Chapter 5 - hAFS cells for liver repair - Final...
Transcript of Chapter 5 - hAFS cells for liver repair - Final...

Chapter 5
HUMAN AMNIOTIC FLUID STEM CELLS AS A SOURCE OF HEPATIC AND ENDOTHELIAL PROGENITORS
FOR IN VIVO CELL THERAPY

89
ABSTRACT
Human amniotic fluid has been utilized in prenatal diagnosis for over 70 years. It has proven to
be a safe, reliable, and simple screening tool for a wide variety of developmental and genetic
diseases. There is now evidence that amniotic fluid may have more utility than only as a
diagnostic tool, and it may be a source of a powerful therapy for a multitude of congenital and
adult disorders. In this chapter, we describe the development of the amniotic fluid in the human,
and the cells that are found within the amniotic fluid. We will further describe previous research
suggesting that amniotic fluid have progenitor cells, and the recent data from our lab
demonstrating a rare population of stem cells from amniotic fluid, termed amniotic fluid stem
(AFS) cells that proliferate readily in culture and differentiate into various cell types. We will
also confirm that these cells don’t form teratomas and show their potential to integrate in
regenerating tissues in vivo. It is possible that in the near future, we will see the development of
therapies utilizing progenitor cells isolated from amniotic fluid for the treatment of newborns
with congenital malformations as well as in adults using cryopreserved amniotic fluid stem cells.

90
5.1 INTRODUCTION
Amniotic fluid derived stem (AFS) cells can be obtained from a small amount of an amniotic
fluid specimen during amniocentesis, a procedure that is frequently performed in many of the
pregnancies in which exists a risk of congenital abnormality of the fetus. Analysis of cell cultures
from this source, provide evidence that they may represent a new source for the isolation of cells
with the potency to differentiate into different cell types, suggesting a new source of cells for
research and regenerative medicine.
5.1.1 DEVELOPMENT AND CHARACTERIZATION OF AMNIOTIC FLUID
Amniotic Fluid in Developmental Biology
Embryonic and fetal cells derived from all three germ layers and extraembryonic tissues have
long been identified in the amniotic fluid1-3
. Regardless of the fact that the specific origins of
many subsets of these cell types still remain to be determined, their origin in the amniotic fluid is
closely tight with the developmental processes unfolding in utero at a particular gestational age.
It is not surprising that the profile of the cellular component of the amniotic fluid varies with
gestational age4.
A major milestone in early post implantation development is gastrulation. At about embryonic
day 15 (E15), gastrulation begins in the posterior region of the human embryo. Pluripotent
epiblast cells are allocated to the three primary germ layers of the embryo (ectoderm, mesoderm,
and endoderm) and germ cells, which are the progenitors of all the tissue lineages as well as the
extraembryonic mesoderm of the yolk sac, amnion, and allantois5. The latter forms the umbilical
cord as well as the mesenchymal part of the labyrinthine layer in the mature chorio-allantoic
placenta. The final positions of the fetal membranes result from the process of embryonic folding
or turning, which occurs around human E21 of gestation and ‘‘pulls’’ the amnion and yolk sac
around the embryo. The amniotic cavity pushes in also at the cranial and caudal ends of the
embryonic disc, thus increasing the degree of longitudinal folding at the head and tail folds. The
amniotic cavity also pinches the connection of the yolk sac and gut to form the narrowed
communication of the vitello-intestinal (or vitelline) duct6.

91
Once formed, the amniotic sac is a tough but thin transparent pair of membranes that holds a
developing embryo (and later fetus) until shortly before birth. The inner membrane, the amnion,
contains the amniotic fluid and the fetus. The outer membrane, the chorion, contains the amnion
and is part of the placenta6, 7
. With all this developmental processes unfolding, it is not surprising
that a wide variety of different origins has been suggested for the mixture of cells within
amniotic fluid1-3, 8-11
.
Amniotic fluid Secretion and Dynamics
In humans, as mentioned above, the amniotic cavity starts to form immediately prior to
gastrulation (E8-9). Once the lining of the amnion is completed, the amniotic cavity fills with
amniotic fluid secreted by the cells in the amniotic membrane. This fluid serves as a shock
absorber for the developing embryo while prevents its desiccation. It also ensures symmetrical
structure development and growth maintaining consistent pressure and temperature. The fluid
permits freedom of fetal movement, important for musculoskeletal development and blood flow6,
7, 12.
In the first half of gestation, most of the amniotic fluid results of active sodium and chloride
transport across the amniotic membrane and fetal skin, with concomitant passive movement of
water13
. In the second half of gestation, most of the fluid comes from fetal urine. An additional
major source of amniotic fluid is secretion from the respiratory tract. Fetal swallowing and
gastrointestinal tract excretions, while not voluminous, also play a role in amniotic fluid
composition14-16
.
These fluid dynamics are responsible for the production and turnover of the amniotic fluid and
are also thought to be important in determining the cell types present in the amniotic cavity. Still,
much remains to be clarified about the ontogeny of many subsets of amniocytes at any
gestational age13, 16
.
Cell Populations in Amniotic Fluid
Amniotic fluid specimens have been long used as a source of human cells in prenatal sex
determination and diagnosis of genetic disorders. These cells also constituted early on a precious

