Embryo Transfer in Cattle Production and Its Principle and ...
Transcript of Embryo Transfer in Cattle Production and Its Principle and ...

Mebratu et al. Int. J. Phar. & Biomedi. Rese. (2020) 7(1), 40-54 ISSN: 2394 – 3726
Copyright © Jan.-Feb., 2020; IJPBR 40
Embryo Transfer in Cattle Production and Its Principle and Applications
Biruh Mebratu1, Haben Fesseha
2* and Eyob Goa
3
1College of Veterinary Medicine, Addis Ababa University, Addis Ababa, Ethiopia
2School of Veterinary Medicine, Wolaita Sodo University, P.O Box 138, Wolaita Sodo, Ethiopia
3College of Veterinary Medicine, Mekelle University, Mekelle, Ethiopia
*Corresponding Author E-mail: [email protected]
Received: 17.12.2019 | Revised: 22.01.2020 | Accepted: 28.01.2020
INTRODUCTION
In any livestock production system, efficient
reproductive performance and monitoring are
imperative for sustainability, especially for
milk, meat, draft, and replacement animals. In
recent times, there has been increasing
challenges for increasing productivity and
disease with altering climate. These targets,
thought to some extent, can be achieved by
conventional reproduction techniques.
Available online at www.ijpbr.net
DOI: http://dx.doi.org/10.18782/2394-3726.1083
ISSN: 2394 - 3726
Int. J. Phar. & Biomedi. Rese. (2020) 7(1), 40-54
ABSTRACT
The demand for animal products has grown several folds as a result of the increasing world
population. In this regard, livestock production has dramatically transformed allowing for
efficiency and improvement in productivity. For the past few years, significant improvements in
livestock productivity were achieved through the application of different biotechnologies,
followed by the extensive uptake of these new techniques. Embryo transfer is the process by
which an embryo is collected from the donor and transferred to another recipient to complete the
gestation period. The most recent advances are made in the areas of embryo transfer, in vitro
fertilization, artificial insemination, cloning, transgenesis and genomics. While some of these
applications are still under continues research scale ups. Bovine embryo transfer technology
involves the selection and management of donor and recipient animals and the collection and
transfer of embryo within a narrow window of time following oestrus. The application of this
method is numerous in cattle production to amplify reproductive rates of valuable females,
genetic improvement, twining, disease control, planned mating, increased farm income, and
others. Because of low reproductive rates and long generation intervals, embryo transfer is
especially useful in this species. However, embryo transfer is still not widely used despite its
potential benefit. The greatest challenges often associated with this in the developing world are
lack of adequate technology due to high cost and technological skills associated with the entire
process. Although this technology is not commercially available in developing countries, ET
technology could provide opportunities for the conservation and the development of minor
breeds.
Keywords: Application, Cattle, Donor, Embryo transfer, Livestock production, Recipient
ReviewArticle
Cite this article: Mebratu, B., Fesseha, H., & Eyob Goa, E. (2020). Embryo Transfer in Cattle Production
and Its Principle and Applications, Int. J. Phar. & Biomedi. Rese. 7(1), 40-54. doi:
http://dx.doi.org/10.18782/2394-3726.1083

Mebratu et al. Int. J. Phar. & Biomedi. Rese. (2020) 7(1), 40-54 ISSN: 2394 – 3726
Copyright © Jan.-Feb., 2020; IJPBR 41
Advent and use of modern reproductive
technologies have opened many avenues to
study, treat and manipulate the reproductive
phenomenon of both in vitro and in vivo to
improve reproductive performance in various
domestic species of livestock (Choudhary et
al., 2016).
Embryo transfer (ET) is a process by
which an embryo is collected from a donor
female and then transferred into a recipient
female where the embryo completes its
development (Stroud, 2012). ET is profitable
for producers of registered pure breed animals.
By far the most common use of embryo
transfer in animal production programs is the
proliferation of so called desirable phenotypes
and production of artificial inseminated (AI)
bulls through planned matting. Many breeders
have identified individual females whose
offspring are most saleable and used them
exclusively in embryo transfer (Fufa et al.,
2016). Selection criteria for the donor animals
are very likely to differ depending on the
reason for doing ET. Selection should be based
on three criteria: genetic superiority,
reproductive ability and market value of the
progeny (Fufa et al., 2016).
From historical perspective on assisted
reproduction, the first successful transfer of
mammalian embryos was performed by Walter
Heape in 1890. The first successful embryo
transfers in cattle were reported by Umbaugh
in 1949. He produced four pregnancies from
the transfer of cattle embryos, but all the
recipients aborted before the pregnancies
reached full term. In 1951, the first embryo
transfer calf was born following the surgical
transfer of an abattoir-derived day-5 embryo
(Mapletoft, 2013).
Developing countries have nearly two
thirds of the world livestock population, but
produce only about a quarter to a third of the
world’s meat, and a fifth of the milk (Rege,
2009). New technologies can help to achieve
high productivity, but need to be transferred to
producers to cause impact (Ehui & Shapiro,
2009; Yang and Honaramooz, 2010).
Naturally, a cow produces about 8 to 10 calves
in her lifetime. But with ET, it is possible to
get 32 embryos per cow per year compared to
the conventional method of breeding where the
farmer has to wait for twelve months for a calf
that could be either male or female (Glenn,
2004; Steel & Hasler 2004; Muchemi, 2011).
This review was organized to review the
principles, applications and the benefit of
embryo transfer in cattle productivity and
production.
2. GENERAL PRINCIPLES OF EMBRYO
TRANSFER
By collecting embryos from genetically elite
females and transferring the harvested
embryos into females of lesser genetic merit, it
is possible to produce more calves from
genetically superior females and fewer calves
from genetically less valuable females
(Youngs, 2007). Therefore, to achieve this
goal the bovine female ovulates multiple,
matured and viable oocytes, which are capable
of being fertilized in-vivo, and which can then
continue to develop into embryos (Smith,
2015).
Embryo collection is undertaken on
day 7 of the oestrous cycle (day 0 = day of
oestrus). Although early embryo transfer
techniques utilized surgical approaches to
embryo collection, now all commercial
embryo collections are non-surgical
procedures requiring trans-cervical
catheterization of the uterine horns (Smith,
2015).
2.1. Selection and Management of Donor
and Recipient Cow
2.1.1. Selection and management of donor cow
and sires
Selection of superior genetic or phenotypic
animals has been the basis of the donor
selection since ET`s inception (Philips and
Jahike, 2016). Genetic superiority animals that
contribute to the genetic objectives of the
programme and likelihood of producing large
numbers of usable embryos are the two broad
criteria for selecting donor cows for most
embryo transfer programmes (Thibier, 2006).
In fact, selecting the male is usually
more important than selecting the donor
female because males will normally be bred to
many females and can be selected more

