Bone Pathology
description
Transcript of Bone Pathology

The Normal Skeleton
- Essential formechanicalsupport and
mineralhomeostasis
- Houseshemopoetic
tissue
- Determinesbody size &
shape
Inorganic65%
Organic35%
206 bonesof varying
size andshape

The Normal SkeletonInorganic Matrix
• Calcium hydroxyapatite[Ca10(PO4)6(OH)2]
- Mineral homeostasis• 99% of calcium stores
• 85% of phosphate stores• 65% of sodium and magnesium stores
- Bone strength

Organic Components of Bone
• Bone Cells- Osteoprogenitor cells Osteoblasts Osteocytes
- Hematopoietic progenitor cells Osteoclasticprecursors Osteoclasts
• Bone Matrix- Type 1 Collagen (29%)
- Non-collagenous proteins (3%)- Serum

Organic MatrixOsteoblasts
• Synthesize proteins
• Initiate mineralization
• Bind hormones
• Make bone
• Regulate osteoclasts
Osteoid (matrix)
12-15 days
Bone (mineralized matrix)

Organic MatrixOsteoclasts
• Large multinucleated cellsresponsible for bone resorption.
• Derived from monocytes/macrophagehematopoietic progenitor cells thatare recruited to the bone
microenvironment where locallyproduced cytokines and growth
factors induce their differentiation intoactively resorbing osteoclasts.
• Bind to the bone surface via integrins,where they form resorption pit
(Howship’s lacunae).

Regulation of OsteoclastsIntercellular Signals - RANK and RANKL
• Receptor Activator of NF-KappaB (RANK) (member of TNF familyof receptors) expressed on the surface of osteoclast precursors.
• RANK ligand (RANKL) is expressed on the surface of osteoblastsand marrow stromal cells.
• Binding of RANK ligand to RANK receptor results in osteoclastmaturation and activation, and thus bone resorption.
• RANK ligand is upregulated by PTHRP, vitamin D3, somemalignancies.
• Osteoprotegrin (OPG), also secreted by osteoblasts/marrowstromal cells (Wnt/β-catenin signaling pathway), blocks RANK
ligand- RANK interaction, thereby inhibits osteoclast maturation andactivation.
• Osteoblasts can enhance or inhibit osteoclast development andfunction by expressing OPG and RANK ligand in various
proportions.

Organic Components of Bone
• Bone Cells• Bone Matrix
- Type 1 Collagen (29%) - 90% by weight oforganic component
- Non-collagenous proteins (3%)
- Minerals 60%
- Water 10%

Non-Collagenous Organic Matrix
• Cell adhesion proteins- Osteopontin, fibronectin,
thrombospondin
• Calcium binding proteins- Osteonectin, bone sialoprotein
• Proteins involved inmineralization
- Osteocalcin
• Enzymes- Collagenase, alkaline
phosphatase
• Growth factors- TGF- β, IGF-1, PDGF
• Cytokines- IL-1, IL-6, RANKL
• Proteins concentrated fromserum- Albumin
- β2-microglobulin

The Normal SkeletonModeling and Remodeling
• Bone is in a dynamic state of formation and resorption inresponse to constantly changing mechanical stresses and the
demands of mineral homeostasis.
• Remodeling occurs continuously throughout life and iscarried out in microanatomical units, bone multicellularunits .
• In these units, a closely coupled sequence of bone resorptionand formation takes place.
• Sequence is initiated by a phase of osteoclastic boneresorption, followed by a phase of reversal ( marked by a
deposition of cement line indicating the extent of bone resorption which hastaken place).
• This is then followed by a phase of bone formation byosteoblasts.

Regulation of Bone Modeling
• The coordinated balance of bone formation andresorption is regulated by systemic hormones, blood-
derived factors and local mediators.
• Remodeling also occurs in response to physical forces,bone being deposited in sites subjected to stress andresorbed from sites where there is little stress.
• The key cell is Osteoblast- Responds to stimulation from osteocytes
- Responds to stimulation from blood borne factors
- Stimulates development of osteoclasts
- Activates osteoclasts

Types of Bone
• Osteoid (unmineralized bone matrix)
• Woven bone (first laid down in fetalskeleton and disease states)
• Lamellar bone (slowly deposited, maturebone)
- Cortical
- Cancellous

Woven (Immature) Bone
• Characterized by the presence of bundles of randomlyarranged, short, thick collagen fibers, best appreciated
by examination under polarized light.
• Osteocytes in woven bone lie in large round lacunae andmore closely packed than in lamellar bone.
• First formed in the developing skeleton (fetal bone).
• In adults, it is seen in pathological conditions: Bonetumors, fracture callus, diseases with high bone turnover
(Paget’s, hyperparathyroidism).
• Formed quickly.
• Woven bone is less strong than lamellar bone.

Types of BoneLamellar (mature) bone
• The matrix is composed ofbundles of thin collagen fibers.
• Each bundle is laid down atapproximately right angles toadjacent bundles, creating thecharacteristic parallelarrangement of alternating lightand dark bands seen onpolarized light.
• This structural organizationprovides the highest level ofdensity of collagen per unitvolume of tissue.
• Deposited slowly.
• Resists unidirectional force.

Cortical Lamellar Bone
• Most of the lamellae areconcentrically arranged around
a central Haversian canal,which is lined by osteoblastsand contains vessels.
• These concentric lamellae areoriented in the long axis of thebone, forming long cylindricalcolumns (Haversian systemsor osteons).
• Small segments of interstitiallamellar bone fills the spacebetween osteons.

Skeletal Development andDevelopmental and Metabolic
Disease States

Skeletal Morphogenesis
• Determined by Homeobox genes encoding fortranscription factors required for the normal
development of the skeleton.
• Most of the embryonic skeleton are first formed as acartilage model or anlage (Exception: cranial vault
bones).
• Cartilage proliferation plays an important role incontinuing skeletal growth and modeling.

Fetal Bone Formation
• Femur and hip joint in a 5-weekfetus.
• Bone is modeled in cartilage andis covered by a condensation ofmesenchymal cells (futureperiosteum), (arrow mark).
• Note that cells in the diaphysis ofthe cartilage model are larger andpaler than those in the upper end.
• Before any bone formation occurswithin the embryonic cartilageskeleton, the cartilage cellstoward the middle of theindividual skeletal parts becomelarger and more separated by
interstitial matrix.

Fetal Bone Formation
• As thechondrocytes in thecenter of the shaftof a long bone
continue to enlarge,the cartilage matrixlying between thecells becomescalcified and thechondrocytes die.