92
source of human cells for the purpose of biological investigation17-19
. Several morphologic cell
types can be found in in vitro cultures of amniotic fluid. These cells have been classified into
three generic different classes, according to their clonal growth and morphology. The most
frequent are the AF-Type of colonies which are constituted by small cells with densely staining
nuclei and only subtly distinct from “classical” fibroblasts. With the greatest in vitro growth
potential of all colony-forming cells are the F-Type colony cells that consist in fibroblast-like
cells. The other clonal cell population usually identified is the E-Type colony cells that have
large polygonal shape with smooth margins and grow in intimate contact with each other. The
morphologies found on some of these cultured cells (e.g. epithelioid, fibroblastoid, etc) also
confirms the putative origin attributed to cells in amniotic fluid as cells with origin in the fetal
amnion, skin, urinary, respiratory, and gastrointestinal tracts that can be shed into the amniotic
cavity and fluid1-3
.
Despite the fact that cell clonal populations were identified in amniotic fluid culture quite early,
it was only in 1993 that small, nucleated, round cells identified as hematopoietic progenitor cells
were found as the first reported progenitor population present in amniotic fluid20
. A study in
1996 was the first to suggest the possibility of multi-lineage potential of non-hematopoietic cells
present in the amniotic fluid, by showing myogenic differentiation of amniocytes21
.
The presence of mesenchymal cells in the amniotic fluid has also been proposed for decades22, 23
.
Nonetheless, the differentiation potential of mesenchymal amniocytes started to be unveiled only
very recently24-27
. In 2001, Kaviani et al reported that only 2 milliliters of amniotic fluid can give
about 20,000 cells, 80 % of which are viable28, 29
. Further, Tsai et al reported that amniotic fluid
cells takes 20 to 24 hours to double the number of cells collected, which is faster than umbilical
cord stem cells (28 to 30 hours) and bone marrow stem cells (over 30 hours)30
. The proliferation
rate of the cells is a very important feature for using the cells for urgent medical conditions. The
isolation success rate for amniotic fluid cells is close to 100 percent, whereas, scientists have
only been able to isolate and differentiate on average just 30 percent of mesenchymal stem cells
(MSC) extracted from a child’s umbilical cord shortly after birth30, 31
. Furthermore, extracting the
cells from one’s own amniotic fluid bypasses the problems associated with a technique called
donor-recipient HLA matching, which involves transplanting cells32
.

93
Similarly, the presence of embryonic stem cells in the amniotic fluid was suggested only in the
last couple of years32-34
. Prusa et al. demonstrated Oct-4 expressing cells and neurogenic cells in
human amniotic fluid35-37
. In 2001, our lab initially reported that we could isolate a
subpopulation in human amniotic fluid that could differentiate into multiple lineages38
. In
January 2007, we reported the isolation of clonal lines of amniotic fluid stem (hAFS) cells that
express embryonic and adult stem cell surface markers39
.
Human amniotic epithelial cells (i.e. cells isolated from the Amnion derived from term placentas,
not from the amniotic fluid) have also shown multipotent potential to differentiate to all three
germ layers - endoderm (liver, pancreas), mesoderm (cardiomyocyte), and ectoderm (neural and
glial cells) in vitro40-43
.
The identification of several stem/progenitor cell populations in amniotic fluid, suggested that
assessment of expression of specific markers is vital to fully elucidate the presence of certain
stem/progenitor cell populations and number of these cells in amniotic fluid. Flow cytometric
analysis of cells obtained from primary cultured cells without expansion of amniocentesis
specimens, revealed low cell numbers for most of the stem/progenitor cell markers analyzed45
(Table 5.1).
Table 5.1. Flow cytometric analysis of cells obtained from primary
cultured cells without expansion of amniocentesis specimens.
Values are mean ± s.e.m. for five independent amniotic fluid
specimens.
Marker Percentage Positive
CD117(c-Kit) 0.9±0.2
CD34 2.8±0.3
CD105 19.5±1.1
CD133 0.9±0.2

94
This result suggested that sorting of specific stem/progenitor cell populations from amniotic fluid
could be made possible using an appropriate method and/or technology. The use of anti c-kit
isolation of AFS cells exposed a new population of clonal stem cells for use in human tissue
engineering and regenerative medicine.
5.1.2 AFS ISOLATION, CHARACTERIZATION AND DIFFERENTIATION
Isolation of AFS cells
AFS cells are separated from the amniotic liquid by centrifugation. AFS cells are allowed to
proliferate in vitro in culture medium (consisting of modified α-Modified Earls Medium, 18 %
Chang Medium B, 2 % Chang C with 15 % embryonic stem cell certified fetal bovine serum,
antibiotics and L-glutamine). In culture, the progenitor cells maintain a round shape for one
week post separation when cultured in non-treated culture dishes. In this state, they demonstrate
a very low proliferative capability. After the first week the cells begin to adhere to the plate and
change their morphology, becoming more elongated and proliferating more rapidly, with a need
for passage every 48-72 hours. No feeder layers are required either for maintenance or
expansion. Once the initial cells are established in culture, a pluripotent subpopulation of
progenitor cells can be isolated through positive selection for cells expressing the membrane
receptor c-kit. We employed immunoselection with magnetic microspheres to isolate the c-Kit –
positive population from many amniocentesis specimens.
C-kit receptor
C-kit is a protoncogene that encodes a 14.5-kD transmembrane receptor, p145 (CD117 or KIT,
or stem cell factor receptor). This receptor belongs to the class III of receptor tyrosine kinase
family45
. It was first identified as a viral transforming gene (v-kit), an oncogene derived from
the feline retrovirus HZ4-FeSV, being c-kit its normal cellular homologue. Due to its feline
origin, that this protoncogene was named as c-kit, for kitten46
. The ligand for this receptor is the
Stem Cell Factor protein, also known as Mast Cell Growth Factor or Steel Factor47
.
KIT plays a key role during fetal development, and its expression is constitutively maintained in
hematopoietic stem cells, mast cells, intraepithelial lymphocytes, germ cells, melanocytes, and
interstitial cells of Cajal. It is associated with immature stages of hematopoiesis, melanogenesis,