Mebratu et al. Int. J. Phar. & Biomedi. Rese. (2020) 7(1), 40-54 ISSN: 2394 – 3726
Copyright © Jan.-Feb., 2020; IJPBR 42
accurately than females. Likewise, it is
necessary to select fertile bulls and fertile
semen which makes it especially important to
use high quality semen (David & Hamilton,
2016). Donors are located either on the farm
under production conditions or at an embryo
transfer centre, frequently under intensive
management. Keeping donors on the farm is
usually the less expensive alternative (George
et al., 2005).
2.1.2. Selection and management of recipient
One of the most important yet
underappreciated aspects of successful ET
program is the recipient. Cows that are
reproductively sound, that exhibit calving
ease, and that have good milking and
mothering ability are recipient prospects. They
must be on a proper plane of nutrition. These
cows also must be on a sound herd health
program (Selk, 2010).
Proper recipient herd management is
critical to ET success. Management of this
herd requires fundamental understanding of
the recipient selection, nutrition, estrus
synchronization, disease management, and
marketing (Schmidt, 2010). Personnel at the
farm must have certain skills and, above all, be
extremely conscientious (George et al., 2005).
2.2. Synchronization of Recipient
To maximize embryo survival in the recipient
female following transfer, conditions in the
recipient reproductive tract should closely
resemble those in the donor. This requires
synchronization of the estrous cycles between
the donor and the recipients, optimally within
one day of each other. Synchronization of the
recipients can be done in a similar manner and
at the same working time as the donor cows
(Galina & Orihuela, 2007).
Recipients synchronized with
prostaglandin F2α (PGF2α) must be treated 12
to 24 hours before donor cows because
PGF2α-induced estrus will occur in recipients
in 60 to 72 hours (Genzebu, 2015) and in super
ovulated donors in 36 to 48 hours (Baruselli
and Moreno, 2002). Synchronizing products
are more effective on recipient females that are
already cycling (Berber et al., 2002). The
synchronization protocol used is shown in
Table 1.
Table 1: Recipient protocol synchronises
Days in sequence Time of the day Activity
1 8.00-10.00 AM Inject 20ml multivitamine
7 8.00-10.00AM Insert CIDR device+2 ml ciderol
12 8.00-10.00 AM Inject Estrumate
14 8.00-10.00 AM Inject Estrumate
15 8.00-10.00 AM Remove CIDR device
16 8.00-10.00 AM Observe heat
24 8.00AM-5.00 PM Transfer
CIDR= (Controlled Internal Drug Release device)
Source: (Ongubo et al., 2015)
2.3. Superovulation of Donor
Superovulation refers to the release of many
oocytes (eggs) during a single estrus period
(Mapletoft, 2003). Once the donor cow is the
selected, the first step is to super ovulate or
produce multiple ova (eggs) for simultaneous
fertilization and subsequent collection.
Initially, the donor female is treated with
gonadotropin hormone called follicle
stimulating hormone (FSH). This hormone is
administered twice daily for four days in the
range of eight to fourteen days while a
functional CL is on the ovary. A PGF2α
injection given on the fourth day of the
treatment schedule will cause CL regression
and estrus to occur approximately 48 hours
later. As the result of treatment multiple
follicles should be developed on the ovaries of
the donor. Multiple numbers of eggs will be
released at estrus, one from each follicle
(Stephen & Blizinger, 2007).

Mebratu et al. Int. J. Phar. & Biomedi. Rese. (2020) 7(1), 40-54 ISSN: 2394 – 3726
Copyright © Jan.-Feb., 2020; IJPBR 43
Fig. 1: Superovulation schedule using CIDRs and GnRH. PGF2α, prostaglandin
Source: (Philipes and Jhanke, 2016)
2.4. General Procedure
The actual embryo transfer process is similar
to the method used for artificial insemination,
except that the transfer gun is passed well up
the uterine horn ipsilateral to the CL (George
et al., 2005). The donor may be inseminated
naturally or artificially and embryos will be
collected non-surgically six to eight days after
breeding. Following collection, embryos must
be identified, evaluated and maintained in a
suitable medium prior to transfer. At this
point, they may also be subjected to
manipulations, such as splitting and sexing,
and may be cooled or frozen for longer periods
of storage (Hasler, 2003).
2.5. In vivo and In vitro Fertilization
2.5.1.In vivo fertilization
During normal in vivo embryonic
development, blastomeres go through a series
of cleavage divisions. The ovum, after
fertilization, divides and develops into a 2-cell
embryo, the 2-cell develops into a 4-cell, the
4-cell into an 8-cell, etc. when the blastomeres
appear like a cluster of a grapes and individual
blastomeres can`t be differentiated, this stage
of embryonic development is known as the
morula stage. As the embryo further develops,
it prepares to undergo its first differentiation
event known as blastulation. Just prior to this
differentiation event, cells of the morula
“compact” and are allocated to the “inside”
and “outside” parts of the embryo (Jahnke et
al., 2014).
2.5.2. In vitro fertilization
Although each ovary contains
hundreds of thousands of oocytes at birth,
most are lost through atresia. This tremendous
loss of genetic material could be reduced by
harvesting oocytes from the ovary and using in
vitro production (IVP) techniques. Bovine IVP
is now a well-established and reasonably
efficient procedure. Moreover, ovum pick up
(OPU) at frequent intervals, in combination
with in vitro fertilization (IVF), has proved its
worth in improving or increasing the yield of
embryos from designated donors (Mapletof &
Hasler, 2005).
This procedure usually comprises four
separate steps in vitro: oocyte maturation,
capacitation of sperm, fertilization, and culture
of embryos until they can be frozen or
transferred to the uterus. The actual IVF step is
the easiest of the four, but success requires that
the other steps work well. Oocyte maturation,
capacitation, and culture of embryos can all be
done in vivo, but as the number of in vivo steps
increases, the practicality decreases greatly
(George et al., 2005).
2.6. Embryo Recovery
In most cases, embryos are recovered six to
eight days after the beginning of oestrus (day
0). Embryos can be recovered non-surgically
as early as four days after oestrus from some
cows, but prior to day 6 recovery rates are
lower than on days 6 to 8. Embryos can also
be recovered on days 9 to 14 after oestrus;