Fetal Bone Formation
• Osteoblasts (perhaps derivedfrom periosteum) are seen toline up on the surface of theremaining calcified cartilagematrix and start making bonymatrix (osteoid).
• This process of cartilagecalcification, vascular invasionand then deposition of bonymatrix on the remaining
calcified cartilage matrix iscalled enchondral
ossification.• Enchondral ossification begins
about 8 th wk of gestation

Fetal Bone Formation
• First laid down bone (core of calcifiedcartilage and primitive bone on the
surface) during endochondral ossificationis called primary spongiosa.
• As the primary spongiosa is remodeledand the calcified cartilage is removed byosteoclasts, the bone trabeculae come tobe formed entirely of lamellar bone byosteoblasts (secondary spongiosa).
• Before birth, conversion of a considerableportion of the shaft of a long bone into
osseous tissue is completed and at birthonly the ends are still formed of cartilage.

Fetal Bone Formation
• During infancy andchildhood, a secondarycenter of ossification is
formed within thecartilagenous ends of thelong bones.
• As the secondary center ofossification grows, onlyremaining cartilages arethose covering the articular
surface and a thin layer(plate) of cartilage
between the secondarycenter of ossification andshaft of the bone.

Fetal Bone Formation
• In intramembranous ossification, boneis directly formed within primitive
mesenchymal tissue.
• Mesenchymal cells proliferate anddifferentiate into osteoblasts that formosteoid, which is then rapidlymineralized to form woven bone.
• This bone formation occurs at severalpoints within the membrane, each focusof ossification enlarging and then fusing
with its neighbors
• Resulting continuous mass of wovenbone is subsequently remodeled tolamellar bone.

Regulation of Bone Modeling
• The key cell is the Osteoblast- Responds to stimulation from osteocytes
- Responds to stimulation from blood bornefactors
- Stimulates development of osteoclasts
- Activates osteoclasts

Intercellular SignalsRANK and RANKL
Receptor Activator of NF-KappaB Ligand(RANKL)
- Expressed on Osteoblasts- Upregulated by PTHRP, vitamin D3, some
malignancies
- Binds to RANK (TNF family) on Osteoclasts andprecursors to activate them
- Function inhibited by Osteoprotegrin (TNFfamily): binds to RANKL acting as a decoypreventing RANK-RANKL interaction

Regulation of Osteoclast
PTH, IL-11, vitamin D3
Osteoblasts (& marrow stromal cells)
paracrine molecular mechanisms
Osteoclasts (activation, proliferation,fusion, differentiation, survival)
M-CSF OsteoclastsOPG (decoy) blocks RANKL andchecks stimulation of osteoclasts

Skeletal Development
• Any failed step in skeletal morphogenesisor modeling may cause disease
- Mesenchyme condensations
- Chondrocyte proliferation
- Defective matrix production
- Defective modeling/remodeling

Skeletal MorphogenesisDefects in Mesenchmyal Condensation
• Examples include synpolydactylyand cleidocranial dysplasia.
• Mutation in homeobox HoxD13gene results in a mesenchymalcondensation abnormality leading
to an extra digit between the thirdand fourth fingers with somedegree of fusion.
• Loss of function mutations in theRUNX2 (CBFA1) causescleidocranial dysplasia (open
fontanelles, delayed closure ofcranial sutures, primitive clavicles,delayed eruption of secondary
teeth).

Skeletal MorphogenesisAchondroplasia
• Caused by an activating mutation infibroblast growth factor receptor 3
(FGFR3) gene.
• Constitutively active FGFR3 inhibitscartilage proliferation in cartilageanlages and growth plates, thussuppress growth.
• Autosomal dominant inheritance,however, 80% of cases are due to newmutations.
• Shortened limbs and ribs, bulgingforehead.
• Normal longevity, intelligence andreproductive status.
Verne Troyer: 2’8”

Abnormalities in Structure andFunction of Bone
• Collagen synthesis- Osteogenesis imperfecta
- Marfan’s syndrome- Ehler Danlos syndrome
- Scurvy• Abnormality of Matrix Turnover
- Osteopetrosis• Metabolic disorders
- Vitamin D deficiency (Rickets, osteomalacia)- Osteoporosis
- Paget’s disease of bone- Defects in types 2, 10, 11 collagen result in fragile cartilage
• Fractures• Severe osteoarthritis
• Defective degradation of cartilage- Mucopolysaccharidoses

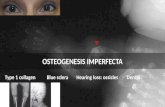
Osteogenesis Imperfecta• Group of phenotypically related disorders caused by
deficiencies in type I collagen synthesis
• Most commonly recognized congenital diseaseaffecting collagen production
• Mutations in the gene encoding α1 and α2 chains oftype I collagen.
- Mutations resulting in decreased synthesis ofqualitatively normal collagen are associated withskeletal abnormalities.
- Mutations resulting in abnormal polypeptides thatcan not be arranged in triple helix causes moresevere phenotypes.

Osteogenesis Imperfecta
• Four types (type I, II,III, IV) based on the clinicalexpression and mode of inheritance.
• Principally affects bone but also impacts other tissuesrich in type I collagen (joints, eyes, ears, teeth, skin).
• Patients make too little bone: Cortex of bones is thinand medulla have thin (attenuated) bony trabeculae;which makes patients vulnerable to fractures with minortrauma (brittle bone disease).

Osteogenesis ImperfectaDefective Osteoblasts and Matrix
ProductionType Clinical Inheritance Biochemical Defect
Features
Type I
Type II
Type III
Type IV
Mild
Blue sclerae
Fractures
Severe/Lethal
Blue ScleraeFractures
Moderate/ severe
Blue sclerae
Fractures
Moderate
Normal scleraeFractures
AD
AR
AD/AR
AD
Type 1 procollagen
Glycine replaced
Deletion in
COL1A1, COL 1A2
Frameshift preventsintegration of
COL1A2 in molecule
Point mutation inCOL1A2

Ehler-Danlos Syndrome
• Heterogeneous group ofconnective-tissuedisorders, recently
classified in diff types• Hyperextensibility of skin,
easy bruising,hypermobile joints, Aorticdissection; blue scleramay be present• Bone is osteopenic,
kyphoscoliosis,spondolisthesis

Marfan’s Syndrome
• Heterogeneous group of inherited (AD) connective tissuedisorder affecting bones, heart, aorta and eyes
• Mutation in locus of fibrillin gene on chromosome 15
• Usually tall with exceptionally long extremities, and longtapering fingers and toes
• Hyperflexible joints, kyphosis, scoliosis, pectusexcavatum
• Eyes: subluxation of lens - ectopia lentis
• CVS: Mitral valve prolapse, Aortic dilatation due to cysticmedionecrosis - AR; Aortic dissection

Abnormality of Collagen Synthesis:Metabolic Bone Disease
Scurvy• Vitamin C deficiency
• Failed cross-linking of collagen
• Fragile capillaries and venules- Subperiosteal hemorrhages
• Defective osteoid synthesis- Microfractures
• Bony deformities

Abnormalities in Matrix Turnover(Modelling/Remodelling)
• Osteopetrosis (too much bone): Marble bone disease
- Group of rare genetic diseases resulting from impairedformation and function of osteoclasts and characterizedby diffuse symmetrical skeletal sclerosis (thickening ofcortical bone and narrowing of the medullary cavity).
- Progressive deposition of bone on pre-existing matrix
• Mutations in carbonic anhydrase II gene interfering with theacidification of resorption pits.
• Mutations in chloride channel gene interfering with the functionof proton pump.
• Mutations in RANK ligand gene resulting in fewer osteoclastso normal.