95
osteoclast and Langerhans cells differentiation, which lose the antigen during differentiation into
more mature cells48-51
.
Several mutations were identified in W mutant mice resulting in anemia, lack of mast cells,
pigmentation defects and infertility, what emphasizes its key role in fetal development and as a
growth factor receptor52, 53
. Altered c-kit levels also occur in a variety of malignancies and
cancers, like leukemias, lymphomas, small cell lung cancer and melanoma, just to mention a
few46, 53
.
Due to the critical role of c-kit in proliferation and survival of several stem/progenitor cell
populations, it was sought as a good strategy to isolate stem/progenitor cells from amniotic fluid
cultured cells.
AFS Cell Culture and Characterization
About 0.8 % to 1.4 % of cells present in amniotic fluid and placenta have been shown to be c-kit
positive. Of these, over 90 % expressed the transcription factor Oct4 which has been associated
with maintenance of the undifferentiated state and the pluripotency of embryonic stem cells54
.
The progenitor cells derived show a high self-renewal capacity with >300 population doublings,
far exceeding Hayflick’s limit. The doubling time of the undifferentiated cells is noted to be 36
hours with little variation with passages.
These cells have been shown to maintain a normal karyotype at late passages and have normal
G1 and G2 cell cycle checkpoints. They demonstrate telomere length conservation while in the
undifferentiated state as well as telomerase activity even in late passages55
. Analysis of surface
markers shows that progenitor cells from amniotic fluid express human embryonic stage specific
marker SSEA4, and the stem cell marker OCT4, and did not express SSEA1, SSEA3, CD4,
CD8, CD34, CD133, C-MET, ABCG2, NCAM, BMP4, TRA1-60, and TRA1-81. These
characteristics demonstrate that AFS cells express some of some key markers of embryonic stem
cell phenotype, but not the full complement of markers expressed by embryonic stem cells. This
may indicate that the amniotic cells are not quite as primitive as embryonic cells, yet are more
primitive than most adult stem cells. Importantly, AFS cells do not form teratomas in vivo when

96
implanted in immunodeficient mice. Lastly, cells, when expanded from a single cell, maintained
similar properties in growth and potential as the original mixed population of the progenitor
cells.
AFS Cell Differentiation
AFS cells have been shown to be pluripotent and differentiate into osteogenic, adipogenic,
myogenic, neurogenic, endothelial, and hepatic phenotypes in vitro. Each differentiation has
been performed through proof of phenotypic and biochemical changes consistent with the
differentiated tissue type of interest.
Adipocytes
To promote adipogenic differentiation, AFS cells can be induced in dexamethasone, 3-isobutyl-
1-methylxanthine, insulin, and indomethacin. The cells cultured with adipogenic supplements
change their morphology from elongated to round within 8 days. This coincides with the
accumulation of intracellular droplets. After 16 days in culture, more than 95 % of the cells have
their cytoplasm filled with lipid-rich vacuoles. Adipogenic differentiation also demonstrates the
expression of peroxisome proliferation-activated receptor γ2 (pparγ2), a transcription factor that
regulates adipogenesis, and of lipoprotein lipase through RT-PCR analysis56, 57
. Expression of
these genes is noted in the cells under adipogenic conditions but not in undifferentiated cells.
Osteocytes
Osteogenic differentiation was induced in the AFS cells with use of dexamethasone, beta-
glycerophosphate, and ascorbic acid-2-phosphate58
. The cells maintained in this medium
demonstrated phenotypic changes within 4 days with a loss of spindle shape phenotype and
development of an osteoblast-like appearance with fingerlike excavations into the cytoplasm. At
16 days, the cells aggregated, showing typical lamellar bone-like structures. In terms of
functionality, these differentiated cells demonstrate a major feature of osteoblasts which is to
precipitate calcium. Differentiated osteoblasts from the AFS cells are able to produce alkaline
phosphatase (AP) and to deposit calcium, consistent with bone differentiation. The
undifferentiated cells lacked this ability. The AFS cells in osteogenic medium express specific

97
genes implicated in mammalian bone development which are AP, core binding factor A1
(cbfa1), and osteocalcin in a pattern consistent with the physiological analog.
Endothelial cells
The AFS cells can be induced to form endothelial cells (EC) by culture in endothelial basal
medium on gelatin coated dishes. Full differentiation is achieved after one month in culture;
however, phenotypic changes are noticed within one week of initiation of the protocol. EC
differentiated AFS cells show expression of human-specific endothelial cell surface marker
(P1H12), factor VIII (FVIII), VEGF Receptor-2 (KDR) and CD31 that are specific for
differentiated endothelial cells. The AFS cells do not stain for endothelial specific markers. The
AFS derived endothelial cells, once differentiated, are able to grow in culture and form capillary-
like structures in vitro.
Hepatocytes
For hepatic differentiation, the AFS cells are seeded on matrigel or collagen coated dishes at
different stages and cultured in the presence of hepatocyte growth factor, insulin, oncostatin M,
dexamethasone, fibroblast growth factor 4, and monothioglycerol for 45 days59, 601
. After 7 days
of the differentiation process, cells exhibit morphological changes from an elongated to a
cobblestone appearance. The cells show positive staining for albumin at day 45 post
differentiation and also express the transcription factor HNF4α, the c-met receptor, the MDR
membrane transporter, albumin, and α-fetoprotein. Hepatic differentiation induced AFS cells
produced urea at a level of 1.21 x 103
ng urea/hour/cell compared with 5.0 x 101 ng
urea/hour/cell for the control cell populations61
.
Myocytes
Myogenic differentiation is induced in the AFS cells by culture in media containing horse serum
and chick embryo extract on a thin gel coat of matrigel62
. In order to initiate differentiation, the
cells are incubated in the presence of 5-azacytidine for 24 hours. Phenotypically, the cells are
organized in bundles which fuse to form multinucleated cells. These cells express sarcomeric
tropomyosin and desmin, both of which are not expressed in the undifferentiated cells.

