Spectroscopy and Photochemistry AOSC 637 R. Dickerson Fall 2011 Copyright © 2011 R. R. Dickerson1.
Copyright © 2015 R.R. Dickerson1 Introduction to Kinetics AOSC 620 Fall 2015 R. Dickerson See...
-
Upload
ashlee-knight -
Category
Documents
-
view
213 -
download
0
Transcript of Copyright © 2015 R.R. Dickerson1 Introduction to Kinetics AOSC 620 Fall 2015 R. Dickerson See...

Copyright © 2015 R.R. Dickerson 1
Introduction to Kinetics
AOSC 620Fall 2015
R. Dickerson
See Finlayson-Pitts Chapter 5Seinfeld and Pandis Chapters 4 & 9

Copyright © 2013 R.R. Dickerson 2
KineticsA. Rates, rate constants, and reaction order. Thermodynamics tells us if a reaction can proceed and gives equilibrium concentration. Kinetics tells us how fast reactions proceed. If thermodynamics alone controlled the atmosphere it would be dissolved in the oceans as nitrates - we would be warm puddles of carbonated water. 1. First Order Reactions
A PRODUCTS
EXAMPLES
222Rn -> 218Po + N2O5 NO2 + NO3 (forward reaction only)

Copyright © 2013 R.R. Dickerson 3
The red line describes first order loss with a rate constant of 1 min-1
The blue line is the rate of formation of the product.
minutes

Copyright © 2013 R.R. Dickerson 4
Radon is important source of indoor air pollution, and N2O5
is nitric acid anhydride, important in air pollution nighttime chemistry. The rate equations take the form:
d[Prod.]/dt = k[A] = -d[React.]/dt
For example:
d[Po]/dt = kRn [Rn] = -d[Rn]/dt
Where k is the first order rate constant and k has units of time-1 such as s-1, min-1, yr-1. We usually express concentration, [Rn], in molecules cm-3 and k in s-1.

Copyright © 2013 R.R. Dickerson 5
Another example, nitric acid anhydride.
d[NO2] /dt = k [N2O5]
and
d[N2O5]/dt = -k [N2O5]
Integrating
ln ([ N2O5 ]t / [ N2O5 ]o) = -k t
If we define the starting time as zero:
[N2O5]t / [N2O5]o = exp(-kt)

Copyright © 2013 R.R. Dickerson 6
The rate constants for these first order reactions are: kRn = 0.182 days-1
kN2O5 = 0.26 s-1 (at room temperature)

Copyright © 2013 R.R. Dickerson 7
2. Second Order Reactions
A + B PRODUCTS
EXAMPLES
NO + O3 NO2 + O2
HCl + OH H2O + Cl
Examples of the rate equations are as follows:
d[NO]/dt = -k[NO][O3]
d[Cl]/dt = k[OH][HCl]
Units of k are {conc-1 time-1}.
1/(molecules/cm3) (s-1) = cm3 s-1

Copyright © 2013 R.R. Dickerson 8
For our second order kinetics examples
Units of k are {conc-1 time-1}. 1/(molecules/cm3) (s-1) = cm3 s-1
Rate constants have the following values: kNO-O3 = 1.8x10-14 cm3 s-1
kHCl-OH = 8.0x10-13 cm3 s-1

Copyright © 2013 R.R. Dickerson 9
3. Third Order Reactions
A + B + C PRODUCTSd[A]/dt = -k[A][B][C]
Examples2NO + O2 2NO2
O + O2 + M O3 + M

Copyright © 2013 R.R. Dickerson 10
2NO + O2 2NO2
O + O2 + M O3 + M
M is any third body (usually N2) needed to dissipate excess energy.
From the ideal gas law and Avg's number:
Where Mo is the molecular number density at STP.
Third order rate constants have units of conc-2 time-1. These are usually (cm-3)-2 s-1.
kNO-O2 = 2.0 x 10-38 cm6 s-1
kO-O2 = 4.8 x 10-33 cm6 s-1

Copyright © 2013 R.R. Dickerson 11
USEFUL IDEA:
For the following reaction:A + B C + D
d[C]/dt = kf [A][B] - kr [C][D]
At steady state d[C]/dt = 0, by definition. Thus:
{ kf }/{ kr } = {[C][D]}/{[A][B]} = Keq

Copyright © 2013 R.R. Dickerson 12
Halflife and Lifetime
Definition: Halflife, t1/2 the time such that:
[A]t1/2 / [A]0 = 1/2
Definition of lifetime or residence time, , comes from kinetics, where k is the first order rate constant with units of time-1.
We know that:
[A]t/[A]0 = exp(-k t)
The lifetime, , is when t = 1/k so 1/k
We can link half-life and lifetime:
t1/2 = ln(2)/k 0.69/k

Copyright © 2013 R.R. Dickerson 13
For radon 222 (222Rn) the lifetime is 5.5 days, but the half-life is only 3.8 days.
For second order reactions we need pseudo first order conditions
For example:
NO + O3 NO2 + O2
k = 1.8 x 10-14 cm3 s-1
ASSUME: [O3] >> [NO] and d[O3]/dt ~ 0.0
Let: = 50 ppb (a reasonable value for air near the surface).
NO = = 1/{1.8 x 10-14 x 50x10-9 x 2.5x1019} = 44 s
CONCLUSION: any NO injected into such an atmosphere (by a car for example) will quickly turn into NO2 , if there are no other reactions that play a role. We will call
k[O3] the pseudo first order rate constant.