Osteopetrosis
• Bones lack medullary canal and ends of bones are bulbous andmisshapen (Erlenmeyer Flask deformity).
• Primary spongiosa , which is normally removed during growthby osteoclasts, persists and fills the medullary cavity.- No mature bone trabeculae in the medullary cavity and no room for
hematopoietic cells (anemia, granulocytopenia, thrombocytopenia,extramedullary hematopoiesis (hepatosplenomegaly)).
• Deposited bone is not remodeled and tends to be woven inarchitecture and prone to fracture with little trauma.
• Obstruction (narrowing) of cranial neural foramina leads tonerve compression & paresis in the cranial nerves.

OsteopetrosisRadiologic Findings
• Marked symmetrical increasein the density of bone
• Normal demarcation ofcancellous (medullary) boneand cortical bone is lost.
• Metaphyseal flaring(Erlenmeyer flask deformity),more prominent around the
knee and hips

Abnormal Skeletal DevelopmentSummary
Any failed step in skeletal morphogenesis maycause disease.
• Abnormal Mesenchymal Condensations- Syndactyly
• Abnormal Chondrocyte Proliferation/ CartilageDevelopment
- Dwarfism
• Abnormal Matrix Production- Osteogenesis imperfecta, Marfan’s, Ehler Danlos, Scury
• Abnormal Modelling/Remodelling- Osteopetrosis

Metabolic Bone Diseases: Vitamin DDeficiency
• Delayed or inadequate mineralization of osteoid formedby osteoblasts.
• This under mineralized bone is weaker and more proneto fracture and deformity.
• Causes Rickets in children and osteomalacia in adults.
• Labs:- low serum calcium, phosphate, vitamin D levels
- raised serum alkaline phosphatase.
• Causes of vitamin D deficiency:- poor dietary intake
- malabsorption from GI tract
- renal disorders causing decreased synthesis of 1,25 dihydroxyvitamin D
- rare inherited disorders.

Osteoporosis

Metabolic Bone DiseaseOsteoporosis
• Diminished bone mass
• Localized or diffuse
• Primary or secondary
• Risk of fracture
• Severe disease of elderly
• Expensive to health care - > $ 14BILLION/YR


Secondary Osteoporosis
• Endocrine
- Hyperparathyroidism
- Hyperthyroidism, Diabetes, Addison’sdisease, Pituitary tumors
• Neoplasia: Carcinomatosis, multiple myeloma,paraneoplastic disease
• Gastrointestinal: Malnutrition, hepaticinsufficiency, vitamin D or C deficiency,malabsorption
• Drugs: Chemotherapy, corticosteroids, alcohol
• Immobilization

Regulation of OsteoclastPTH, IL-11, vitamin D3
Pathogenesis• PTH Stimulation ofosteoblasts ( RANKL)to activate osteoclasts
• Osteoclastic Activity Massive bony resorption

Metabolic Bone DiseaseHyperparathyroidism: Pathology
Unabated Osteoclastic
Activity
Giant cell tumor-like mass
Neo-vascularization, Hemorrhage
(Brown tumor)

Renal Osteodystrophy
• Chronic renal failure → hyperphosphatemia →Hypocalcemia → Secondary PTH
• Reason for Hypocalcemia• Decreased vitamin D metabolism in kidney (inhibition ofconversion of vitamin D to active metabolites by phosphate)
• Diminished intestinal absorption of vitamin D
• Iron and aluminum accumulation in bone (fromdialysate) prevents further bone deposition

Renal Osteodystrophy
•PTH → Osteoclastic activity → Boneresorption
• Matrix mineralization (osteomalacia)
• Osteoporosis
• Growth retardation

Paget’s DiseaseDisease of Osteoclasts
• Etiology- Virally induced [Paramyxovirus (measles, RSV) - nucleocapsid
antigens identified in osteoclasts)(Paramyxovirus- slow virus disease)
- Genetic predisposition - mutation - p62• Pathogenesis
- Virus stimulates IL-6- IL-6 & M-CSF Activate osteoclasts
- Osteoclasts hyper-responsive to RANKL & vit. D- p62 - RANK/RANKL SIGNALLING - Osteoclasts
(till date, no virus has been isolated from affected bone)

Paget’s Disease
Disease of stagesOsteolytic Stage:Osteoclastic activity-patchy, florid
Mixed Lytic andBlastic Stage:
PredominantlyOsteoblastic
Osteosclerotic(burnt-out) Stage:
End stage: bonemass

Paget’s Disease• More common in whites in England, France,
Austria, US, Germany, New Zealand,Australia
• Less in Japan, China, Scandinavia, Africa• 5-11% of whites, Adults, M = F
• Diagnosis:- X-ray
- ↑Serum Alkaline Phosphatase- ↑Urinary Hydroxyproline
• May be monostotic or polyostotic

Paget’s DiseaseComplications
• Deformities - Pain (compressed nerves)• Fracture/Microfractures (chalk-stick #) Pain
• Degenerative Joint Disease PainRarely:
• High-Output cardiac failure (osteoblastic phase)• Tumors
- Sarcoma- 5-10%, High grade, Lethal- Giant cell tumor
- Extra-osseous hematopoiesis

SGU Medical School Bone Pathology Course, November, 2011
Lecture #2
Fracture, Osteomyelitis and Arthritis
Ashraf Khan, MD, FRCPath
University of MassachusettsMedical School
Worcester, MA

Fractures
Etiology:- Traumatic• Significant Trauma
• Stress #: slowly develops with repetitivephysical activity (aerobics, marching)
- Pathologic• Metabolic Bone disease
• Tumors and other bone pathology (cysts etc.)