98
The development profile of AFS cells differentiating into myogenic lineages mirrors a
characteristic pattern of gene expression reflecting that is seen with embryonic muscle
development63, 64
. With this protocol, Myf6 is expressed at day 8 and suppressed at day 16. MyoD
expression is detectable at 8 days and suppressed at 16 days. Desmin expression is induced at 8
days and increases by 16 days65, 66
.
Neuronal
The AFS cells were induced in DMSO, butylated hydroxyanisole (BHA), and neuronal growth
factor67, 68
After 2 days the cells were returned to AFS growth medium lacking DMSO and BHA
but still containing NGF. The cells cultured in neurogenic conditions change their morphology
within the first 24 hours. Two different cell populations are apparent: morphologically large flat
cells and small bipolar cells. The bipolar cell cytoplasm retracts towards the nucleus, forming
contracted multipolar structures. Over the subsequent hours, the cells display primary and
secondary branches and cone-like terminal expansions. The induced cells show a characteristic
sequence of expression of neural-specific proteins. At an early stage the intermediate filament
protein, nestin, which is specifically expressed in neuroepithelial stem cells, is highly expressed.
The expressions of βIII-tubulin and glial fibrillary acidic protein (GFAP), markers of neuron and
glial differentiation, respectively, increases over time and seems to reach a plateau at about 6
days69
. The cells cultured under neurogenic conditions show the presence of the neurotransmitter
glutamic acid in the collected medium. Glutamic acid is usually secreted in culture by fully
differentiated neurons70
.
To induce the differentiation of AFS cells into dopaminergic cells a two-stage induction
procedure has been designed. AFS cells are first seeded on fibronectin coated plates and
incubated in DMEM/F12 medium supplemented with N2 and bFGF for 8 days. Under these
conditions over 80% of cells showed expression of nestin, a marker of neural stem cells. The
cells then were transferred to conditions biasing to production of dopaminergic neurons 71
. Under
these conditions, a fraction of these cells assumed a characteristic pyramidal morphology. Gene
expression analysis determined the expression of GIRK2 gene, a marker of dopaminergic
neurons. After induction with another neurogenic differentiation protocol using NGF, AFS cells
acquired the ability to secrete the excitatory neurotransmitter L-glutamate in response to
stimulation by potassium ions.

99
5.1.3 IMPLANTATION AFS CELLS IN VIVO AND IN ANIMAL MODELS OF REGENERATION
To investigate if differentiated hAFS cells can truly contribute to the regenerative process of a
damaged organ or tissue or to new tissue growth, several animal models were used.
Although before any in vivo experiment or therapeutical application, non-tumorigenic behavior
in vivo needs to be confirmed. To investigate this, immunodeficient mice were used and cells
implanted in an immunoprivileged site, the testis, in order to avoid any residual immune
surveillance that these mice could have.
Certain cells differentiated in culture from human AFS cells are capable of integrating into
normal appearing tissue structures when transplanted in vivo, under conditions promoting
regeneration or new growth. Endothelial differentiated hAFS cells were challenged to participate
in the neovascularization of a tumour72
. Myoblast differentiated hAFS were investigated in a
skeletal muscle injury model induced by cardiotoxin I73
. Similarly, hepatocyte differentiated
hAFS were challenged to contribute to liver regeneration after partial hepatectomy74
.

100
5.2 MATERIALS AND METHODS
5.2.1 Teratoma analysis
Cells of one cloned human AFS cell line (hAFS-A1 at passage 14) labelled with MMP-nlacZ,
pseudotyped with VSV-G viral vector (Harvard Gene Therapy Initiative, Harvard Medical
School, Boston, MA) and mouse embryonic stem cells (mES 129 at passage 25) were injected
into the scrotal pouch of 8-week-old male rag2-/-
C57BL/6 mice (Taconic Inc., Hudson, NY,
USA). All animal care and experimental procedures were done in full compliance with the Wake
Forest University Institutional Animal Care and Use Committee, as well as all State and Federal
Guidelines Each mouse was injected with 5x106 cells of each cell line using 0.9% sodium
chloride solution (Abbott Laboratories, North Chicago, IL, USA) as vehicle. One to three months
after injection, dependent on tumour appearance, the mice were sacrificed and the scrotum
removed. Testis and tumours (where present) were fixed for 2h at 4ºC with 2 %
paraformaldehyde (Polysciences Inc., Warrington, PA, USA), 0,2 % glutaraldehyde, 0,01 %
sodium deoxycholate and 0,02 % NP-40 (Sigma-Aldrich, St. Louis, MO, USA) in PBS 1x
(Gibco-Invitrogen, Carlsbad, CA, USA). After fixation, tissues were developed for 24 h at 30 ºC
in solution with 0,1 % X-Gal (Roche Applied Sciences, Indianapolis, IN, USA) 2mM
magnesium chloride, 0,02 % NP-40, 0,01% sodium deoxycholate, 5 mM potassium ferricyanide
and 5 mM potassium ferrocyanide (Sigma-Aldrich, St. Louis, MO, USA).
After X-Gal solution development, tissue was processed in a Shandon Citadel 1000 Tissue
Processor (Thermo Electron Corporation, Waltham, MA, USA) and paraffin embedded for
sectioning. Histological examination was done using hematoxylin and eosin statining.
5.2.2 Myoblast Implantation
Cloned hAFS-A1 cells were cultured in induction medium promoting myogenic differentiation
(as described previously) and 5x105 cells previously labeled with Ad6-nlacZ vector, encoding β-
galactosidase (β-Gal) modified with a nuclear localization signal (Harvard Human Gene Therapy
Initiative, Harvard Medical School, Boston, MA) were injected using Matrigel HC (BD
Biosciences, San Jose, CA, USA) as a vehicle in male nu/nu (nude) mice (Charles River
Laboratories Inc, Wilmington, MA) 8 weeks old and 24h after injection with 30 µl of
Cardiotoxin I solution (Sigma-Aldrich, St. Louis, MO, USA) at 10-5
M in deionized water on
both hindlimbs posterior tibialis skeletal muscle. Right hindlimb muscle was used as control.