Mebratu et al. Int. J. Phar. & Biomedi. Rese. (2020) 7(1), 40-54 ISSN: 2394 – 3726
Copyright © Jan.-Feb., 2020; IJPBR 44
however, they hatch from the zona pellucida
on day 9 or 10, making them more difficult to
identify and isolate and more susceptible to
infection. After day 13, embryos elongate
dramatically and are sometimes damaged
during recovery or become entangled with
each other. Procedures for cryopreservation
and bisection have been optimized for day 6–8
embryos, which is another reason for choosing
this time (George et al., 2005).
Fig. 2: Diagram of the embryo flushing and recovery procedure
Source: (Selk, 2010).
2.7. Embryo Handling, Evaluation and
Storage
2.7.1. Embryo handling
Embryos are normally held in the same or a
similar medium to that in which they were
collected (IVIS, 2006) as shown in Table 2.
Careful handling of embryos between
collection and transfer is necessary to prevent
the transmission of pathogens. The use of
aseptic techniques, sterile solutions, and sterile
equipment is essential (Peregrino et al., 2000).
Table 2: Recommended culture condition for bovine embryo
PH 7.2-7.6
Osmolality 270-310 mOs M/kg
Humidity 100 %
Temperature Room temperature(15-25oC ) or 37
oC in incubator
Buffer Phosphate or bicarbonate ion (later must be maintained under 5% CO2 atmosphere)
Source: (George et al., 2008).
2.7.2. Embryo evaluation
Embryos are classified and evaluated by
morphological examination at 50 to 100 X
magnification according to the Manual of
the International Embryo Transfer Society
(IETS) (Givens et al., 2010). The overall
diameter of the bovine embryo is 150 to
190 µm, including a zona pellucida
thickness of 12 to 15 mm. The overall
diameter of the embryo remains virtually
unchanged from the one cell stage until
blastocyst stage (Bekele et al., 2016).
Generally the major criteria for
quality evaluation include; regularity of
shape of the embryo, compactness of the
blastomeres (the dividing cells within the
boundaries of the embryo), variation in
cell size, color and texture of the
cytoplasm (the fluid within the cell wall),
overall diameter of the embryo, presence
of extruded cells, thickness and regularity
of the zona pellucida (the protective layer
of protein and polysaccharides around the
single celled embryo) and presence of

Mebratu et al. Int. J. Phar. & Biomedi. Rese. (2020) 7(1), 40-54 ISSN: 2394 – 3726
Copyright © Jan.-Feb., 2020; IJPBR 45
vesicles (small bubble like structures in the cytoplasm) (Bekele et al., 2016).
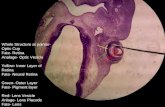
Fig. 3: Illustration of a blastocyst stage bovine embryo.
Source: (Jahnke et al., 2014)
2.7.3. Embryo storage
Procedures such as ET, IVF, sex
determination, and cloning depend on
maintaining the viability of embryos for hours
to days outside of the reproductive tract. For
many applications, the storage system must
not only maintain the viability of the embryo,
but must also support continued development
(Sauvé, 2002).
2.8. Transfer of Bovine Embryo
Transfer of embryos in the cow will result in a
high pregnancy rate providing the preceding
estrus in the donor and recipient occurred
within 24 h of each other (Mapletoft, 2006;
Smith, 2015). The successful utilization of
both surgical and non-surgical embryo transfer
in animal breeding programs represents a
significant new approach for animal breeders
(Baruselli et al., 2006).
George et al., (2005) suggested that,
both surgical and non-surgical methods of
embryo transfer can be made to work well.
Under most circumstances, non-surgical
transfer is greatly preferred, although surgical
transfer can be done quite rapidly, even in
rather primitive circumstances as described in
Table 3.
Table 3: Comparison of surgical and nonsurgical methods for recovery of embryo
Method of recovery
End point Surgical Nonsurgical
Anesthesia General Epidural
Fasting Required Non required
Ability to recovery embryos at any stage Excellent Limited
Ability to accurately assess number of ovulation Excellent Poor
Risk of accurate complication to donor Definite possibility Virtually nil
Risk to future reproductive performance of donor Yes Probably none
Embryos recovery rate Excellent Good
Source: (Betteridge, 2003)
3. APPLICATIONS OF EMBRYO
TRANSFER IN CATTLE PRODUCTION
The widespread use of embryo transfer
technology in animal breeding schemes is
relatively recent. Genetic engineering and
related new technologies will only increase its
utilization (Mapletof, 2006). Common uses of
embryo transfer technology in cattle

