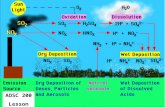
Biogeochemical Cycles of Methane and Nitrous Oxide AOSC ...
Transcript of Biogeochemical Cycles of Methane and Nitrous Oxide AOSC ...

Biogeochemical Cycles of Methane and Nitrous Oxide
AOSC 433/633 & CHEM 433/633
Ross Salawitch
Class Web Site: http://www.atmos.umd.edu/~rjs/class/spr2013
Goals :
• CH4
sources and sinks
lifetime
recent reduction in d(CH4)/dt
• N2O
sources and sinks
• Connection of CH4 and N2O to stratospheric O3
Lecture 06
12 February 2013
1Copyright © 2013 University of Maryland.
This material may not be reproduced or redistributed, in whole or in part, without written permission from Ross Salawitch or Tim Canty.
Radiative Forcing of Climate, 1750 to 2005
2Copyright © 2013 University of Maryland.
This material may not be reproduced or redistributed, in whole or in part, without written permission from Ross Salawitch or Tim Canty.
CH4 & N2O have contributed
about 38% of the RF of CO2

IPCC 2007 FAQ
What do these time series resemble ?
3Copyright © 2013 University of Maryland.
This material may not be reproduced or redistributed, in whole or in part, without written permission from Ross Salawitch or Tim Canty.
CO2, CH4, & N2O
Chapter 3, Chemistry in Context
4Copyright © 2013 University of Maryland.
This material may not be reproduced or redistributed, in whole or in part, without written permission from Ross Salawitch or Tim Canty.

CH4 is the most reduced form of carbon
CO2
Carbon dioxide
+4
CO
Carbon Monoxide
CH2O
Formaldehyde
CH4
Methane
+20-4
CO2
Carbon dioxide
+4
CO
Carbon Monoxide
CH2O
Formaldehyde
CH4
Methane
+20-4
Decreasing oxidation number (reduction reactions)Decreasing oxidation number (reduction reactions)
Increasing oxidation number (oxidation reactions)Increasing oxidation number (oxidation reactions)
Oxidation state represents number of electrons:
added to an element (negative #) or removed from an element (positive #)
H
H
C HH
C in CH4: has received an electron from each H atom.
All electrons are paired and hence this compound
is relatively stable
5Copyright © 2013 University of Maryland.
This material may not be reproduced or redistributed, in whole or in part, without written permission from Ross Salawitch or Tim Canty.
Atmospheric Time Series of CH4 – Recent Data
Data shown in ORANGE are preliminary
http://www.esrl.noaa.gov/gmd/dv/iadv/graph.php?code=MLO&program=ccgg&type=ts
6Copyright © 2013 University of Maryland.
This material may not be reproduced or redistributed, in whole or in part, without written permission from Ross Salawitch or Tim Canty.

Biological Production of CH4
CH4 produced by “methanogenic” bacteria:
• grow only in low O2 environments
• fermentation of cellulose and other organic material
• swamps, marshes, rice paddy fields
• rumina of cows and sheep.
Warneck, Chemistry of the Natural Atmosphere, 2000
7Copyright © 2013 University of Maryland.
This material may not be reproduced or redistributed, in whole or in part, without written permission from Ross Salawitch or Tim Canty.
Sources and Sinks of CH4
Human
Evans, New Phytologist, 2007.
8Copyright © 2013 University of Maryland.
This material may not be reproduced or redistributed, in whole or in part, without written permission from Ross Salawitch or Tim Canty.

Sources and Sinks of CH4
Chapter 3, Global Warming, Houghton
9Copyright © 2013 University of Maryland.
This material may not be reproduced or redistributed, in whole or in part, without written permission from Ross Salawitch or Tim Canty.
Latitudinal Distribution of CH4
Warneck, Chemistry of the
Natural Atmosphere, 2000
A nice animation of CH4 vs latitude, as time evolves, is at
http://www.esrl.noaa.gov/gmd/ccgg/globalview/ch4/ch4_intro.html
10Copyright © 2013 University of Maryland.
This material may not be reproduced or redistributed, in whole or in part, without written permission from Ross Salawitch or Tim Canty.

CH4 Lifetime
We have stated (e.g., Lecture 1, Slide 19) that CH4 has a lifetime of ~9 years.
Chemistry in Context (Table 3.2) and Houghton (page 44) give 12 years for the
lifetime of CH4 and Jacobson (page 71) states “the e-folding lifetime of CH4 due
to chemical reaction is about 8 to 12 years”.
What is meant by lifetime?
Which value is correct?
11Copyright © 2013 University of Maryland.
This material may not be reproduced or redistributed, in whole or in part, without written permission from Ross Salawitch or Tim Canty.
CH4 is lost by reaction with OH
Computer models indicate tropospheric OH maximizes in tropics because:
a) overhead O3 column lowest in the tropics, allowing more UV light
to reach lower atmosphere
b) warmer air holds more H2O: OH produced by O(1D)+H2O
12Copyright © 2013 University of Maryland.
This material may not be reproduced or redistributed, in whole or in part, without written permission from Ross Salawitch or Tim Canty.