101
Before cell injection, both hindlimbs were γ-irradiated with 25 gray by a JL Shepherd Mark II
Cesium-137 Irradiator. 100 µl of 0,9 % sodium chloride solution (Abbott Laboratories, North
Chicago, IL, USA) were injected in the right hindlimb muscle as control.
Samples were collected for histological analysis after 1 (n=3) and 3 weeks (n=3).
The dissected posterior tibialis skeletal muscles (hAFS injected and control) were developed in
X-Gal solution (as described above). After development, tissue was processed and sectioned.
Sections were stained with hematoxylin and eosin and immunostained with mouse anti-
sarcomeric tropomyosin (Sigma-Aldrich, St. Louis, MO, USA).
5.2.3 Endothelial Cell Implantation
Cloned hAFS-A1 cells were cultured in induction medium promoting endothelial differentiation,
and then 5x105 cells were injected together with 2x10
6 cells of human pancreatic carcinoma cells
Hs-776T (HS-VF), engineered to over-express VEGF, into immune deficient nu/nu (nude) mice
(Charles River Laboratories Inc, Wilmington, MA). The human cells were marked with the
nlacZ gene with a nuclear localization β-galactosidase, introduced via a defective adenoviral
vector (Harvard Human Gene Therapy Initiative, Harvard Medical School, Boston, MA), to
allow their detection using a chromogenic β-galactosidase (β-Gal) substrate (X-gal).
After 1 and 4 weeks tumours were collected for histological analysis. All samples were
developed with X-Gal solution, before tissue processing and sectioning as described above.
Sections were stained with hematoxylin and eosin. To detect mouse blood vessels, sections were
immunostained with rat monoclonal anti-mouse CD31 antibody (BD Pharmingen, Franklin
Lakes, NJ, USA).
5.2.4 Hepatocyte Implantation
Cloned hAFS-A1 cells were cultured in induction medium promoting hepatic differentiation (as
desbribed previously). Immune deficient nu/nu (nude) mice (Charles River Laboratories Inc,
Wilmington, MA) were submitted to a partial hepatectomy (70 %) before injection of 5x105 cells
previously marked with the nlacZ gene with a nuclear localization β-galactosidase, introduced
via a defective adenoviral vector (Harvard Human Gene Therapy Initiative, Harvard Medical
School, Boston, MA). These cells were injected in the parenchyma of the remaining liver lobe

102
(right lobe) using 0.9 % sodium chloride solution (Abbott Laboratories, North Chicago, IL,
USA) as vehicle.
Samples were collected for histological analysis after 1 and 3 weeks. All samples were
developed with X-Gal solution, before tissue processing and sectioning as described above.
Sections were stained with hematoxylin and eosin and immunostained with mouse monoclonal
anti-albumin antibody (Sigma-Aldrich, St. Louis, MO, USA).

103
5.3 RESULTS
Tumor formation was observed in the scrotal pouch of mice implanted with mES 129 cells as
early as 4 weeks. As expected, all mice injected with mES 129 generated massive tumors. After
histological examination, several differentiated structures/tissues were observed within the
tumors (Figure 5.1A and 5.1B), undeniably confirming their embryonic origin and their
classification as teratomas. The mice injected with hAFS, didn’t develop any tumors within the 3
month range of the study. No β-galactosidase positive cells could be observed in the histological
sections of the testis and surrounding tissues (Figure 5.1C). Nonetheless, some scattered small
vesicular X-Gal (blue) staining could be observed in some areas of the epididymis (Figure 5.1D).
This blue staining was not nuclear localized, what indicates some sort of staining artifact. This
experiment confirms the non-tumorigenic nature of hAFS cells that even in immunodeficient
mice in immunoprivileged sites don’t generate tumors.
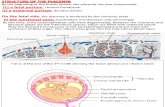
Figure 5.1 | Histological analysis of teratomas and testis of mice injected with mES and hAFS.
Teratomas were found in mice injected with mES cells and were analyzed by histological
examination and stained with hematoxylin and eosin, showing a stratified epithelium tubular

104
structure (A) and a cell mass of chondroid tissue (B). Mice injected with hAFS didn’t form any
tumors and histological analysis of testis, revealed normal tissue architecture without stem cell
incorporation (C). In some areas of the epididymis, non specific scattered X-Gal staining could
be observed, probably due to a staining artifact.
When hAFS are differentiated into myoblasts, β-Gal positive myofibers could be clearly
observed in histological sections of the posterior tibialis skeletal muscle damaged with
cardiotoxin I (Figure 5.2A) one week after cell implantation. Immunohistochemical staining with
antibody anti-sarcomeric tropomyosin confirmed the skeletal muscle identity of these labeled
myofibers (Figure 5.2B). The integration of hAFS in the damaged skeletal muscle of this animal
model shows that differentiated hAFS can identify the microenvironmental cues produced in
regenerating muscle and contribute to the repair of the injured tissue. The exact
mechanism/biology used by these cells in this regenerative process remains to be elucidated.
Figure 5.2 | Histological analysis of injured skeletal muscle and VEGF secreting tumor cells
injected with hAFS cells differentiated into myoblasts and endothelial cells, respectively. After
cardiotoxin I injection, the hAFS cells injected into mice skeletal muscle incorporated in the

105
damaged myofibers area. β-gal positive myocytes can be clearly observed in a hematoxylin and
eosin staining (A). These blue myofibers express also sarcomeric tropomyosin, confirming their
skeletal muscle identity (B). In the retrieved tumors, it could be observed some β-gal positive
cells (C). After CD31 staining to identify vascular structures, it could be observed blue staining
cells aligned with the CD31 expressing cells, indication of possible contribution of hAFS cells
into these newly formed vascular structures (D).
The implanted pancreatic cancer cells developed a detectable tumour as early as 2 weeks after
injection and up to 4 weeks. Blood vessels growing in the tumour were visualized by
immunohistochemical staining with an antibody to mouse CD31. Staining for β-Gal (blue)
shows that human endothelial cells derived from hAFS cells, co-localize with these vascular
structures. This provides some evidence that hAFS cells have the potential to integrate into these
new blood vessels (Figure 5.2D) and contribute to tumour angiogenesis.
One week after hAFS cell injection into a regenerating liver, several extensive clusters of β-Gal
positive cells could be observed throughout the whole liver (Figure 5.3A and 5.3B).
Immunohistochemical staining for albumin showed intense expression within one of the clusters
of β-Gal positive cells (data not shown). Although cell nuclei imaging with PI was quite difficult
in these cell clusters, due to the intensity and darkness of the blue staining. Nevertheless, this
experiment showed that hAFS differentiated into hepatic lineage engrafted in regenerating
hepatic tissue after partial hepatectomy. However, due to the substantially different mechanisms
of hepatic tissue repair, other injury models should be used to clarify and expand the potential of
hAFS in liver disease/regeneration75
.

106
Figure 5.3 | Histological analysis of regenerating mouse liver after hAFS cell injection. One
week after partial hepatectomy and hAFS cell injection, extensive clusters of β-Gal positive cells
could be observed in eosin (A) and hematoxylin and eosin stainings (B).