FracturesStages of Healing
• Inflammatory Phase (1 wk)
• Reparative Phase (2-3 wk)
• Remodeling Phase

Inflammatory Phase
• Immediately following a fracture, there is a disruption ofblood vessels at the medulla, cortex, periosteum of the
bone and surrounding soft tissues.• This results in hematoma formation, which lies between
the fractured ends of a bone and extends beneath theperiosteum and into the surrounding soft tissues.Fracture hematoma favors bone repair and its removalretards healing.
• Fibrin mesh seals the fracture site and providesscaffolding for other cells.
• The bone marrow near the fractured ends of a boneshows hemorrhage and fat necrosis.
• Vascular injury leads to ischemia, which in turn leads tonecrosis of the ends of bone.

Inflammatory Phase
• There is granulation tissue formation with in-growthfrom the surrounding viable bone of fibroblasts and
capillaries into the fibrin mesh (clot) between thefractured bone ends.
• This is accompanied by an inflammatory infiltrates,initially containing neutrophils, but later containing more
chronic inflammatory cells including macrophages.• Macrophages remove red cells, necrotic fat and tissue
debris.• Migrating inflammatory cells and degranulated platelets
release cytokines (FGF, PDGF, IL, TGF-β familyincluding bone morphogenic proteins ), which activatethe osteoprogenitor cells in the periosteum, medullarycavity and surrounding soft tissues and activateosteoblastic and osteoclastic activity.
• Thus a soft tissue callus (Procallus) is formed towardthe end of inflammatory phase: Organizing fibrin +
granulation tissue + inflammatory cells. No structural

Bone Morphogenetic Proteins
• They are normally present within the bone andmarrow and may be elevated in fracture.
• Polypeptides responsible for post-fetal differentiationof mesenchymal cells into chondrocytes and
osteoblasts.• More than 16 human Bone Morphogenetic Proteins
(BMPs) have been identified.
• Osteoinductive BMP (BMP 2 and BMP 9) provide asignal for the differentiation of mesenchymal cells into
osteoblasts.• They are also critical for chondrogenesis -
cartilaginous callous formation - and bone formationby enchondral ossification

Reparative Stage
• During this stage, primary callus (skeletal repairtissue that unites the fractured bone ends) is formed
and consists of newly formed bone and cartilage.• Activated osteoprogenitor cells deposit trabeculae of
woven bone within medullary cavity and beneath theperiosteum.
• Subperiosteal trabeculae of woven bone are orientedperpendicular to the cortical axis.
• Activated mesenchymal cells in the bone and softtissue surrounding the fracture site also differentiateinto chondroblasts that make fibrocartilage and
hyaline cartilage.• Cartilage formed in fracture callus undergoes
endochondral ossification and is usually surroundedby organized woven bone.

Reparative Stage• Callus which lies within the reparative tissue in the
medulla is called internal callus.• External callus develops around the fractured bone
ends and is in contact with the surrounding softtissues, including the muscle.
• Normal callus contains variable amount of bone,cartilage and fibrous tissue depending on stability,
vascularity and extent of injury.• When the fracture site is unstable and poorlyvascularized, an abundance of cartilage is seen.
• Internal callus, which is relatively well vascularizedand mechanically more stable contains less cartilageand more woven bone compared to external callus.
• In an uncomplicated fracture, primary callus reachesits maximal girth at the end of second or third week,
which helps stabilizes the fracture site.

Remodeling Phase• Secondary callus is the term used to describe the
mature lamellar bone which gradually replaces thewoven bone of primary callus. In this way, the normalcortical and cancellous bone architecture is restoredfollowing injury.• In this stage, osteoblasts lay down new bone in
apposition on the surface of the woven bone inprimary callus.
• At the same time, there is resorption of primary callusby osteoclasts.• As the callus matures and is subjected to weight-
bearing forces, the portions that are not physicallystressed are resorbed. Thus, the callus is reduced insize until the shape and outline of the fractured bonehave been re-established.
• Similarly, the medullary cavity is also restored.• This process of gradual remodelling occurs over a
period of several months and is more rapid in

FracturesRemodeling Phase
- Callus slowly resorbed andremodeled to the shape &contour of normal bone.
- Woven bone replaced bylamellar bone.
- Complete resolution:Fracture site may not beapparent.

Fracture Healing by InternalFixation
• Absence of cartilage from the healing process at thefracture site.
• Absence of subperiosteal reaction or peripheral(external) callus formation.
• Apposition of new bone on trabecular surfaces at anearly period after fracture
• Rapid healing
• Absence of significant osteoporosis due to earlymobilization

FracturesComplications
• Delayed union- Displaced or comminuted fractures
- Devitalized bone resorbed slowly
- Interposition of soft tissue impedes callusformation - resection of soft tissue necessary
• Inadequate immobilization• Permanent deformity
• Infection: in Open or Comminuted #

FracturesComplications - Pseudoarthrosis
Poor immobilizationCallus composed offibrous tissue- Bonycallus does not form Non-unionCystic degeneration
- cyst lined bysynovium-like cells(pseudoarthrosis)
False joint impedescallus formationResection required
for complete healing

Complications of Fractures
• Fractures can lead tosystemic complications,including shock
syndrome andmyoglobunuria, the latteroccurring when there issignificant muscle injury.
• Fractures can cause fatembolization through thedamaged venous systemby disrupting the bonemarrow which contains
adipose tissue. Fatembolization becomes aclinical problem in severemultiple fractures and
Lung tissue with fat globulesstained with oil red O stain.
extensive orthopedicsurgery.

FracturesDeterminants of Healing
- Age
- Fracture type
- Fracture site
- Extent of soft tissueinjury
- Local factors• vascular supply
• mechanical forces
- Overall Health &Nutritional status
• Diabetes,
• Calcium &phosphorous levels(osteoporosis,
osteomalacia)
• Vitamin deficiency
• Infection

FractureSummary
• Repair occurs in phases
• Ongoing bony remodeling → completeresolution
• Impeded repair due to infection or instability→ severe complications

OsteomyelitisInflammation of Bone or Marrow
• Implies infection
• Stages
Acute Chronic
• Etiology- Hematogeneous
(seeding fromsystemic disease)
- Direct inoculation
• Organisms- Bacterial
- Fungal
- Viral
- Protozoal

Pyogenic OsteomyelitisUsually Bacterial
• No organism isolated in 50%.
• S. aureus (80-90%) - receptors for bonematrix components e.g. collagen
• Gram - rods (E. coli, Klebsiella,Pseudomonas) - GU infection, IV drug
abuse
• Mixed bacterial (direct inoculation)
• H. influenza (neonates)
• Group B Streptococcus (neonates)
• Salmonella (sickle cell disease)

Acute Hematogenous Osteomyelitis
• Epidemiology- Young, growing children
- Adults (> 50 years)- Gender M:F = 2:1
- Location• Long tubular bones (femur, tibia, humerus)
• Vertebral bodies
- Predisposing factors: catheter, trauma,infection, underlying disease, IV drugabuse

Acute Hematogenous Osteomyelitis
• Hematogenousosteomyletis usually beginsin the metaphysis of a bone.
• Thought to be initial site ofinfection for anatomic
reasons:- Nutrient arteries terminate in
venous sinusoids (ideal lakesfor bacterial seeding) ratherthan forming anastomosiswith veins through capillaries.
- These vascular loops andterminal branches have lowoxygen tension and inhibitedphagocytosis, conducive to
bacterial growth.