Mebratu et al. Int. J. Phar. & Biomedi. Rese. (2020) 7(1), 40-54 ISSN: 2394 – 3726
Copyright © Jan.-Feb., 2020; IJPBR 46
production are as follows (Table 4) (Lucero, 2015).
Table 4: Contribution of embryo transfer in animal production
Contribution Role in animal production
Rapid gene pool expansion Expand gene pools of rare breeds or in controlled geographical
region.
Increased selection intensity
in females
Approximately double selection intensity among offspring of
selected dams in beef cattle
Increased twining rate Increased number of calves produced per gestation.
Facilitate seed stock transport To aid transport of diploid genome of frozen or cultured embryos
with reduced disease hazard from country to country.
Utilize prepubrtal cocytes and
in vitro fertilization
Reduce generation time and utilize genetic potential of more oocytes
present in bovine ovary.
Gene–environment
interaction studies
Facilitate research into maternal-fetal interaction with reference to
reproductive rate, pharmacological and disease states
Source: (Can, 2018)
3.1. Genetic Improvement
Genetic progress has generally been
considered to be slower with embryo transfer
than with conventional artificial insemination,
especially on a national herd basis. However,
with increased selection intensity and
shortened generation intervals, i.e.,
transferring female offspring, genetic gain can
be made on a within-herd basis (Bekel et al.,
2016). This has resulted in the term MOET
(multiple ovulation and embryo transfer)
(Thomas, 2007).
In several countries around the world
nucleus herds are now being developed and
heifer offspring are being subjected to
"Juvenile MOET", while male offspring are
being selected for the next generation of AI
bulls. In this way, it has been estimated that
genetic gains can be doubled. On the other
hand, it has been estimated that the production
of about six offspring per donor cow could
double selection intensity and the rate of
response to genetic selection for traits such as
growth that can be measured in both sexes
(Mapletof, 2006).
ET is now commonly used to produce
AI sires from proven donor cows and bulls in
AI service. In addition, new genomic
techniques are being used increasingly to
select embryo donors; genomic analysis has
become essential for the selection of bull dams
to be used in embryo transfer (Seidel, 2010).
Although economics would not at this time
support the use of embryo transfer techniques
for anything but seed stock production, the
commercial cattle industry can benefit by the
use of bulls produced through well-designed
MOET programs (Bekel et al., 2016).
3.2. Planned Mating
By far the most common use of embryo
transfer in animal production programs is the
proliferation of so-called desirable phenotypes.
As AI has permitted the widespread
dissemination of a male's genetic potential,
embryo transfer provides the opportunity to
disseminate the genetics of proven, elite
females. ET also permits the development of
herds of genetically valuable females
(Mapletof, 2006).
Many breeders have identified
individual females whose offspring are most
saleable and used them exclusively in ET.
Embryo transfer has also been used to rapidly
expand a limited gene pool. The production of
AI bulls through embryo transfer is the most
common application of planned mating (Fufa
et al., 2016).
3.3. Disease Control
Even though ET is better and efficient
technique, the risk of transmitting genetic
disease the same as that involved in natural
mating or AI so, wise selection of dams and
sires is mandatory, no matter how cattle are
propagated. There is no increased incidence of
abnormal offspring due to these procedures.
With this technology, however, there may be

Mebratu et al. Int. J. Phar. & Biomedi. Rese. (2020) 7(1), 40-54 ISSN: 2394 – 3726
Copyright © Jan.-Feb., 2020; IJPBR 47
greater temptation to amplify reproduction of
cows with a very high market, some kinds of
infertility with a genetic basis can be
circumvented and because the sale of the
offspring can be very profitable (George et al.,
2005).
Embryo transfer procedures can be
used to control large-scale transmission of
genetic diseases such as syndactyly by
screening young bulls for undesirable
recessive Mendelian characteristics before
semen is distributed for AI. Also embryos
from parents with abnormal karyotypes can be
biopsied and karyotyped, and only the normal
ones transferred (George et al., 2005). Risk of
infectious disease transmission is less by IVP
embryos, providing embryo handling
procedures were done correctly (Stringfellow
et al., 2004). In fact, the IETS has categorized
disease agents based on the risk of
transmission by a bovine embryo (Stringfellow
& Givens, 2000; Mapletoft & Hasler, 2005).
Category 1 diseases, for which
sufficient evidence has accrued to show that
the risk of transmission is negligible, provided
that embryos are properly handled between
collection and transfer. This includes
inspection of the zona pellucida at >50X
magnification and washing/trypsin treatment
procedures. Category 1 diseases include
Enzootic bovine leucosis, Foot and mouth
disease, Bluetongue, Brucella abortus,
Infectious bovine rhinotracheitis /IBR/,
Haemophils somnus, Bovine genital
campylobacteriosis, Trichomoniasis,
Leptospira, Chlamydia, Genital Mycoplasma
and Bovine spongioform encephalopathy can
be transmitted through ET. Consequently, it
has been suggested that ET be used to salvage
genetics in the face of a disease outbreak,
which could be a useful alternative in
establishing disease-free herds (Wrathall et al.,
2004; Mapletoft, 2006).
3.4. Circumvent Infertility
It is possible to obtain offspring from
genetically valuable cows that have become
infertile due to injury, disease, or age by
means of superovulation and ET. Infertile
heifers and cows with genetically caused
subfertility should not be propagated (George
et al., 2005). Although success rates are low, it
is possible to recover oocytes from genetically
valuable, moribund cows, fertilize them in
vitro, transfer them, and obtain offspring as
listed in Table 5 (Marahall et al., 2002).
Table 5: Therapy for various types of infertility based on embryo transfer procedures
Cause of infertility Procedure
Uterine infection
In cases of persistent pyometra in which volumes of fluid and debris
build up in the uterus, it is often efficacious to flush the uterus with 0.9
percent NaCl until the recovered fluid is clear, and then to administer
PGF2α. Penicillin or oxytetracycline may be infused for three to four
days but, if not done carefully, can do more harm than good. Normal
embryo transfer procedures can be followed after the next oestrus. In
cases of subclinical or recurrent endometritis, repeated superovulation
and ET may result in the “rescue” of viable embryos from the toxic
environment to develop in the healthy uterus of the recipient.
Repeat breeder
Heifers in this category should not be propagated. With normally
cycling, parous cows one should try at least three times to recover a
single ovum to diagnose if there is ovulation, and if the ovum has been
fertilized. It may be helpful to use raw semen. If a morphologically
normal embryo at the expected stage of development is recovered,
consider progesterone therapy during gestation.
Aged repeat breeder
Problem likely due to “worn-out” uterus; normal superovulatory
treatment and transfer of embryos to uterus of younger recipient is
often effective.
Cystic ovarian disease Often superovulatory treatments based on the insertion and removal of
a progestin implant rather than on PGF2α injection are effective. If the