107
5.4 DISCUSSION
The use of embryonic stem cells has been an intense debate with ethical controversies. Stem
cells in amniotic fluid may represent an attractive alternative to embryonic and adult stem cells
because of their apparent advantages of accessibility and multipotentiality over embryonic and
adult stem cells, respectively.
There are many clinical situations that one could predict amniotic fluid stem cells be used for.
For example, that one could foresee amniotic fluid stem cells being used to repair congenital
anomalies. The cells could be used for surgical reconstruction of birth defects in the neonatal
period76
. A small amount of amniotic fluid may be sufficient to yield enough cells to prepare and
engineered tissue construct for implantation ready upon birth77, 78
. An example is a recent study
showing experimental diaphragmatic hernia repair with autologous tendon engineered from
mesenchymal amniocytes79
.
The in vitro and in vivo experiments completed so far provide solid evidence of the safety and
regenerative capability of these stem cells80
, although further in vivo studies need to be
performed to expand the potential of hAFS cells in human medicine. In the particular case of the
liver, combination of hAFS derived endothelial and hepatic cells constitute a new alternative
source for liver cell therapies and liver tissue engineering. This is further emphasized by the fact
that the most important factor affecting clinical hepatocyte transplantation is a lack of donor
availability81
. Also, clinical evidence shows that adult hepatocyte transplantation benefit is
transient in its therapeutic effects, due to the limited capacity of hepatocyte proliferation, reduced
cell engraftment after transplantation and immune surveillance82
. Hepatocyte progenitor cells
constitute potentially a better approach for transplantation. Considering the properties of hAFS,
differentiation of these cells in hepatic progenitor cells is of capital importance, potentially
increasing cell availability and immune compatibility. We expect that in the near future, amniotic
fluid stem cells will become a relevant, vital tool in stem cell research, tissue engineering, and
regenerative medicine.

108
5.5 CONCLUSION
AFS cells isolated from amniotic fluid are an exciting contribution to the field of stem cell
biology and regenerative medicine. The discovery of these cells has been recent, and a
considerable amount of work remains to be done on the characterization and use of these cells in
vivo. In the future, banking of these stem cells could provide a convenient source for autologous
therapy by matching of histocompatible donor cells with recipients. AFS cells could represent an
attractive and abundant, noncontroversial source of stem cells for regenerative medicine.

109
5.6 BIBLIOGRAPHY
1. Hoehn,H., Bryant,E.M., Fantel,A.G., & Martin,G.M. Cultivated cells from
diagnostic amniocentesis in second trimester pregnancies. III. The fetal urine as a
potential source of clonable cells. Humangenetik. 29, 285-290 (1975).
2. Hoehn,H., Bryant,E.M., Karp,L.E., & Martin,G.M. Cultivated cells from
diagnostic amniocentesis in second trimester pregnancies. I. Clonal morphology and
growth potential. Pediatr. Res. 8, 746-754 (1974).
3. Hoehn,H. & Salk,D. Morphological and biochemical heterogeneity of amniotic
fluid cells in culture. Methods Cell Biol. 26, 11-34 (1982).
4. Torricelli,F. et al. Identification of hematopoietic progenitor cells in human
amniotic fluid before the 12th week of gestation. Ital. J. Anat. Embryol. 98, 119-126
(1993).
5. Bianchi,D.W., Wilkins-Haug,L.E., Enders,A.C., & Hay,E.D. Origin of
extraembryonic mesoderm in experimental animals: relevance to chorionic mosaicism
in humans. Am. J. Med. Genet. 46, 542-550 (1993).
6. Developmental Biology, Gilbert SF (Ed.) Sinauer Associates Inc., Sunderland,
(2003).
7. Human Embryology, Larsen,W.(Ed.) Churchil Livingstone, New York, (1993).
8. In 't Anker,P.S. et al. Amniotic fluid as a novel source of mesenchymal stem cells
for therapeutic transplantation. Blood. 102, 1548-1549 (2003).
9. Kaviani,A. et al. The amniotic fluid as a source of cells for fetal tissue
engineering. J. Pediatr. Surg. 36, 1662-1665 (2001).
10. Prusa,A.R., Marton,E., Rosner,M., Bernaschek,G., & Hengstschlager,M. Oct-4-
expressing cells in human amniotic fluid: a new source for stem cell research? Hum.
Reprod. 18, 1489-1493 (2003).
11. Torricelli,F. et al. Identification of hematopoietic progenitor cells in human
amniotic fluid before the 12th week of gestation. Ital. J. Anat. Embryol. 98, 119-126
(1993).
12. Baschat,A.A. & Hecher,K. Fetal growth restriction due to placental disease.
Semin. Perinatol. 28, 67-80 (2004).
13. Beall,M.H., van den Wijngaard,J.P., van Gemert,M.J., & Ross,M.G. Amniotic
Fluid Water Dynamics. Placenta. 28, 824-32 (2007).
14. Mescher,E.J. et al. Ontogeny of tracheal fluid, pulmonary surfactant, and plasma
corticoids in the fetal lamb. J. Appl. Physiol. 39, 1017-1021 (1975).