CH4 is lost by reaction with OH
CH4 + OH H2O + CH3
44
CHProduction Loss = Production [OH][CH ]
dk
dt = − −
Arrhenius Expression for rate constant:
12 1775 / 3 12.45 10 cm secTk e− − − = × ×
44
4
[CH ]Abundance 1Lifetime of CH
Loss [OH][CH ] [OH]k k = = =
4 15 3 1 6 3
9 1
1Lifetime of CH
10 cm s 1 10 molecules cm
yrs
− − −
− −
= = 3.59 × ⋅ ×
1 = 8.9 3.59 × 10
Commonly T = 272 K and [OH] = 1 × 106 molec cm 3 are used (see Box 1-3 of
http://www.unep.ch/ozone/Assessment_Panels/SAP/Scientific_Assessment_2010/03-Chapter_1.pdf)
yielding :
13Copyright © 2013 University of Maryland.
This material may not be reproduced or redistributed, in whole or in part, without written permission from Ross Salawitch or Tim Canty.
CH3CCl3 (methyl chloroform) is lost by reaction with OH
& its atmospheric abundance / industrial production are well known
CH3CCl3 + OH CH2CCl3+H2O
3 33 3
CH CClProduction Loss = Production [OH][CH CCl ]
dk
dt = − −
Fig 1-1, WMO/UNEP (2010) Fig 1-2, WMO/UNEP (2010)
Methyl chloroform (1,1,1-trichloroethane) is an excellent solvent and one of the least toxic of the chlorinated hydrocarbons. Prior to the
Montreal Protocol, it was widely used for cleaning metal parts and circuit boards, as an aerosol propellant, and as a solvent for inks, paints,
and adhesives. It was also the standard cleaner for photographic film (movie/slide/negatives, etc.). Methyl chloroform was also used as a
thinner in correction fluid products such as Liquid Paper.
The Montreal Protocol banned the use of methyl chloroform and is use was rapidly phased out, throughout the world.
See http://en.wikipedia.org/wiki/1,1,1-Trichloroethane
14Copyright © 2013 University of Maryland.
This material may not be reproduced or redistributed, in whole or in part, without written permission from Ross Salawitch or Tim Canty.

Ozone Depletion and Halocarbons
15Copyright © 2013 University of Maryland.
This material may not be reproduced or redistributed, in whole or in part, without written permission from Ross Salawitch or Tim Canty.
ODP (species " ") =i
3
3
global loss of O due to unit mass emission of " "
global loss of O due to unit mass emission of CFC-11
i
where :is the global atmospheric lifetimeτ
is the molecular weightMW
is the number of chlorine or bromine atomsn
is the effectiveness of ozone loss by bromineαrelative to ozone loss by chlorine α = 60 12 1775 / 3 1
OH+CH4
15 3 1
12 1520 / 3 1
OH+CH3CCl3
15 3 1
4
2.45 10 cm sec
3.59 10 cm sec @ 272 K
10 cm sec
6.14 10 cm sec @ 272 K
6.14CH lifetime 5 yr
3.59
T
T
k e
k e
− − −
− −
− − −
− −
= × × =
×= 1.64× × =
×
≈ × = 8.6 yr
Br Cl CFC-11
CFC-11
( + )
3
i
i
n n MW
MW
α ττ
≈
So Why Does IPCC Give a Lifetime for CH4 of 12 Years?
http://www.grida.no/publications/other/ipcc%5Ftar/?src=/climate/ipcc_tar/wg1/134.htm
16Copyright © 2013 University of Maryland.
This material may not be reproduced or redistributed, in whole or in part, without written permission from Ross Salawitch or Tim Canty.

CH4 and Stratospheric Ozone
• Oxidation of CH4 in the stratosphere produces CO2 and H2O:
Tropospheric CH4 is an important source of stratospheric H2O
Dessler, The Chemistry and Physics of Stratospheric Ozone, 2000
17Copyright © 2013 University of Maryland.
This material may not be reproduced or redistributed, in whole or in part, without written permission from Ross Salawitch or Tim Canty.
CH4 and Stratospheric Ozone
• Oxidation of CH4 in the stratosphere produces CO2 and H2O:
Tropospheric CH4 is an important source of stratospheric H2O
• If tropospheric CH4 continues to rise, stratospheric H2O should increase:
As stratospheric H2O rises:
a) polar stratospheric clouds easier to form larger O3 hole
b) more HOx radicals more efficient loss of O3 most of strat
18Copyright © 2013 University of Maryland.
This material may not be reproduced or redistributed, in whole or in part, without written permission from Ross Salawitch or Tim Canty.