Mebratu et al. Int. J. Phar. & Biomedi. Rese. (2020) 7(1), 40-54 ISSN: 2394 – 3726
Copyright © Jan.-Feb., 2020; IJPBR 48
cystic ovarian condition appears to be hereditary, no propagation
should be attempted.
Adhesions of ovaries or
blocked oviducts
Superovulation increases the chances that at least a few ova will be
picked up if there are adhesions. A combination of superovulation with
surgical recovery or laparoscopy helps to diagnose the cause of the
infertility and can result in recovery of viable embryos for transfer.
Some conditions can be corrected surgically (e.g., flushing plugs of
debris from the oviduct), although relieving adhesions is rarely of
lasting benefit
Chronic abortion Recovery of the embryo before the abortion-causing condition is
active can often circumvent the resulting infertility.
Source: (George et al., 2005)
3.5. Twinning in Cattle
Cattle twinning for beef production could offer
impressive economic advantages where
nutrition is not a limiting factor and intensive
management is possible. Since about 70
percent of nutrients consumed by dams are for
body maintenance and the other 30 percent go
to producing the foetus and milk to feed the
calf, it should theoretically be possible to
produce twice as many calves with only 30
percent more nutrients if cattle had twins.
Probably a 60 percent increase in feed costs is
more realistic due to higher morbidity and
mortality and slower growth rates with twins.
In practice, one would probably decrease cow
numbers and increase calf numbers (due to
twins) so that the amount of nutrients used per
farm would remain constant (George et al.,
2005).
3.6. Embryo Transfer as Part of other
Biotechnology
As mentioned by George et al., (2005),
detection of carriers of undesirable Mendelian
recessive traits via ET is very effective for
both cows and bulls. For certain traits like
syndactyly and dwarfism, there is a shortage of
homozygous, fertile females to use as mates
for suspected carrier bulls. Embryo transfer is
an obvious means of amplifying gamete (and
embryo) production of such females so that
bulls can be tested for carrier status.
ET also provides a method of testing
daughters of carrier bulls to determine which
half does not have the deleterious allele. Since
at least seven defect-free calves are required to
be 99 percent certain that a given animal is not
a carrier, it would normally take longer than
the average reproductive lifespan of a cow to
test this; furthermore, all the calves produced
during the test would be carriers because of
using semen from a double recessive bull.
With superovulation and ET, one or two
courses of superovulation will provide enough
embryos to test most cows; moreover,
recipients can be twinned and the fetuses
examined at about two months of gestation to
diagnose many of these defects. Thus, with
embryo transfer a quick answer is possible to a
problem that is otherwise intractable (George
et al., 2005).
Exploitation of other technologies that require
manipulating the oocyte or embryo in vitro
depends on good embryo transfer techniques
for success. Such technologies include IVF,
sexing, production of transgenic animals,
bisection of embryos and cloning by nuclear
transplantation (George et al., 2005).
4. ECONOMIC IMPORTANCE OF
EMBRYO TRANSFER
In approximately for 40 years, commercial
embryo transfer in cattle has become a well-
established industry with more than 500,000
embryos being transferred on an annual basis
through the world (Mapletoft, 2012). ET is
being used for genetic improvement,
especially in the industry and most semen used
today comes from bulls produced ET (Fufa et
al., 2016).
4.1. Cost of Embryo Transfer
Costs of embryo transfer vary greatly from
country to country, and within countries,
depending on a variety of factors. However,
two generalizations can be made. First, no
matter how it is done, embryo transfer
programmes are relatively expensive. The
costs of the actual ET services or technology

Mebratu et al. Int. J. Phar. & Biomedi. Rese. (2020) 7(1), 40-54 ISSN: 2394 – 3726
Copyright © Jan.-Feb., 2020; IJPBR 49
may be quite low; however, labor and feed
costs averaged over the number of calves
produced are high since normal, healthy cows
or heifers are kept out of production in order to
be available as recipients. Second, costs per
calf are lowest when success rates are high,
because costs are spread over more calves.
Costs will be listed assuming that embryo
recovery and transfer services are purchased.
In many cases, these services will be provided
by a government, a cooperative or the
company owning the cattle, when the costs
should be determined in a different way; they
will still be real costs, nonetheless (George et
al., 2005).
4.2. Import and Export
The desire to improve herds of cattle, to
increase variation in the gene pool, and to
introduce new breeds, has motivated the
importation/exportation of breeding stock. In
the past, trade has been primarily either in
young animals with outstanding pedigrees or
semen. Animals have the advantage of being
100 percent of the desired new genotype and
are usually of breeding age so that impact on
the herd is immediate. The disadvantages are
that costs, especially for transportation, are
very high and that there is a high morbidity
rate if the new environment is markedly
different in management, climate, or endemic
pathogens. Moreover, if cows are imported,
the genetic influence on the general population
is limited until their bull calves reach breeding
age (George et al., 2005).
The intercontinental transport of live
animals also costs thousands of dollars,
whereas an entire herd can be transported, in
the form of frozen embryos, for less than the
price of a single plane fare (Mapletof, 2013).
Additional benefits of frozen embryos for the
international movement of animal genetics
compared to live animals includes reduced risk
of disease transmission, reduced quarantine
costs, a wider genetic base from which to
select, the retention of the original genetics
within the exporting country, and adaptation
(Table 6) (Stroud, 2012).
Table 6: Comparison of importing germplasm as postparturient animals, as semen or as embryos
Advantage Disadvantage
Postparturient animals
Animals productive quickly Expensive
Animals often succumb to disease
Chance of introducing exotic disease
Complex transportation logistics
Limited immediate genetic influence if females are
imported
Semen
Inexpensive Need to grade up to get pure-bred animals
Low risk of disease transmission Need for Al technology
Hybrid vigour, F1 and F2 Long wait until animals productive
Simple transportation logistics
Passive immunity from native dam
Embryo
Very low risk of disease transmission Need for ET technology
Costs may be lower than animals Long wait until animals productive
Simple transportation logistics
Passive immunity from native dam
Source: (George et al., 2005)
4.3. Farm Income Increased
Reproduction can have a multitude of impacts
on a farm, from altering culling policies,
increasing retention of better replacements,
moving primiparous cows into a more
productive the second lactation and improving
milk production. Because production accounts
for more than 88% of the gross income of a
dairy farm (Santos et al., 2010) it is no
surprise that most attention paid to