110
15. Olver,R.E. & Strang,L.B. Ion fluxes across the pulmonary epithelium and the
secretion of lung liquid in the foetal lamb. J. Physiol. 241, 327-357 (1974).
16. Muller,F. et al. Amniotic fluid digestive enzymes: diagnostic value in fetal
gastrointestinal obstructions. Prenat. Diagn. 14, 973-979 (1994).
17. Jacobson,C.B. & Barter,R.H. Intrauterine diagnosis and management of genetic
defects. Am. J. Obstet. Gynecol. 99, 796-807 (1967).
18. Sachs,L., Serr,D.M., & Danon,M. Prenatal diagnosis of sex using cells from the
amniotic fluid. Science. 123, 548 (1956).
19. Serr,D.M., Sachs,L., & Danon,M. The diagnosis of sex before birth using cells
from the amniotic fluid (a preliminary report). Bull. Res. Counc. Isr. 5B, 137-138
(1955).
20. Torricelli,F. et al. Identification of hematopoietic progenitor cells in human
amniotic fluid before the 12th week of gestation. Ital. J. Anat. Embryol. 98, 119-126
(1993).
21. Streubel,B., Martucci-Ivessa,G., Fleck,T., & Bittner,R.E. [In vitro transformation
of amniotic cells to muscle cells-background and outlook]. Wien. Med. Wochenschr.
146, 216-217 (1996).
22. Hurych,J., Macek,M., Beniac,F., & Rezacova,D. Biochemical characteristics of
collagen produced by long term cultivated amniotic fluid cells. Hum. Genet. 31, 335-
340 (1976).
23. Macek,M., Hurych,J., & Rezacova,D. Letter: Collagen synthesis in long-term
amniotic fluid cell cultures. Nature. 243, 289-290 (1973).
24. In 't Anker,P.S. et al. Amniotic fluid as a novel source of mesenchymal stem cells
for therapeutic transplantation. Blood. 102, 1548-1549 (2003).
25. Kaviani,A. et al. The amniotic fluid as a source of cells for fetal tissue
engineering. J. Pediatr. Surg. 36, 1662-1665 (2001).
26. Kaviani,A. et al. The placenta as a cell source in fetal tissue engineering. J.
Pediatr. Surg. 37, 995-999 (2002).
27. Kaviani,A. et al. Fetal tissue engineering from amniotic fluid. J. Am. Coll. Surg.
196, 592-597 (2003).
28. Kaviani,A. et al. Fetal tissue engineering from amniotic fluid. J. Am. Coll. Surg.
196, 592-597 (2003).
29. Kaviani,A. et al. The amniotic fluid as a source of cells for fetal tissue
engineering. J. Pediatr. Surg. 36, 1662-1665 (2001).

111
30. Tsai,M.S., Lee,J.L., Chang,Y.J., & Hwang,S.M. Isolation of human multipotent
mesenchymal stem cells from second-trimester amniotic fluid using a novel two-stage
culture protocol. Hum. Reprod. 19, 1450-1456 (2004).
31. In 't Anker,P.S. et al. Amniotic fluid as a novel source of mesenchymal stem cells
for therapeutic transplantation. Blood 102, 1548-1549 (2003).
32. Tsai,M.S., Lee,J.L., Chang,Y.J., & Hwang,S.M. Isolation of human multipotent
mesenchymal stem cells from second-trimester amniotic fluid using a novel two-stage
culture protocol. Hum. Reprod. 19, 1450-1456 (2004).
33. De Coppi P,F.R.S.S.Y.J.A.A. Human embryonic and fetal stem-cell isolation
from amniotic fluid and placenta for tissue reconstruction. J.Am.Coll.Surg. 195, S93.
2002.
Ref Type: Abstract
34. Prusa,A.R., Marton,E., Rosner,M., Bernaschek,G., & Hengstschlager,M. Oct-4-
expressing cells in human amniotic fluid: a new source for stem cell research? Hum.
Reprod. 18, 1489-1493 (2003).
35. Prusa,A.R. & Hengstschlager,M. Amniotic fluid cells and human stem cell
research: a new connection. Med. Sci. Monit. 8, RA253-RA257 (2002).
36. Prusa,A.R. et al. Neurogenic cells in human amniotic fluid. Am. J Obstet.
Gynecol. 191, 309-314 (2004).
37. Prusa,A.R. & Hengstschlager,M. Amniotic fluid cells and human stem cell
research: a new connection. Med Sci Monit. 8, RA253-RA257 (2002).
38. Prusa,A.R., Marton,E., Rosner,M., Bernaschek,G., & Hengstschlager,M. Oct-4-
expressing cells in human amniotic fluid: a new source for stem cell research? Hum.
Reprod. 18, 1489-1493 (2003).
39. DeCoppi,P. Human Fetal Stem Cell Isolation from Amniotic Fluid. Proceedings
of the American Acadamy of Pediatrics National Conference, 210-211. 2001. San
Francisco, CA.
Ref Type: Abstract
40. De Coppi,P. et al. Isolation of amniotic stem cell lines with potential for therapy.
Nat Biotech 25, 100-106 (2007).
41. Miki,T., Lehmann,T., Cai,H., Stolz,D.B., & Strom,S.C. Stem cell characteristics
of amniotic epithelial cells. Stem Cells. 23, 1549-1559 (2005).
42. Sakuragawa,N., Thangavel,R., Mizuguchi,M., Hirasawa,M., & Kamo,I.
Expression of markers for both neuronal and glial cells in human amniotic epithelial
cells. Neurosci. Lett. 209, 9-12 (1996).

112
43. Sakuragawa,N. et al. Human amniotic epithelial cells are promising transgene
carriers for allogeneic cell transplantation into liver. J. Hum. Genet. 45, 171-176
(2000).
44. Takahashi,N. et al. Transplantation of amniotic epithelial cells into fetal rat liver
by in utero manipulation. Cell Transplant. 11, 443-449 (2002).
45. De,C.P. et al. Isolation of amniotic stem cell lines with potential for therapy. Nat.
Biotechnol. 25, 100-106 (2007).
46. Canonico,B., Felici,C., & Papa,S. CD117. J. Biol. Regul. Homeost. Agents. 15,
90-94 (2001).
47. Besmer,P. et al. A new acute transforming feline retrovirus and relationship of its
oncogene v-kit with the protein kinase gene family. Nature. 320, 415-421 (1986).
48. Williams,D.E. et al. Identification of a ligand for the c-kit proto-oncogene. Cell.
63, 167-174 (1990).
49. Rappold,I. et al. Functional and phenotypic characterization of cord blood and
bone marrow subsets expressing FLT3 (CD135) receptor tyrosine kinase. Blood. 90,
111-125 (1997).
50. Matsui,Y., Zsebo,K.M., & Hogan,B.L. Embryonic expression of a haematopoietic
growth factor encoded by the Sl locus and the ligand for c-kit. Nature. 347, 667-669
(1990).
51. Matsui,Y. et al. Effect of Steel factor and leukaemia inhibitory factor on murine
primordial germ cells in culture. Nature. 353, 750-752 (1991).
52. Nocka,K. et al. Molecular bases of dominant negative and loss of function
mutations at the murine c-kit/white spotting locus: W37, Wv, W41 and W. EMBO J. 9,
1805-1813 (1990).
53. Ashman,L.K. The biology of stem cell factor and its receptor C-kit. Int. J.
Biochem. Cell Biol. 31, 1037-1051 (1999).
54. Shamblott,M.J. et al. Derivation of pluripotent stem cells from cultured human
primordial germ cells. Proc. Natl. Acad. Sci. U. S. A 95, 13726-13731 (1998).
55. Bryan,T.M., Englezou,A., Dunham,M.A., & Reddel,R.R. Telomere length
dynamics in telomerase-positive immortal human cell populations. Exp. Cell Res. 239,
370-378 (1998).
56. Medina-Gomez,P. & del Valle,M. [The culture of amniotic fluid cells. An
analysis of the colonies, metaphase and mitotic index for the purpose of ruling out
maternal cell contamination]. Ginecol. Obstet. Mex. 56, 122-126 (1988).