CH4 and Stratospheric O3
19Copyright © 2013 University of Maryland.
This material may not be reproduced or redistributed, in whole or in part, without written permission from Ross Salawitch or Tim Canty.
CH4 constant @ year 2000 level
CH4 doubling by year 2100
Portmann and Solomon, GRL, 2000
CH4 and Stratospheric Ozone
• As tropospheric CH4 rises, stratospheric CH4 will also rise,
reducing efficiency of chlorine catalyzed loss of O3
due to acceleration of Cl+CH4 sink of chlorine radicals:
O3
Production : CFCs +hν→ Inorganic chlorine
Reservoir : HCl
Cl ClO
O
CH4
O3
Production : CFCs +hν→ Inorganic chlorine
Reservoir : HCl
Cl ClO
O
CH4
ClO & Cl catalyze loss of stratospheric O3:
Cl + O3 → ClO + O2
ClO + O → Cl + O2
Net: O3 + O → 2 O2
ClO & Cl catalyze loss of stratospheric O3:
Cl + O3 → ClO + O2
ClO + O → Cl + O2
Net: O3 + O → 2 O2
20Copyright © 2013 University of Maryland.
This material may not be reproduced or redistributed, in whole or in part, without written permission from Ross Salawitch or Tim Canty.

CH4 and Stratospheric Ozone
• As tropospheric CH4 rises, stratospheric CH4 will also rise,
reducing efficiency of chlorine catalyzed loss of O3
due to acceleration of Cl+CH4 sink of chlorine radicals
• Since stratospheric CH4 is most abundant in the lower
part of the stratosphere where CO is still high, the larger
abundance of HOx radicals associated with rising CH4 produces
O3 via this set of reactions:
OH + CO → CO2+ H
H + O2+ M → HO2 + M
HO2 + NO → OH + NO2
NO2 + hν → NO + O
O + O2
+ M → O3
+ M
Net: CO + 2 O2
→ CO2
+ O3
21Copyright © 2013 University of Maryland.
This material may not be reproduced or redistributed, in whole or in part, without written permission from Ross Salawitch or Tim Canty.
CH4 and Stratospheric Ozone
• Oxidation of CH4 in the stratosphere produces CO2 and H2O:
Tropospheric CH4 is an important source of stratospheric H2O
• If tropospheric CH4 continues to rise, stratospheric H2O should increase:
As stratospheric H2O rises:
a) polar stratospheric clouds easier to form larger O3 hole
b) more HOx radicals more efficient loss of O3 most of strat
• As tropospheric CH4 rises, stratospheric CH4 will also rise,
reducing efficiency of chlorine catalyzed loss of O3
due to acceleration of Cl+CH4 sink of chlorine radicals
• Since stratospheric CH4 is most abundant in the lower
part of the stratosphere where CO is still high, the larger
abundance of HOx radicals associated with rising CH4 produces
O3 via traditional “smog” chemical reactions
Computer models project stratospheric column O3 will increase as CH4 rises
22Copyright © 2013 University of Maryland.
This material may not be reproduced or redistributed, in whole or in part, without written permission from Ross Salawitch or Tim Canty.

Atmospheric CH4 and Energy from Gas, Rice, Cattle Trends
Data from http://cdiac.ornl.gov/ftp/trends/atm_meth
Ice Cores
Modern
Air
Dlugokencky et al., GRL, 2009
(kindly updated)
Data from
Data from http://cdiac.ornl.gov
http://faostat.fao.org
23Copyright © 2013 University of Maryland.
This material may not be reproduced or redistributed, in whole or in part, without written permission from Ross Salawitch or Tim Canty.
Recent trends in CH4
Two recent papers offer conflicting views recent reduction in d(CH4)/dt:
• Aydin et al. (Nature, 2011) suggest “rising economic value of natural gas”
and “development of cleaner technologies” have led to a sharp decline in
unintentional release of CH4 by the petroleum industry, based on the
temporal evolution of CH4 and ethane (C2H6)
• Kai et al. (Nature, 2011) suggest changes in agricultural practices,
particularly in China, including new high yield rice species, use of more
fertilizer, and most importantly shorter water inundation periods have led
to a sharp decline in microbial release of CH4, based on the temporal
evolution of the isotopic composition of CH4
Can learn more from the Heimann News & Views piece in supplemental
reading
24Copyright © 2013 University of Maryland.
This material may not be reproduced or redistributed, in whole or in part, without written permission from Ross Salawitch or Tim Canty.