Acute HematogenousOsteomyelitis
• Once infection takeshold, it spreads to
adjacent trabecular andcancellous bone.
• Continuing infectionresults in the formationof abscesses within themedulla and beneaththe periosteum.

Acute HematogenousOsteomyelitis
• Inflammatory swelling leads tothe loss of both endosteal and
periosteal blood supply resultingin necrosis of nearby bonytrabeculae including a segmentof cortex.• This necrotic bone is called
sequestrum.• After infection reaches
periosteum, it is elevated,becomes reactive and starts
forming new bone.• A sleeve of new bone
(involucrum) develops aroundthe necrotic cortex and medulla.
• Subperiosteal abscesseventually ruptures into softtissue, forming soft tissue
abscess and ultimately draining

Vertebral Pyogenic Osteomyelitis
• Most commonly arises inlumbar spine, less
frequently thoracic andcervical spine.
• Predisposing factorsinclude urinary tractinfection, diabetes and IV
drug abuse.• Begins in vertebral bodythen spreads through the
vertebral end plate toinvolve the disc (discitis).
• Disc destruction is morecommon in pyogenic thannon-pyogenic infections of
the spine.

Vertebral Pyogenic Osteomyelitis
• More than one vertebralsegment may be
involved.• May breach the anterior
cortex and ligamentousstructures to formparavertebral soft tissueabscesses(retrophryngeal abscess,psoas abscess extending
to groin and politealfossa).
• May spread posteriorly toinvolve the posterior arch
and the neural canal,

Pyogenic OsteomyelitisPathology
• The initial response to infection withpyogenic organisms is acute
inflammation, resulting in fluid exudatecontaining neutrophils and fibrin.
• Continuing exudation raises the tissuepressure, which comprises the vascularspace, leading to bone death (boneunable to expand to relieve the pressureon vascular space, no swelling as in soft
tissue).
• Major problem in treating patients with OMis the extent of osteonecrosis, whichinterferes with the access of antibiotics.

Pyogenic OsteomyelitisPathology
• After infection reachesperiosteum, it is elevated,
becomes reactive and startsforming new bone.
• A sleeve of new bone(involucrum) develops aroundthe necrotic cortex.• This involucrum is initiallycomposed of reactive woven
bone trabeculae, but later,may become organized into aneocortex of compact bone.

Pyogenic OsteomyelitisSummary of Pathology
• Acute inflammation
• Bone necrosis
• Subperiosteal abscess
• Progressive ischemia leads to segmentalbone necrosis (sequestrum) surrounded byviable new bone (involucrum) formation
• Draining sinus tracts
• Extension into joint space (acute septicarthritis)

Acute OsteomyelitisTreatment
• Treat early• IV antibiotics 4-6 weeks
- Exception: Children with hematogenousspread
• Oral therapy if organism is susceptible• Good compliance• Rapid response
• Consider surgical debridement

Evolution to Chronic Osteomyelitis
• 5-25% of Acute Osteomyelitis do notresolve Chronic Osteomyelitis
- Delay in diagnosis
- Inappropriate treatment:• Inappropriate antibiotics
• Therapy is too short
- Inadequate surgical debridement - extensivenecrosis
- Underlying medical condition

Chronic Osteomyelitis
• Pathology- Chronic Inflammation
- Resorption of Dead bone- Deposition of Woven bone
• Brodie abscess: Intracortical abscess• Sclerosing OM of Garré: Jaw - extensivenew bone obscuring the underlying bone

Chronic OsteomyelitisSequelae
• Recurrent acute exacerbations
• Pathologic fracture
• Secondary amyloidosis
• Endocarditis
• Sepsis
• Septic Arthritis
• Rarely, malignant complications- Squamous cell carcinoma of fistula tract
- Sarcoma of infected bone

Tuberculous Osteomyelitis
• 1-3% of patients with pulmonary TB
• Immunocompetent or compromised
• Location- Spine (Pott’s spine) > knees > hips
• Burrowing abscesses withcalcification

Skeletal Tuberculosis
• Very Destructive• Spreads throughmedullary cavitycausing extensive
necrosis• Extends thru IV discsinvolving multiple
bones• Difficult to control

Pathology of Joint Diseases

The Normal JointComponents - Synovium & Cartilage
Synovial cells: Mesenchymal -cuboid or fibroblast like cells - 1-4
cell thickProduce:
- Hyaluronic acid- Proteins
- Secrete fluid into joint spaceArticular cartilage: Shock absorberChondrocytes regulate matrix turn over:Synthesis:
-Type II collagen-Proteoglycans
Breakdown: Matrix degrading enzymesDevoid of vessels, nerves
1-4 mm thickNourished by synovial fluid

Arthritis
• Osteoarthritis
• Rheumatoid arthritis
• Other: gout, pseudogout (covered earlier)
• Seronegative arthritis
• Infectious

ArthritisMultiple Etiologies
Infection Immune-mediatedInjury
Inflammation of the Joint
Crystal deposition Degenerative

OsteoarthritisDegenerative Joint Disease
• Most common form of arthritis
• Progressive destruction of articularcartilage
• Not true inflammatory arthritis
• Billions of health care dollars/lost workdays

OsteoarthritisDegenerative Joint Disease
• Primary- Aging
phenomenon
- Oligoarticular
- 80-95% of peopleover 65 years
• Secondary- Younger patients
- Predisposition• Diabetes
• Hemachromatosis• Ochronosis
• Obesity• Congenital deformity
- Polyarticular- Severe

Primary OA
• Most common form of arthritis
• Primary OA typically involvesvariable number of joints in
characteristic locations, as shown*
• Age: 75% of persons over age 70have OA
• Female sex
• Obesity
• Hereditary
• Secondary:- Trauma
- Neuromuscular dysfunction- Metabolic disorders
*Exceptions to these locations should trigger consideration of secondary causes of OA.