Copyright © 2013 R.R. Dickerson 14
For example:
O + O2 + M O3 + M
k = 4.8x10-33 cm6s-1
ASSUME: d[O2]/dt = d[M]/dt = 0.0
We know that [O2] = 0.21 and that [M] ~ [O2] + [N2] ~ 1.00. At RTP P02 = 0.21
atm and PM ~ 1.0 atm. Therefore the lifetime of O atoms is
= [4.8x 10-33 x 0.21 x 1.0 x (2.5x1019) 2]-1
= 1.6x10-6 s VERY SHORT!
For third order reactions we must assume that two components are constant.

Copyright © 2013 R.R. Dickerson 15
Example 2
Same reaction at stratospheric temperature and pressure.
P30km ~ P0 exp(-30/7) = 0.014 atm
= [4.8x10-33 x 0.21 x 1.0 x (0.014 x 2.5x1019) 2]-1 = 2.1x10-3 s
The lifetime of an O atom is is still short, but it is a thousand times longer than in the troposphere! The pressure dependence has a major impact on the formation and destruction of tropospheric and stratospheric ozone.

Copyright © 2013 R.R. Dickerson 16
If two of the reactants in a third order reaction are the same, we can derive a useful expression for the rate of loss of the reactant.
A + A + B PROD
For a great excess of B:
d[A]/dt = -(2k[B])[A] 2
[A] -2 d[A] = -(2k[B])dt
Integrating from 0 to time t
[A]-2 d[A] = -(2k[B])dt
-[A]t-1 + [A]0
-1 = -(2k[B])t
[A]t
-1 = 2k[B]t + [A]0-1
Now we can calculate the concentration at any time t in terms of the initial concentration and the rate constant k.

Copyright © 2013 R.R. Dickerson 17
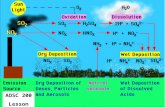
Transport and Evolution of a Pollution Plume from Northern China:A Satellite-based Case Study
Can Li1, Nick Krotkov2, 3, Russ Dickerson1, Zhanqing Li1, 4, and Mian Chin2
1AOSC, UMD 2GSFC, NASA 3GEST, UMBC 4ESSIC, UMD
Why China? A lot of emissions… Local, regional, and global effects
Why transport and evolution? Key factors determining the large-scale impact of air pollution (e.g., conversion from SO2 to sulfate aerosols, adding CCN to the system)
Why satellites? Transport episodes associated with synoptic weather system, and are of regional scale – new satellite sensors provide great spatial coverage, daily observation, and sensors of different strengths can be combined

Copyright © 2013 R.R. Dickerson 18
On April 5,2005 on the aircraft:Lots of SO2
Lots of dust
~1 hr later and from space:Lots of SO2
Lots of dust

Copyright © 2013 R.R. Dickerson 19
Trajectory model projects movement of the plume, satellite sensors take snapshots every day
Trajectory Projection
Satellite Snapshots
AMF Correction to operational product, combination of satellites and models, and MODIS AOD data
SO2 lifetime: 1-4 daySO2 to sulfate: ~0.1-0.2 increase in AOD near plume core in one day
Uncertainties? Details? Suggestion?Please come to see our poster.

The comparison demonstrates that operational OMI algorithm can distinguish between heavy pollution ( April 5 ahead of cold front ):
SO2
OMI SO2AQUA- MODIS RGB

…and background SO2 conditions (on April 7 behind cold front)
SO2
OMI SO2MODIS RGB

y = -0.26x - 0.01
-0.6
-0.5
-0.4
-0.3
-0.2
-0.1
0
0 0.5 1 1.5 2 2.5
Days after 5 April 2005
ln(C
/C0)
SO2 lifetime estimate
By following the path of the air mass, and correcting for the altitude effect, the amount of SO2 lost to dry deposition and chemical
reactions was approximated.
Ln (C/C0) = -kt
The mass decreases from the 5th to the 7th looks to be first order with a lifetime of ~4 d.
The mass on April 8 shows no change from April 7 because the air mass was very disperse (large box) and mixed with other plumes.

SO2 + OH prod (H2SO4)
If attack by OH is the only sink for SO2, and if the lifetime is ~4 d =~ 3x105 s.
Lifetime = = ([OH]*M*k)-1
k = 2x10-12 cm-3 s-1
Molecular number density of OH = 1.4x106 cm-3