CH4 Hydrates and Permafrost
Chapter 3, Chemistry in Context
We describe and quantify an important source of CH4 - ebullition (bubbling) from northern
lakes - that has not been incorporated in previous regional or global methane budgets…
Extrapolation of measured fluxes from 16 sites to all lakes north of 45°N suggests emission
of 24.2 ±10.5 Tg CH4 yr 1. Thermokarst lakes http://en.wikipedia.org/wiki/Thermokarst
have particularly high emissions because they release CH4 produced from organic matter
previously sequestered in permafrost.
Schematic depicting future of CH4 emissions from
northern lakes, as the north changes from a permafrost-
rich landscape to a landscape free of surface permafrost.
25Copyright © 2013 University of Maryland.
This material may not be reproduced or redistributed, in whole or in part, without written permission from Ross Salawitch or Tim Canty.
http://www.sciencenews.org/view/feature/id/343202/description/The_Facts_Behind_the_Frack
CH4 (or natural gas) production
from fracking
Fracking
Airplane measurements of CH4 obtained by Anna Karion, Colm Sweeney, et al. over
Uintah Basin, Utah indicate fugitive emission of CH4 is ~9% of total fracking yield.
Break even point for “climate” is 3.2 % (we’ll explore this later)
EPA projects fugitive emission = 2.4% of total U.S. natural gas production
Can learn more from Tollefson News In Focus piece in supplemental reading
26Copyright © 2013 University of Maryland.
This material may not be reproduced or redistributed, in whole or in part, without written permission from Ross Salawitch or Tim Canty.

Nitrous Oxide: N2O
N2O Lifetime ≈ 114 yrs
27Copyright © 2013 University of Maryland.
This material may not be reproduced or redistributed, in whole or in part, without written permission from Ross Salawitch or Tim Canty.
Oxidation state represents number of electrons:
added to an element (negative #) or removed from an element (positive #)
HNO3
Nitric acid
NO3-
Nitrate
+5
NO2
Nitrogen
dioxide
+4
HONO
Nitrous acid
NO2-
Nitrite
NO
Nitric
oxide
N2O
Nitrous
oxide
N2NH3
Ammonia
+3+2+10-3
HNO3
Nitric acid
NO3-
Nitrate
+5
NO2
Nitrogen
dioxide
+4
HONO
Nitrous acid
NO2-
Nitrite
NO
Nitric
oxide
N2O
Nitrous
oxide
N2NH3
Ammonia
+3+2+10-3
Decreasing oxidation number (reduction reactions)Decreasing oxidation number (reduction reactions)
Increasing oxidation number (oxidation reactions)Increasing oxidation number (oxidation reactions)
See http://guweb2.gonzaga.edu/faculty/cronk/chemistry/L00-index.cfm?L00resource=Lewis_structures
for Lewis Dot Structure of N2O … please note we will not ask questions
about Lewis Dot Structures on exams !
28Copyright © 2013 University of Maryland.
This material may not be reproduced or redistributed, in whole or in part, without written permission from Ross Salawitch or Tim Canty.

Sources and Sinks of N2O
http://www.esrl.noaa.gov/gmd/hats/combined/N2O.html
Chapter 7, IPCC 2007
29Copyright © 2013 University of Maryland.
This material may not be reproduced or redistributed, in whole or in part, without written permission from Ross Salawitch or Tim Canty.
N2O and Stratospheric O3
30Copyright © 2013 University of Maryland.
This material may not be reproduced or redistributed, in whole or in part, without written permission from Ross Salawitch or Tim Canty.
N2O constant @ year 2000 level
N2O rising above 400 ppb in year 2100
Portmann and Solomon, GRL, 2000

N2O and NOy
Chapter 6, WMO 1998 Ozone
Assessment Report.
Loss of N2O occurs mainly in the stratosphere, due to:
photolysis − main sink
reaction with electronically excited O(1D) − minor sink
Minor sink for N2O loss has a path that results in “fixed
nitrogen”:
N2O + O(1D) NO + NO
This is critical: source of stratospheric total fixed
nitrogen (NOy) is crucial to stratospheric chemistry
We’ll later see that nitrogen oxides catalyze loss of O3 &
participate in a series of chemical reactions that
affect partitioning of chlorine radicals, etc.
Minschwaner, Salawitch, and McElroy, JGR, 1993
31Copyright © 2013 University of Maryland.
This material may not be reproduced or redistributed, in whole or in part, without written permission from Ross Salawitch or Tim Canty.
Nitrous Oxide (N2O): The Dominant Ozone-Depleting Substance
[to be] Emitted in the 21st Century
32Copyright © 2013 University of Maryland.
This material may not be reproduced or redistributed, in whole or in part, without written permission from Ross Salawitch or Tim Canty.
http://www.sciencemag.org/content/326/5949/123.full