Mebratu et al. Int. J. Phar. & Biomedi. Rese. (2020) 7(1), 40-54 ISSN: 2394 – 3726
Copyright © Jan.-Feb., 2020; IJPBR 50
improvement in reproduction evolve around
altering milk production during the productive
life of cows.
In most cases, altering milk production has to
be considered per day of calving interval, as
improvements in reproduction increase the
time a cow spends in the dry period, which is
considered a nonproductive stage of the
lactation cycle. Improving reproduction often
times results in greater availability of
replacement animals, which increases herd
turnover (Ribeiro et al., 2012).
5. APPLICATION OF BOVINE EMBRYO
TRANSFER IN DEVELOPING
COUNTRY
Livestock production is one of the fastest
growing agricultural subsectors in developing
countries, where it accounts for more than a
third of agricultural gross domestic product
(GDP) (FAO, 2006). Livestock production is
expected to grow tremendously in line with the
projected demand for animal products.
Therefore, the methods of livestock production
must change to allow for efficiency and
improvement in productivity (Kahi & Rewe,
2008).
Biotechnology is important if the
world is to respond to the pressure to produce
more food from animals for the ever-growing
human population. In general, biotechnology
in livestock production can be categorized as
the biological, chemical and physical
techniques that influence animal health
(survival), nutrition, breeding and
reproduction. These techniques have been
applied mostly in developed countries but their
application in Africa is minimal due to reasons
related to economic growth such as poor
infrastructure, technical and educational
capacity (Kahi & Rewe, 2008).
Quantitative information on the
current status of use of animal biotechnologies
in developing countries is lacking, except the
use of some assisted reproductive
biotechnologies such as AI, ET and molecular
markers (FAO, 2010). Artificial insemination
and embryo transfer are probably the most
popular methods that have been adopted in
developed and developing livestock industries.
Embryos produced in vitro have led to
successful births of buffalo and cattle calves in
developing countries (Madan, 2005). The
animal species in which the technology has
been applied are cattle, buffaloes, horses and
goats (FAO, 2007). ET is limited to highly
commercialized livestock production systems
and more popular with cattle than any other
species (Robinson & McEvoy, 1993).
After artificial insemination and oestrus
synchronization, embryo transfer is the third
most commonly used biotechnology (Cowan,
2010). ET from one mother to a surrogate
mother makes it possible to produce several
livestock progenies from a superior female.
However, ET is still not widely used despite
its potential benefits. An evaluation of country
report (FAO, 2007) shows that only five of the
African countries providing information (Cote
d`Ivore, Kenya, Madagascar, Zambia and
Zimbabwe) use ET technology, all on a very
limited scale. The use of ET also been
independently reported in South Africa
(Greyling et al., 2002).
5.1. Challenges of Embryo Transfer
In Africa, livestock are still being reared in
natural pastures and environments that are
acceptable by most consumers worldwide. The
potential for Africa in the international
livestock industry is therefore promising as
demonstrated by the encouraging
developments in stable countries in the south
(Republic of South Africa), east (Kenya) and
west (Ghana) (Kahi & Rewe, 2008). The
utilization of embryo transfer in Africa is
mainly an issue of cost benefit analysis and
infrastructure. The majorities of farmers are
smallholder and have found that returns do not
measure to the investment in ET technology
considering that ET involves cryopreservation
of embryos as well as in-vitro maturation,
fertilization and culture, which are expensive.
The transport systems and technological
capacity is still too poor in some countries of
Africa to sustain the spread of reproductive
biotechnologies (Kahi & Rewe, 2008).
The major constraints of animal
biotechnology in developing countries
includes: insufficient access to land and other

Mebratu et al. Int. J. Phar. & Biomedi. Rese. (2020) 7(1), 40-54 ISSN: 2394 – 3726
Copyright © Jan.-Feb., 2020; IJPBR 51
productive resources, unfavorable terms of
trade for food products, especially for animal
products, lack of database on livestock and
animal owners , uniqueness of animal breeds,
lack of trained scientists, technicians and field-
workers, absence of coordination between
industry, universities and institution for
technology transfer, expensive technology to
be purchased from developed world, high cost
of technological inputs, poor bio-safety
measures, negligible investment in animal
biotechnology, lack of clear policy and
commitment from the government, disregards
for indigenous knowledge and local
agricultural resources management (Seidel &
Seidel, 1992).
In addition, factors such as lack of
political stability and poor conditions of the
human population like poverty, malnutrition,
disease, poor hygiene and unemployment are
also the constraints and limitation of
biotechnology in animal production in
developing countries (Madan, 2003).
5.2. Embryo Transfer in Ethiopia
The first successful embryo transfer in
Ethiopia, resulted in the birth of a Holstein-
Jersey calf at the Adami Tulu Animal
Research Center in the beginning of May,
2010 and five more calves had born. In April
2010, eighty frozen embryos that were
imported the previous August were implanted
in the native cows (Madan, 2003).
Ethiopia is a home of diverse
indigenous cattle breeds and Boran cattle are
one of them. However, effort made so far to
increase the productivity on Boran cattle in
Ethiopia was fully based on crossing with
exotic breeds either through AI or natural
breeding. As a result, crossbreeding of Boran
with Holstein has resulted in improved growth,
milk production and reproductive
performance, and these traits exhibited an
increasing trend with increasing exotic
inheritance level (Demeke et al., 2000; Haile
et al., 2009).
The Boran is a Bos indicus cattle
breed, and under ideal management conditions
it is generally accepted that Bos indicus cattle
are less fertile and have lower levels of milk
production than Bos taurus breeds. However,
Bos indicus breeds typically are better adapted
to harsh environmental conditions and
therefore are more likely to reproduce
successfully (Degefa et al., 2016).
Boran cattle breed is also the only
indigenous breed exposed to assisted
reproductive technology other than AI in the
process of breed improvement, even though
reproductive technologies are mostly
developed for Bos taurus breeds. Boran breeds
showed a relatively better potential for
application of embryo technology in terms of
ovarian follicular growth, superovulatory
response, and embryo production (Degefa,
2016).
6. CONCLUSION AND
RECOMMENDATIONS Embryo transfer in cattle has grown into a
mature international business with high
success rates and it becomes a well-established
industry. Its impact is large because of the
quality on animals being produced. Embryo
transfer is now being used for real genetic gain
and most semen used today comes from bulls
that have been produced by embryo transfer. It
also offers other important contribution to
increase numbers of offspring by production of
twines. Although embryo transfer is generally
costly in both developed and developing
countries, it is profitable. However, embryo
transfer is still not widely used despite its
potentials. In developing countries such as
Ethiopia, could only make use of the
technology in teaching and research purpose
due to absence of the necessary facilities and
infrastructure. Since the technology upgrades
and maximizes the genetic potential and hence
the productivity of animals, it is best if used in
our local breeds. In conclusion, Coordination
should be existing between industries,
universities and institutions to create well
trained technician for embryo transfer
technology. Ina ddition, awareness should be
created in the community and companies about
the profitability of embryo transfer.
REFERENCES

