cpb-us-e1.wpmucdn.com · Web viewThe Chemistry of Carbon Carbon has exactly _____ total electrons...
Transcript of cpb-us-e1.wpmucdn.com · Web viewThe Chemistry of Carbon Carbon has exactly _____ total electrons...

v Unit 2: Biochemistry –Macromolecules
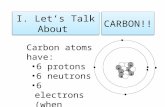
The Chemistry of Carbon• Carbon has exactly _____ total electrons but ____ of
those electrons are in its outer shell.o This makes it easy for Carbon to
____________________ electrons with other elements.
o This forms ____________________compounds.
• There are millions of molecules that contain carbon. These are called ___________________________. Macromolecules
• ________________________ are large organic molecules made of thousands of atoms of carbon and other elements can be bonded together.
• The four classes of organic molecules are:• ____________________________________• ____________________________________• ____________________________________• ____________________________________• Every organic molecule except lipids is a
_____________________, or a molecule made up of repeating parts.
The Diversity of Polymers• An immense variety of __________________ can
be built from a small set of ___________________.• From the same three types of simple sugars you can
make:_____________________ (energy storage in plants)___________________ (energy storage in animals)______________________ (table sugar)______________________ (milk sugar)__________________________ (plant fiber)_______________________ (insect exoskeletons)
Carbohydrates• Carbohydrates include sugars and the polymers of
sugars• Made of ______________, ________________,
and _________________• Carbohydrates are the main source of energy for
all life.o _____________________o _____________________o _____________________
Sugars• ____________________________ are the simplest carbohydrate, made from only ____________________ .
o ___________________ (Blood sugar)o ___________________ (Part of milk sugar)o ___________________(Fruit sugar)
• ____________________________ serve as a major fuel for cells and as raw material for building molecules • Polysaccharides are polymers (__________________________________________) of sugars.• Polysaccharides have two main purposes in living things:
o Providing _______________________o Energy _______________________

Storage Polysaccharides• Starch, a storage polysaccharide of ____________.
o Made entirely of __________________ __________________ joined together.
• Plants ___________________________ starch within their cells.
• ___________________ makes the strongest part of the ___________________of plants.
• Allows plants to be ____________ and _________.• Cellulose is very similar to starch and is also made
from __________________________________.• The bonds between the glucose molecules are
slightly different.• These molecules are only digestible by__________
________________________.o Many herbivores, from cows to termites,
have ________________________ with bacteria to help them digest cellulose.
• ___________________, another structural polysaccharide, is found in the exoskeleton of _________ and the cell walls of ___________.
• Chitin can be used as ____________________ ____________________ because it is gradually reabsorbed by the body.
• Chitin is ______________________________; only species that eat mainly _______________ can break it down easily.
Lipids• Lipids do not form __________________.• Lipids are considered _________________ because they are non-polar and cannot dissolve in water.• The most biologically important lipids are _______, ____________________, and ________________.
Phospholipids• Phospholipids make up ______________________• The two fatty acid ______________ are hydrophobic, but the
phosphate ____________is hydrophilic.• When phospholipids are added to water, they self-assemble into
a _________________.• The phospholipid ________________ form the outer part that is
in contact with the water (hydrophilic= likes water)• The fatty acids ________________ form the inner part that is
away from water. (hydrophobic= fears water) • This phospholipid bilayer creates the basic structure of ______
cell membranes. •Proteins• Proteins account for more than 50% of the dry mass of most cells• Protein functions include
o ____________________________________ o ___________________, ____________________o _____________________________________________o ________________________________________o _______________________________________________________

Protein Structure• Proteins are made of chains of _________________
_________________________.• There are only _____ amino acids, but they can be
combined in nearly infinite ways.• The ___________________ of amino acids
determines the _________________ of the protein.• The ___________________ of the protein is the
biggest factor that determines its ______________________.
• “FORM DICTATES FUNCTION!”
Polypeptides• Polypeptides are _____________________
with ______________________________.o Poly = _________________o Peptides = _________________o Polypeptides is another way to say
__________________________
4 Levels of Protein Structure• _______________________________ is the
_____________________ of amino acids in a protein.
• Compare to the order of letters in a long word.• Primary structure is directly _____________
_________________________in the nucleus.
Sickle Cell Anemia• A slight change in __________________________
_____________________can drastically change the _________________________________ of the protein.
• When a protein changes shape, it ________________ work the same.
• ________________________________ is a genetic disease that occurs when red blood cells are shaped like crescents or sickles instead of saucers.
o Result: Not enough oxygen gets through the body, making the person become tired very easily.
• The single gene that is affected changes ______________________________ in the ___________________________________ of hemoglobin.
• This affects the ______________________ structure, which affects the __________________________ structure, which affects the ____________________ structure.
What determines protein conformation?• Protein shape can also be affected by
________________ and ________________.• When a protein loses its normal shape, this is
called _____________________.• A denatured protein is __________________
___________________________.• Denaturing is usually __________________
and _______________________________.