OsteoarthritisPathogenesis
• Cartilage is made up of chondrocytes and matrix.The matrix is composed of water, proteoglycans, typeII collagen and glycoproteins (fibronectin,chondronectin).
• The mechanisms leading to OA are complex and notentirely clear.
• It is thought to start with chondrocyte injury bygenetic, biochemical factors and aging.• Chondrocytes respond to injury by proliferating
(cloning) and secreting proteases and inflammatorymediators in addition to collagen and proteoglycans.Eventually, there is remodeling of the cartilagenous
matrix. In early OA, water content of the matrixincreases and concentration of proteoglycansdecreases.

Osteoarthritis (OA)
• Subsequently, superficial layers of cartilage isdegraded. This manifests itself as vertical andhorizontal cracks (splits).
• Later, deeper fissures and cracks develops on thearticular cartilage which becomes reduced in
thickness as chondrocytes in the superficial layer dieand portions of the superficial cartilage are sloughed
off.• Often chondrocytes in the deeper portion of the
cartilage proliferate adjacent to the fissures.• The underlying subchondral bone plate shows
thickening and there is reduplication of the tidemark.• Eventually, cartilage may be lost in the weight-
bearing regions of the affected joint.

Osteoarthritis (OA)
• The exposed subchondral bone plate becomes the newarticular surface, and friction with the opposing
degenerated articular surface polishes the exposed bone(eburnation).• Small fractures in the articulating bone allows the jointfluid to be forced into subchondral regions, leading to theformation of subchondral cysts. Hypermetobolic state ofthe subchondral bone also contributes to subchondral
cyst formation.• Subchondral cysts have a fibrous wall and are usually
filled with myxoid or fibromyxoid material.

Osteoarthritis (OA)
• In non-weight-bearing areas of the joint and around themargins of the joints, ostoecartilagenous outgrowthsdevelop (osteophytes) by endochondral ossification.

Osteoarthritis (OA)
• Part of the eroded cartilage may break off and formloose bodies (joint mice). Loose bodies, which lie
freely within the joint cavity, have a central core ofnecrotic bone. The covering cartilage remains viable as itis nourished from the joint fluid.

OsteoarthritisPathology- Joint Mice
Microfractures
chunks of
dislodged bone
and cartilage
loose bodies
(joint mice)

Inflammatory ArthritisPathogenesis
Inflammation of synovium
Cytokine release
Edema, granulation tissue
Synovial overgrowth
Diminished nutrition to cartilage
Cartilage breakdown

Rheumatoid Arthritis

Rheumatoid arthritis
Systemic disease manifested by polyarthritis:pain, inflammation, swelling, destruction
• Prevalence estimated at ~0.5 - 1%• Female:male ratio = 2.5:1
• Genetic predisposition• Symmetrical arthritis, typically of the hands and
feet, also often involving ankles, knees, wrists,elbows and shoulders
• Joint destruction occurs early and is a marker fordisease progression
Hochberg MC, Spector TD. Epidemiol Rev. 1990;12:247-52; Doran MF, et al.. Arthritis Rheum.2002:46:625-31.

Rheumatoid Arthritis
• Erosion of cartilage withingrowth of synovium
• Osteoclastic activity (due toelevated levels of RANKL
produced by activated T cellsand synovial fibroblasts)
• Subchondral cysts,Subchondral Osteoporosis
(localized and systemic) asopposed to osteoarthritis.

Rheumatoid Arthritis
• Eventually, after the cartilage has beendestroyed, the pannus bridges the opposingbones in a joint, leading to fixation (stiffness)in the fused joint (fibrous ankylosis, fibrous
obliteration of the joint).

Rheumatoid Arthritis
Unlike OA, there is little reparative activity,osteophyte formation, new boneformation,
or bone sclerosis.

Rheumatoid Arthritis: Summary
• Papillary synovial hyperplasia• Chronic inflammation
• Organizing fibrin• Pannus
• Erosion of underlying cartilage• Narrow (fused) joint space

Systemic Pathology in RA
• Soft tissue- Rheumatoid nodules
• Lung involvement
• Vasculitis
• Uveitis- Usually in juvenile RA

Rheumatoid Nodule
• Found in 25% of RA patients.• Non-tender firm nodule most commonly found inregions of skin (subcutaneous fibroadipose tissue)
subject to pressure (over bony prominences):ulnar aspect of forearm, elbow, occiput,lumbosacral area.
• Also develop in joints, tendons, soft tissue, lung,heart (peri-, myo-, endocardium), aorta, spleen,viscera.
• Histologically, it is characterized by itsirregular shape and a central zone of necroticfibrinoid material surrounded by histiocytesand chronic inflammatory cells.
• May be the result of vascular damage.

Pulmonary Involvement in RA30% - 40% of patients
• Pleura:- Chronic pleuritis
- Pleural effusion
- Pleural rheumatoidnodules
• Intrapulmonaryrheumatoid nodules
• Parenchyma- Diffuse interstitialpneumonitis & fibrosis
- End-stage lung disease
• Pulmonaryhypertension

Vasculitis in RA
• Acute necrotizing vasculitis - Can affect anyorgan
- Heart - Myocardial Infarction
- Brain - Cerebrovascular Occlusion
- Kidneys - Renal Failure
- Mesentery - mesenteric and intestinal infarction
- Gangrene of digits

Complications in RA
• Life Expectancy by 3-7 yrs
• End-stage lung disease
• Vasculitis & its complications
• Systemic amyloidosis
• Iatrogenic effects of Tx- Immunosuppression

Differences: OA vs. RA
• AM stiffness:- OA< 30 minutes,
- RA>1 hour• Sxs:
- OA worse with activity- RA better with activity
• Joint distribution:- OA: weight bearing joints
and PIP & DIP- RA: wrist /MCP/PIP
• RA is systemic disease -fever, weight loss
• OA is non-inflammatory,RA is inflammatory
• OA has reparative activityand new bone formation
- osteophytes,subchondral sclerosis
• RA - No reparative boneformation - periarticular
osteopenia

Crystal Deposition DiseaseGout
• Acute gouty arthritis- Red, painful, swollen
• Chronic tophaceous gout- Fibrosis, crystal deposits, joint destruction
• Gouty tophus- Large crystalline masses with associatedtissue reaction

Crystal Deposition DiseaseGout
• Epidemiology- Hyperuricemia
• Primary (genetic predisposition)• Secondary (increased nucleic acid turnover:leukemia, alcohol, obesity, drugs, renal disease,
purine rich diet)
- Peak in 5th decade- Linked to duration of hyperuricemia (20-30years)

