontogenic keratocystsarise from quiescent epithelial rests and are associated withderegulated hedgehog signaling in mice and humans. Am JPathol. 2006;169:806-814.
20. de Vicente JC, Torre A, Gutierrez AM, Lequerica P. Immuno-histochemical comparative study of the odontogenic keratocystsand other odontogenic lesions. Med Oral Patol Oral Cir Bucal.2010;15:E709-E715.
21. Mitrou GK, Tosios KI, Kyroudi A, Sklavounou A. Odontogenickeratocyst expresses vascular endothelial growth factor: animmunohistochemical study. J Oral Pathol Med. 2009;38:470-475.
22. Poomsawat S, Punyasingh J, Vejchapipat P. Immuno-histochemicalexpression of p53 protein and iNOS in odontogenic cysts. J MedAssoc Thai. 2009;92:952-960.
23. Gadbail AR, Chaudhary M, Patil S, Gawande M. Actual prolif-erating index and p53 protein expression as prognostic marker inodontogenic cysts. Oral Dis. 2009;15:490-498.
24. de Oliveira MG, Lauxen Ida S, Chaves AC, Rados PV, Sant’AnaFilho M. Immunohistochemical analysis of the patterns of p53and PCNA expression in odontogenic cystic lesions. Med OralPatol Oral Cir Bucal. 2008;13:E275-E280.
25. Ayoub MS, Baghdadi HM, El-Kholy M. Immunohistochemicaldetection of laminin-1 and Ki-67 in radicular cysts and kerato-cystic odontogenic tumors. BMC Clin Pathol. 2011;11:E4-E8.
26. Gadbail AR, Patil R, Chaudhary M. Co-expression of Ki-67 andp53 protein in ameloblastoma and keratocystic odontogenictumor. Acta Odontol Scand. 2012;70:529-535.
27. Kuroyanagi N, Sakuma H, Miyabe S, et al. Prognostic factorsfor keratocystic odontogenic tumor (odontogenic keratocyst):analysis of clinico-pathologic and immunohistochemical findingsin cysts treated by enucleation. J Oral Pathol Med. 2009;38:386-392.
28. Goncalves CK, Fregnani ER, Leon JE, Silva-Sousa YT,Perez DE. Immunohistochemical expression of p63, epidermalgrowth factor receptor (EGFR) and notch-1 in radicular cysts,dentigerous cysts and keratocystic odontogenic tumors. BrazDent J. 2012;23:337-343.
29. Alaeddini M, Salah S, Dehghan F, Eshghyar N, Etemad-Moghadam S. Comparison of angiogenesis in keratocysticodontogenic tumours, dentigerous cysts and ameloblastomas.Oral Dis. 2009;15:422-427.
30. Seifi S, Shafigh E, Allaie A. Quantitative and qualitative analysisof argyrophilic nuclear organizer regions in follicular cyst, kera-tocystic odontogenic tumor and ameloblastoma. J Cancer ResTher. 2011;7:280-285.
31. Okamoto E, Kikuchi K, Miyazaki Y, et al. Significance ofpodoplanin expression in keratocystic odontogenic tumor. J OralPathol Med. 2010;39:110-114.
32. Caetano AD, Tjioe KC, Faustino SE, et al. Immunolocalization ofpodoplanin in benign odontogenic tumours with and withoutectomesenchyme. Arch Oral Biol. 2013;4:408-415.
33. Wang YP, Liu BY. High expression of osteopontin and CD44v6in odontogenic keratocysts. J Formos Med Assoc. 2009;108:286-292.
34. Tsuneki M, Yamazaki M, Cheng J, Maruyama S, Kobayashi T,Saku T. Combined immunohistochemistry for the differentialdiagnosis of cystic jaw lesions: its practical use in surgicalpathology. Histopathology. 2010;57:806-813.
35. Senguven B, Oygur T. Investigation of interleukin-1 alphaand interleukin-6 expression and interleukin-1 alpha genepolymorphism in keratocystic odontogenic tumors and ame-loblastomas. Med Oral Patol Oral Cir Bucal. 2011;16:e467-e472.