Mebratu et al. Int. J. Phar. & Biomedi. Rese. (2020) 7(1), 40-54 ISSN: 2394 – 3726
Copyright © Jan.-Feb., 2020; IJPBR 52
Baruselli, P., De Sa′ Filho, M., Martins, C.,
Nasser, L., Nogueira, M., Barros, C., &
Bo., G. (2006). Superovulation and
embryo transfer in Bos indicus cattle.
Theriogenology, 65, 77–88.
Baruselli, P. & Moreno, D. (2002). The control
of follicular wave development for
selfappointed embryo transfer
programs in cattle. Theriogenology, 57,
53-72.
Bekele, T., Mekuriaw, E. & Walelign, B.
(2016). Bovine embryo transfer and its
application: A review. An International
Peer-Reviewed, 26, 48-60.
Betteridge, K. (2003). A history of farm
animal embryo transfer and some
association techniques. Animal
Reproductive Science, 79, 203-244.
Can, J. (2018). The role of embryo transfer in
cattle improvement program. Animal
Science, 57, 33-45.
Choudhary K., Kavya K., Jerome, A., &
Sharma, R. (2016). Advances in
reproductive biotechnologies;
Published Online 2016 Apr 18.
Document, 9(4), 388-395.
Cowan, T. (2010). Biotechnology in animal
agriculture: status and current issues.
Analyst in natural resources and rural
development. Congressional Research
Service, pp: 3.
David, A. & Hamilton, S. (2016). Hamco
cattle co. 18thAnnual angus bull sale.
Glenboro, Manitoba Canada.
Degefa, T. (2016). Ovarian follicular
dynamics, super-ovulatory response,
and in vivo embryo production
potential of Boran (Bos indicus) and
Boran *Holstein cross cattle in
Ethiopia. PhD Thesis, Addis Ababa
University, Faculty of Veterinary
Medicine, Bishoftu, Ethiopia.
Degefa, T., Lemma, A., Tegegne, A. and
Youngs, C. (2016). Superovulation of
Boran Cattle in Ethiopia: A
Preliminary Report. Animal Industry
Report, l662, 1-28
Demeke, S., Neser, F., Schoeman, S.,
Erasmus, G., Van Wyk, J. &
Gebrewolde, A. (2000). Crossbreeding
Holstein-Friesian with Ethiopian Boran
cattle in a tropical highland
environment: preliminary estimates of
additive and heterotic effects on milk
production traits. South African
Journal of Animal Science, 30(1), 32-
33.
Ehui, S. & Shapiro, B. (2009). Research and
Technology transfer for livestock
development. Journal of Animal
Science, 87, 801-812.
FAO (2010). Current status and options for
livestock biotechnologies in
developing countries.
Fufa, N., Abera, D. & Kabeta, T. (2016).
Review on bovine embryo transfer.
European Journal of Biological
Science, 8(3), 79-84.
Galina, C. & Orihuela, A. (2007). The
detection of estrus in cattle raised
under tropical conditions: What We
Know and What We Need To Know
Hormones and Behavior, 52, 32-38.
Genzebu, D. (2015). A review of embryo
transfer technology in cattle. Global
Journal of Animal Scientific Research,
3(2), 562-575.
George, E., Seidel, J., & Sarah, M. (2008).
Training manual for embryo transfer in
cattle (FAO). Animal reproduction
laboratory: pp: 79.
George, E. Seidel, J., & Sarah, M. (2005).
Training manual for embryo transfer in
cattle (FAO) animal production and
health paper, pp: 77.
Glenn, S. (2004). Embryo transfer in cattle.
Oklahoma State University, Division
of Agriculture, USA.
Grayling, J., Van der, N., Schwalbach, L., &
Muller, T. (2002). Super ovulation and
embryo transfer in South African Boer
and indigenous feral goats. Small
Ruminant Reproductive, 43, 45-51.
Haile, A., Joshi, B., Ayalew, W., Tegegne, A.,
& Singh, A. (2009). Genetic evaluation
of Ethiopian Boran cattle and their
crosses with Holstein Friesian in