Crystal Deposition DiseaseTophaceous Gout
• Deposition in areasof low temperature
• Incite inflammatoryresponse
• Bone and jointdestruction
• May ulcerate skin

Crystalline DiseasesCalcium Pyrophosphate
• Epidemiologyseudogout)- >50 years of age
- 30-60% of people over 85 years
- M=F
- Equal race distribution

Crystalline DiseasesCalcium Pyrophosphate
• Hereditary (Pseudogout)- Linked to chr 8 (ANKH gene, encodes transmembrane
pyrophosphate transport channel)
• Secondary:- trauma, diabetes, amyloidosis, hyperparathyroidism,
hemochromatosis
• Idiopathic (sporadic)

Crystalline DiseasesCalcium Pyrophosphate
(Pseudogout)• Pathogenesis
- Unknown
- Altered matrix enzymes thatmodulate phosphate metabolism
- Deposits ingested by PMNs
- Cytokine release

Crystalline DiseasesCalcium Pyrophosphate
• Clinical courseeudogout)- Acute, subacute, or chronic arthritis
- Mimic gout, osteoarthritis, rheumatoidarthritis
- Mono-articular or poly-articular
- 50% of patients suffer severe jointdamage

Crystalline DiseasesHydroxyapatite
• “Tumoral Calcinosis”
• Mechanism unknown- May be seen in children
• Massive accumulations ofhydroxyapatite crystals
• Form nodules in soft tissues nearjoints

Crystal Deposition DiseaseSummary
• Crystal deposits due to hereditaryand environmental factors
• Cause arthritis, bone and softtissue destruction
• Elicit an inflammatory response thatresults in injury
• Distinguishable by histologiccharacteristics of crystals

Infectious Arthritis
• Organisms- Bacteria: Rapid joint destruction
• Gonococcal: Late adolescents, adults, women• Non-Gonococcal:
- S. Aureus (older children, adults)- H. influenza, Streptococcus: Children <2 yr
- Gram-: E. Coli, Salmonella (sickle cell anemia), Pseudomonas- Mycobacteria (Tuberculous arthritis)
- Borrelia (Lyme disease)
- Virus:• Parvovirus B19, Rubella, Hepatitis C

Septic Arthritis
• Etiologic microorganisms are pyogenic bacteria suchas staphylococcus, Streptoccoccus, Neisseria.
• Usually a monarticular infection most commonlyinvolving the hip in infants and young children and
the knee in older children and adults.• Contributing predisposing factors:
- Chronic diseases (diabetes, alcoholism, renal disease)- Chronic joint diseases (rheumatoid arthritis, SLE)
- Immunosuppressed states (AIDS)

Clinical Presentation• Symptoms include fever, malaise, pain (arthralgia).
• Physical examination reveals that the joint is warm, painful,erytematous and distended, with a decreased range of motion.
• The most common sites: Knee > hip > shoulder, ankle, elbow> wrist.
• Workup of a patient with septic arthritis include repeated jointaspiration for culture and fluid analysis including gram stain,
white cell count (elevated), protein level (elevated) andglucose level (decreased).
• Because infection of synovium precedes joint fluid infection,etiologic diagnosis by joint fluid aspiration may be delayedand repeated joint aspiration over several days may be required
to confirm the diagnosis.• A biopsy is indicated if clinical suspicion continues in the
setting of nonconfirmatory lab tests.

Septic Arthritis-Pathology
• Microscopic examinationshows that the synovial lining
is usually ulcerated andcovered by an acute
inflammatory fibrinousexudate containing numerous
neutrophils.• The underlying subintima iscomposed of granulation tissuecontaining heavy inflammatoryinfiltrates. Organisms may beidentified by gram stain in the
inflamed synovium in theacute stage.

Septic Arthritis-Pathology
• The articular cartilage showsloss of matrix staining and
necrosis of cartilage cells inthe superficial zone. Cartilageis particularly susceptible toenzymes released by bacteriaand disintegrating neutrophils
and is rapidly destroyed.

Septic Arthritis-Pathology
• If left untreated, a pannus ofinflamed fibrous tissue extends
over the articular cartilagefrom the inflamed synovium.• This tissue may becomeorganized and lead to fibrous
ankylosis of the joint.

Complications of Septic Arthritis
• Soft tissue inflammation
• Osteomyelitis
• Sepsis
• Degenerative Joint Disease
• Fibrous and bony ankylosis

Tuberculous Arthritis
• Is a chronic progressive monoarticular disease involving theknee or hip and most commonly affecting middle-aged orolder persons.
• Usually develops as a complication of adjoining osteomyelitisor after hematogenous spread from a visceral (lung) site ofinfection.
• Synovial tissue reveals yellow cheese-like areas of necrosis.
• Microcopic examination shows granulomas with caseousnecrosis. Inflamed synovial tissue extends pannus-like oversynovial tissue, causing cartilage and bone destruction.Chronic disease results in severe destruction with fibrousankylosis and obliteration of the joint space.

Lyme Arthritis
• Caused by infection with spirochete BorreliaBurgdorferi.
• Approximately, 60-80% of untreated patients withLyme disease develop joint symptoms within a fewweeks to two years after onset of the disease.
• Serologic or molecular tests are usually required forconfirmation of diagnosis.

Lyme Arthritis
• There is thickening of thesynovial lining characterizedby chronic papillary synovitiswith synoviocyte hyperplasia,fibrin deposition, onion-skinthickening of the arterial walls
and mononuclear cellinfiltrates (lymphocytes andplasma cells).
• Silver stains may sometimesreveal small numbers of
microorganisms (Spirochetes)in the vicinity of blood vessels.

Viral Arthritis
• Arthritis can occur in a setting of a variety of viral infections:Hepatitis C, Hepatitis B, Rubella, Mumps, Epstein-Barr virus,Parvovirus B19.
• The biopsy findings reveal only nonspecific chronicinflammation.
• The pathogenesis is unclear. Changes could be due to- Direct infection of the joint by the virus. (Rubella, some alpha viruses)
- Autoimmune reaction induced by the virus (HIV-associated chronicarthritis).

