Electron as Wave, Orbital Type and Electron Configuration for Students
-
Upload
glen-mangali -
Category
Documents
-
view
237 -
download
0
Transcript of Electron as Wave, Orbital Type and Electron Configuration for Students
-
8/6/2019 Electron as Wave, Orbital Type and Electron Configuration for Students
1/25
Understand the concept of Bohr and electron
configuration
Describe quantum number and itsimportance
Identify and predict the atomic, period and
group number
-
8/6/2019 Electron as Wave, Orbital Type and Electron Configuration for Students
2/25
-
8/6/2019 Electron as Wave, Orbital Type and Electron Configuration for Students
3/25
small bits of energy
-
8/6/2019 Electron as Wave, Orbital Type and Electron Configuration for Students
4/25
designated as by letter n and can have
positive integral values 1, 2,3,4.....
-
8/6/2019 Electron as Wave, Orbital Type and Electron Configuration for Students
5/25
-
8/6/2019 Electron as Wave, Orbital Type and Electron Configuration for Students
6/25
Maximum number of electrons = 2n2
-
8/6/2019 Electron as Wave, Orbital Type and Electron Configuration for Students
7/25
What is the maximum number of electrons in
a given shell is given by 2n2. For the fifth
level, n=5, and so we have
2 x 52 = 2 x 25 = 50
-
8/6/2019 Electron as Wave, Orbital Type and Electron Configuration for Students
8/25
Hydrogen (Z=1) the single goes into the first
shell
Helium (Z=2) both goes to the first shell Lithium ( Z=3) two goes to the first and other
goes to the second shell
-
8/6/2019 Electron as Wave, Orbital Type and Electron Configuration for Students
9/25
Flourine and Aluminum
Flourine has a symbol F ( Z=9); two of theseelectron goes into the first shell and the
remaining seven go into the second levelF 2, 7
-
8/6/2019 Electron as Wave, Orbital Type and Electron Configuration for Students
10/25
Flourine and Aluminum
Aluminum ( Z=13) has 13 electronAl 2, 8, 3
-
8/6/2019 Electron as Wave, Orbital Type and Electron Configuration for Students
11/25
Where group does flourine belongs?What
about aluminum?
What have you observe with the groupings?
-
8/6/2019 Electron as Wave, Orbital Type and Electron Configuration for Students
12/25
What is the maximum number of electrons in
the fourth shell ( fourth energy level)?
32 electrons
-
8/6/2019 Electron as Wave, Orbital Type and Electron Configuration for Students
13/25
-
8/6/2019 Electron as Wave, Orbital Type and Electron Configuration for Students
14/25
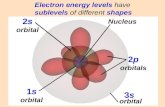
l = 0 designated by s and the shape of the
electron standing wave is spherical-like
l = 1 designated by p and the shape is dumbbell-like
l = 2 designated by d and the shape is four-lobedl = 3 designated by f and the shape is very
complicated
-
8/6/2019 Electron as Wave, Orbital Type and Electron Configuration for Students
15/25
Principal quantum number n corresponds to
the main energy level in which the electron
moves, that is 1,2,3 or K,L,M Azimuthal quantum number 1 gives and
measures the angular momentum of anelectron in its motion about the nucleus
-
8/6/2019 Electron as Wave, Orbital Type and Electron Configuration for Students
16/25
n l ml
Number of
orbitals
Orbital
Name
Number of
electrons
1 0 0 1 1s 22 0 0 1 2s 2
1 -1, 0, +1 3 2p 6
3 0 0 1 3s 2
1 -1, 0, +1 3 3p 6
2-2, -1, 0, +1,
+25 3d 10
4 0 0 1 4s 2
1 -1, 0, +1 3 4p 6
2-2, -1, 0, +1,
+25 4d 10
3-3, -2, -1, 0,+1, +2, +3
7 4f 14
-
8/6/2019 Electron as Wave, Orbital Type and Electron Configuration for Students
17/25
Magnetic quantum number m indicates the
behavior of the electrons in a magnetic field;
m may value from -1 to +1 including 0. Spin quantum number s indicates the spin of
an electron about its own axis in a clockwise(+) or counter clockwise (-) direction.
-
8/6/2019 Electron as Wave, Orbital Type and Electron Configuration for Students
18/25
The number of orbital types in a given shell is
equal to the shell number. Assume that n=
shell number or energy level, if n= 1, orbitaltype is 1.
-
8/6/2019 Electron as Wave, Orbital Type and Electron Configuration for Students
19/25
The different types of orbitals in order of
increasing complexity of shape as s,p,d,f.
The number of orbital s of a given typeincreases from type to type according to a
series of odd numbers beginning from 1
Paulis exclusion principles states that no less
than two electrons can occupy a given orbitaltype.
-
8/6/2019 Electron as Wave, Orbital Type and Electron Configuration for Students
20/25
The electron population of each shell or
energy level may be determined using the
formula 2n2 where n is equal to the numberof shell or energy level.
-
8/6/2019 Electron as Wave, Orbital Type and Electron Configuration for Students
21/25
ApartmentHouse Rules Electron Rules
From the Bottom Up:Rooms must be filled from
the ground floor up. Fill theone room on the first floorbefore starting to put new
tenants on the second floor.Then fill the s room before thep rooms. At higher floors theorder might change a bit.
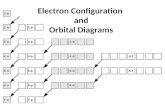
Aufbau Principle:the electrons fill the available
orbitals from lowest energy tohighest energy. In the groundstate all the electrons are in
the lowest possible energylevel.
-
8/6/2019 Electron as Wave, Orbital Type and Electron Configuration for Students
22/25
Singles First:
the owner of the buildingwants to have the tenants
spread out as much aspossible. For that reasonsingles are placed in rooms
before couples. If couplesmust be placed into a roomthen all of the other rooms onthat floor must already have asingle in them.
Hunds Rule:
The electrons must be placedinto the orbitals in such a way
that no pairs are put togetherunless absolutely necessary.That is, single electrons must
be placed into boxes first andthen paired up if necessary.
-
8/6/2019 Electron as Wave, Orbital Type and Electron Configuration for Students
23/25
-
8/6/2019 Electron as Wave, Orbital Type and Electron Configuration for Students
24/25
The first main energy level can have a
maximum number of two electrons.
The second main energy level can have amaximum number of thirty-two electrons (2s
orbitals, 6p, 10d, and 14 f orbitals)
An s-orbital can only have 2 electrons, a p-
orbital 6, and 1 d-orbital 10, and an f-orbital14
-
8/6/2019 Electron as Wave, Orbital Type and Electron Configuration for Students
25/25
The valence number is determined by
counting the number of electrons in the last
main energy level. A particular energy level may have orbitals
which may not be filled up due to highenergy.