Mebratu et al. Int. J. Phar. & Biomedi. Rese. (2020) 7(1), 40-54 ISSN: 2394 – 3726
Copyright © Jan.-Feb., 2020; IJPBR 53
central Ethiopia: milk production traits.
Animal Science, 3, 486-493.
Hasler, J. (2003). The current status and future
of commercial embryo transfer in
cattle. Animal Reproduction Science,
79, 245-264.
IVIS (2006): Reviews in Veterinary Medicine.
Kunkel, J. (2002). Embryo Transfer.
Dairy Integrated Reproductive
Management (IRM). Pp. 26
Jahnke, M., West, J., & Youngs C. (2014).
Evaluation of in vivo derived embryos.
In: Hopper RM, editor. Bovine
reproduction. Hoboken (NJ): Wiley
and Sons. Pp. 735.
Kahi, A. and Rewe, T. (2008): Biotechnology
in livestock production: Overview of
possibilities for Africa. Animal
Breeding and Genetics, 7(25), 498-
499.
Lucero, M. (2015). Artificial insemination and
embryo transfer. New Mexico youth
ranch management camp beef day 4-H
agriculture agent.
Madan, M. (2005). Animal biotechnology:
applications and economic implications
in developing countries. Review
Science Technology, 24(1), 127-139.
Madan, M. (2003). Opportunities and
constraints for using gene-based
technologies in animal agriculture in
developing countries. In: FAO/IAEA
international symposium on
applications of gene based technologies
for improving animal production and
health in developing country, Vienna,
Austria, 6-10 October.
Mapletoft, R. & Hasler, J. (2005). Assisted
reproductive technologies in cattle: a
review. Review Science Technology,
24(1), 393-403.
Mapletoft, R. (2013). History and perspectives
on bovine embryo transfer. Animal
Reproduction, 10(3), 168-173.
Mapletoft, R. (2012). Perspective on Bovine
Transfer. WCDS Advances in Dairy
Technology, 24, 83-93.
Mapletoft, R. (2006). Bovine embryo transfer.
In: I.V.I.S. (ed.): IVIS Reviews in
veterinary medicine, Saskatchewan,
Canada.
Marahall, D. & Minyard, J. (2002). Embryo
transfer in beef cattle. College of
Agriculture & Biological sciences /
South Dakota State University /
USDA.
Muchemi, J. (2011). New embryo transfer
technology to boost dairy farmers’
fortune, Sunday Nation, Kenya.
August pp. 26-27.
Ongubo, M., Rachuonyo, H., Lusweti1, F.,
Kios, D., Kitilit, J., Musee, K.,
Tonui,W., Lokwaleput, I. & Oliech, G.
(2015): Factors affecting conception
rates in cattle following embryo
transfer. Uganda Journal of
Agricultural Sciences, 16(1), 19 – 27.
Peregrino, G. (2000). Technical aspects of the
recovery, handling and transfer of
embryos. National headquarters and
gene pool Philippine Carabao center
muñoz, nueva ecija 3120 Philippines.
Philips, P. & Jahinke, M. (2016). Embryo
transfer (Technique, Donors, and
Recipients). Articles in Veterinary
Clinics of North America Food animal
practice. Pp. 366-384.
Rege, J. (2009). Biotechnology options for
improving livestock production in
developing countries with special
reference to Sub-Saharan Africa.
Society of Animal Science, 34, 123-
131.
Ribeiro, E., Galvao, W., Thatcher, J. & Santos,
J. (2012). Economic aspect of
applying reproductive technologies to
dairy. Herd Animal Reproductive, 9,
370-380.
Robinson, J. and McEnvoy, T. (1993).
Biotechnology – the possibilities.
Animal Production, 57, 335-352.
Santos, J., Bisinotto, E., Riberio, F., Lima, L.,
Greco, C. & Staples, C. (2010).
Applying nutrition and physiology to
improve reproduction in dairy cattle.
Social Reproductive Fertilization
Supply, 67, 387-403.

Mebratu et al. Int. J. Phar. & Biomedi. Rese. (2020) 7(1), 40-54 ISSN: 2394 – 3726
Copyright © Jan.-Feb., 2020; IJPBR 54
Sauvé, R. (2002). Embryo Transfer. Available:
http://www.embryobec.com/TEang.ht
ml. URL
Schmidt, J. (2010). Thriving, surviving, and
nose-diving in beef recipient
management. In: proceeding of
America Embryo Transfer
Association, Champaign, IL; p. 35-42
Seidel, G. (2010). Brief introduction to whole
genome selection in cattle using single
nucleotide polymorphisms.
Reproductive Fertilization
Development, 22, 138-144.
Seidel, G. & Seidel, S. (1992), Analysis of
applications of embryo transfer in
developing countries. ATSAF
(Council for tropical and subtropical
research) Bonn, Germany.
Selk, G. (2010). Embryo transfer in cattle.
Division of Agriculture and Natural
Resource, 3158, 1-4
Smith, A. (2015). Principles of embryo
transfer. Proceeding of the Australian
Reproduction Veterinarian (ARV)
Steel, R. & Hasler, J. (2004). Pregnancy rates
resulting from transfer of fresh and
frozen Holstein and Jersey embryos.
Reproduction, Fertility and
Development, 16, 182-183.
Stephen, B. & Blizinger, A. (2007). Embryo
transfers becoming more popular with
procedures, cattle today. Amazon
Stringfellow, D., Givens, M., & Waldrop, J.,
(2004). Biosecurity issues associated
with current and emerging embryo
technologies. Reproductive
Fertilization Development, 16, 93-102.
Stringfellow, D. and Givens, M. (2000).
Epidemiologic concerns relative to in
vivo and in vitro production of
livestock embryos. Animal
Reproduction Science, 60(61), 629-
642.
Stroud, B. IETS., (2012). Statistics and Data
Retrieval Committee Report. The year
2011 worldwide statistics of embryo
transfer in domestic farm animals.
Embryo Transfer News Letter, 30, 16-
26.
Thibier, M. (2006). Data retrieval committee
annual report. Embryo Transfer
Newsletter, 24(4), 12-18.
Thomas, H. (2007). Embryo transfer can
maximize best genetics. Cattle today,
Inc.
Wrathall, E., Simmons, H., Bowles, D. &
Jones, S. (2004). Biosecurity strategies
for conserving valuable livestock
genetic resources. Reproductive
Fertilization Development, 16(2), 103-
112.
Yang, C. & Honaramooz, C. (2010). Effects of
medium and hypothermic temperatures
on preservation of isolated porcine
testis cells. Reproduction, Fertility and
Development 22, 523-532.
Youngs, C. (2007). Embryo transfer in beef
cattle. Proceedings of applied
reproductive strategies in beef cattle.