SeronegativeSpondyloarthropathies
• Heterogeneous group of diseases
• Arthritis one of several manifestations
• Associated with identified infectiousagents
• In part, immune mediated
• Similar, but milder disease thanrheumatoid arthritis
• HLA-B27 associated

SeronegativeSpondyloarthropathy
Sex Relapse Extraskeletal HLA-Disease B27
AnkylosingSpondylitis
Reiter’s Syndrome
Psoriatic Arthritis
EnteropathyAssociated Arthritis
M>F Yes Uveitis, Aortitis 90%
M>F Rarely Conjunctivitis, 80%Aortitis,
Urethritis/Cervicitis
M>F Yes Uveitis, YesConjunctivitis
M>F Rarely Uveitis, IBD Yes

Seronegative SpondyloarthropathyPathology
• Chronic synovitis• Destruction of articular cartilage
• Fibrosis and narrowing of joint space(fibrosing ankylosis)*
• Ossification of fibrous tissue (bonyankylosis)*
• Joint immobility**Most common is ankylosing spondylitis

Immune-mediated ArthritisSummary
• Heterogeneous group of systemic disorders thatshare common features
• Most associated with a genetic predisposition- HLA-DR in rheumatoid arthritis
- HLA-B27 in seronegative diseases
• Probable link to environmental trigger- Likely infectious in nature
• Histopathologically similar, though RA tends tobe more severe
• All are progressive diseases

BONE TUMORS

Bone TumorsGeneral Principles
• Incidence- Most common malignant tumor is a
METASTASIS- 2,500 new primary bone tumors/year U.S.
- True incidence unknown, most asymptomatic,not biopsied
- Sarcomas result in 1,300 deaths/year in U.S.

Normal Tissue Benign Tumor Malignant Tumor
Bone Osteoma OsteosarcomaOsteoid Osteoma Osteoblastic
Osteoblastoma Chondroblastic
Telangiectatic
Dedifferentiated
Cartilage Enchondroma
Osteochondroma
Chondromyxoid fibroma
Chondroblastoma
Chondrosarcoma
Conventional
Clear cell
Mesenchymal
Dedifferentiated
Fibro-osseous Fibrous dysplasia AdamantinomaOsteofibrous dysplasia
No NormalCounterpart
Giant cell tumor
Aneurysmal bone cyst
Malignant giant cell tumor
Ewing’s sarcoma

Bone TumorsPredisposing Factors-Usually None
• Rarely arises as a complication of:- Osteomyelitis (sarcoma, SCC)
- Gaucher’s disease (sarcoma)
- Bone grafts/prostheses (sarcoma)
- Radiation (sarcoma)
- Bone infarcts (sarcoma)
- Paget’s disease (sarcoma)

Bone TumorsClinical Symptoms
• Benign tumors usually asymptomatic• Malignant tumors are aggressive
• Symptoms- Pain
- Pathologic fracture- Metastases
• Radiology- Site and appearance clues to diagnosis

Bone Tumors
Benign- Well-circumscribed
- “Scalloped border”
- No destructive growth
- No invasion of other tissuesor joint
- Usually small
- Typical in younger patients

Malignant Bone TumorsPathologic Features
• Histologic grade: Usually high grade, poorprognosis
- Important predictor of behavior
- Determines likelihood of adjuvant therapy
• Tumor stage- Predicts clinical outcome

Benign Bone Forming TumorsOsteoid Osteoma
• Tumor of young adults• Male predilection• Predilection for
appendicular skeleton• Classic clinical
presentation:Nocturnal pain alleviated
by aspirin

Benign Bone Forming TumorsOsteoma (Bone Island)
- Benign
- Mature bone
- Predilection forcraniofacial bones
- Gardner’sSyndrome

Osteosarcoma
• Epidemiology- Most common bonesarcoma (20%)
- Bimodal age distribution• Elderly (Paget’s,
radiation)• Children, young adults
- Location• Metaphyseal (long bones)in youth
• Flat bones in elderly

OsteosarcomaGenetic Alterations
• Hereditary tumors- p53 mutations (LiFraumeni)
- Retinoblastoma gene (hereditary Rb)
• Sporadic- P53 mutations
- MDM2 (inactivates apoptotic capacity of p53)- Rb mutations are rare

OsteosarcomaPathology
• Anatomic location- Intramedullary (usually low grade)
- Intracortical (high grade)- Juxtacortical (low or high grade)
• Grade- Grade 1: mild cytologic atypia
- Grade 2: intermediate- Grade 3: high grade, pleomorphic

Cartilage TumorsGeneral Features
• Most are benign
• Types of cartilage:- Hyaline (found in tumors and normal)
- Myxoid (found only in tumors)
- Fibrocartilage (rare in tumors)
- Elastic (extremely rare in tumors)

Osteochondroma
- Benign cartilagetumor of metaphysis
- Young adults- Occur only in bones
with enchondralossification

Enchondroma
• Benign- Hyaline and myxoid cartilage
• Metaphyseal and diaphyseal• Medullary cavity
• May erode (but not invade) cortex• Present with pathologic fracture• Solitary or syndromic (Ollier’s or
Mafucci’s disease)• May give rise to chondrosarcoma

Multiple Enchondromatosis
• Ollier’s Disease- Disfiguring
- 20% develop chondrosarcoma
• Mafucci’s Disease- Enchondromas and soft tissue
vascular tumors
- 20% develop chondrosarcoma
- 100% develop anotherextraskeletal malignancy

Chondrosarcoma• Typically occurs in older adults
• Pelvis, humerus, proximal femur• May arise in enchondroma (low
grade)• Low grade (1/3) do not
metastasize, but may recur anddedifferentiate
• High grade metastasize

Giant Cell Tumor of Bone
• Benign locally aggressivetumor
• 4-5% of all primary bonetumors
• Age 20-40 yrs
• Epiphysis• End of long bones
• X-ray: expanding lytic lesion“soap bubble appearance”

Ewing’s Sarcoma & PrimitiveNeuroectodermal Tumor (PNET)
• Family of small blue cell tumorswith a characteristic t(11;22) andt(21;22)
• 6-10% of all primary malignantbone tm.
• Age: youngest among malignantbone tm
• Diaphysis of long bones
• Prognosis: dismal, improves bychemo

Metastatic Tumors to Bone
• Metastatic carcinoma, mostcommon malignant tumor of
bone• May be blastic or lytic• Multifocal or solitary
• Common Primary sites:- B(breast)- L(lung)
- T(thyroid)- And a (adrenal)- Kosher (kidney)- Pickle (prostate)

Metastatic Tumors to Bone
• Should consider- Multifocal disease
- Location (vertebrae, diaphysis)
- Older patient
- Pertinent history

Pathology of Bone TumorsSummary
• Primary bone tumors classified according to matrixproduction
• Tumor site key to differential diagnosis
• Benign tumors are well-circumscribed, do notinfiltrate bone
• Malignant tumors are radiographically andhistologically infiltrative
• Metastatic disease most common, may mimicprimary bone tumors