113
57. Cremer,M., Schachner,M., Cremer,T., Schmidt,W., & Voigtlander,T.
Demonstration of astrocytes in cultured amniotic fluid cells of three cases with neural-
tube defect. Hum. Genet. 56, 365-370 (1981).
58. Jaiswal,N., Haynesworth,S.E., Caplan,A.I., & Bruder,S.P. Osteogenic
differentiation of purified, culture-expanded human mesenchymal stem cells in vitro. J.
Cell Biochem. 64, 295-312 (1997).
59. Schwartz,R.E. et al. Multipotent adult progenitor cells from bone marrow
differentiate into functional hepatocyte-like cells. J. Clin. Invest 109, 1291-1302
(2002).
60. Dunn,J.C., Yarmush,M.L., Koebe,H.G., & Tompkins,R.G. Hepatocyte function
and extracellular matrix geometry: long-term culture in a sandwich configuration.
FASEB J 3, 174-177 (1989).
61. Hamazaki,T. et al. Hepatic maturation in differentiating embryonic stem cells in
vitro. FEBS Lett. 497, 15-19 (2001).
62. Rosenblatt,J.D., Lunt,A.I., Parry,D.J., & Partridge,T.A. Culturing satellite cells
from living single muscle fiber explants. In Vitro Cell Dev. Biol. Anim 31, 773-779
(1995).
63. Bailey,P., Holowacz,T., & Lassar,A.B. The origin of skeletal muscle stem cells in
the embryo and the adult. Curr. Opin. Cell Biol. 13, 679-689 (2001).
64. Rohwedel,J. et al. Muscle cell differentiation of embryonic stem cells reflects
myogenesis in vivo: developmentally regulated expression of myogenic determination
genes and functional expression of ionic currents. Dev. Biol. 164, 87-101 (1994).
65. Hinterberger,T.J., Sassoon,D.A., Rhodes,S.J., & Konieczny,S.F. Expression of the
muscle regulatory factor MRF4 during somite and skeletal myofiber development. Dev.
Biol. 147, 144-156 (1991).
66. Patapoutian,A. et al. Disruption of the mouse MRF4 gene identifies multiple
waves of myogenesis in the myotome. Development 121, 3347-3358 (1995).
67. Woodbury,D., Schwarz,E.J., Prockop,D.J., & Black,I.B. Adult rat and human
bone marrow stromal cells differentiate into neurons. J. Neurosci. Res. 61, 364-370
(2000).
68. Black,I.B. & Woodbury,D. Adult rat and human bone marrow stromal stem cells
differentiate into neurons. Blood Cells Mol. Dis. 27, 632-636 (2001).
69. Guan,K., Chang,H., Rolletschek,A., & Wobus,A.M. Embryonic stem cell-derived
neurogenesis. Retinoic acid induction and lineage selection of neuronal cells. Cell
Tissue Res. 305, 171-176 (2001).

114
70. Carpenter,M.K. et al. Enrichment of neurons and neural precursors from human
embryonic stem cells. Exp. Neurol. 172, 383-397 (2001).
71. Perrier,A.L. et al. From the Cover: Derivation of midbrain dopamine neurons
from human embryonic stem cells. PNAS 101, 12543-12548 (2004).
72. Schuch,G., Kisker,O., Atala,A., & Soker,S. Pancreatic tumor growth is regulated
by the balance between positive and negative modulators of angiogenesis.
Angiogenesis. 5, 181-190 (2002).
73. Muguruma,Y. et al. In vivo and in vitro differentiation of myocytes from human
bone marrow-derived multipotent progenitor cells. Exp. Hematol. 31, 1323-1330
(2003).
74. Zhan,Y.T. et al. Differentiation of rat bone marrow stem cells in liver after partial
hepatectomy. World J. Gastroenterol. 12, 5051-5054 (2006).
75. Cantz,T., Manns,M.P., & Ott,M. Stem cells in liver regeneration and therapy. Cell
Tissue Res. 331, 271-282 (2008).
76. Fauza DO Fetal Tissue Engineering , Academic Press, San Diego, 353-368
(2000).
77. Kaviani,A. et al. The amniotic fluid as a source of cells for fetal tissue
engineering. J. Pediatr. Surg. 36, 1662-1665 (2001).
78. Kaviani,A. et al. Fetal tissue engineering from amniotic fluid. J. Am. Coll. Surg.
196, 592-597 (2003).
79. Fuchs,J.R. et al. Diaphragmatic reconstruction with autologous tendon engineered
from mesenchymal amniocytes. J Pediatr. Surg. 39, 834-838 (2004).
80. De,C.P. et al. Isolation of amniotic stem cell lines with potential for therapy. Nat.
Biotechnol. 25, 100-106 (2007).
81. Organ Procurement and Transplantation Network Database. OPTN . 2008. 2008.
Ref Type: Electronic Citation
82. Fox,I.J. & Chowdhury,J.R. Hepatocyte transplantation. Am. J. Transplant. 4
Suppl 6:7-13 (2004).