36. Wang HC, Li TJ. The growth and osteoclastogenic effects offibroblasts isolated from keratocystic odontogenic tumor. OralDis. 2013;19:162-168.
37. Henriques AC, Vasconcelos MG, Galvao HC, de Souza LB, deAlmeida Freitas R. Comparative analysis of the immunohisto-chemical expression of collagen IV, MMP-9, and TIMP-2 inodontogenic cysts and tumors. Oral Surg Oral Med Oral PatholOral Radiol Endod. 2011;112:468-475.
38. Rud J, Pindborg JJ. Odontogenic keratocysts: a follow-up studyof 21 cases. J Oral Surg. 1969;27:323-330.
39. Donoff RB, Harper E, Guralnick WC. Collagenolytic activity inkeratocysts. J Oral Surg. 1972;30:879-884.
40. Harris M. Odontogenic cyst growth and prostaglandin-inducedbone resorption. Ann R Coll Surg Engl. 1978;60:85-91.
41. Pitak-Arnnop P, Chaine A, Oprean N, Dhanuthai K, Bertrand JC,Bertolus C. Management of odontogenic keratocysts of the jaws:a ten-year experience with 120 consecutive lesions. J Cranio-maxillofac Surg. 2009;107:452-457.
42. Stoelinga PJ. Long-term follow-up on keratocysts treatedaccording to a defined protocol. Int J Oral Maxillofac Surg.2001;30:14-25.
43. Marker P, Brondum N, Clausen PP, Bastian HL. Treatment oflarge odontogenic keratocysts by decompression and later cys-tectomy: a long-term follow-up and a histologic study of 23 cases.Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;82:122-131.
44. Dammer R, Niederdellmann H, Dammer P, Nuebler-Moritz M.Conservative or radical treatment of keratocysts: a retrospectivereview. Br J Oral Maxillofac Surg. 1997;35:46-48.
45. Chapelle KA, Stoelinga PJ, de Wilde PC, Brouns JJ,Voorsmit RA. Rational approach to diagnosis and treatment ofameloblastomas and odontogenic keratocysts. Br J Oral Max-illofac Surg. 2004;42:381-390.
46. Bataineh AB, al Qudah M. Treatment of mandibular odontogenickeratocysts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod.1998;86:42-47.
47. Stoelinga PJ. The treatment of odontogenic keratocysts by exci-sion of the overlying, attached mucosa, enucleation, and treatmentof the bony defect with Carnoy solution. J Oral Maxillofac Surg.2005;63:1662-1666.
48. Ribeiro O Jr, Borba A, Alves C, de Gouveia M, Coracin F,Guimaraes J Jr. Keratocystic odontogenic tumors and Carnoy’ssolution: results and complications assessment. Oral Dis.2012;18:548-557.
49. Sivanmalai S, Kandhasamy K, Prabu N, Prince CN, Prabu CS.Carnoy’s solution in the management of odontogenic keratocyst.J Pharm Bioallied Sci. 2012;4:S183-S185.
50. Zhao Y, Liu B, Cheng G, Wang SP, Wang YN. Recurrent ker-atocystic odontogenic tumours: report of 19 cases. Dentomax-illofac Radiol. 2012;41:96-102.

OOOO ORIGINAL ARTICLE
Volume 116, Number 1 Finkelstein et al. 83
51. Johnson NR, Batstone MD, Savage NW. Management andrecurrence of keratocystic odontogenic tumor: a systematicreview. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;115:515-522.
52. Clark P, Marker P, Bastian HL, Krogdahl A. Expression ofp53, Ki-67, and EGFR in odontogenic keratocysts beforeand after decompression. J Oral Pathol Med. 2006;35:568-572.
53. Borgonovo AE, Di Lascia S, Grossi G, Maiorana C. Two-stage treatment protocol of keratocystic odontogenic tumour inyoung patients with GorlineGoltz syndrome: marsupialization
and later enucleation with peripheral ostectomy. A 5-year-follow-up experience. Int J Pediatr Otorhinolaryngol. 2011;75:1565-1571.
Reprint requests:
Steven D. Vincent, DDS, MSUniversity of IowaCollege of Dentistry, Iowa CityIA 52242-1001, [email protected]