Advances in Local Anesthetics - dentalcetoday.com · Advances in Local Anesthetics: ... Handbook of...
Transcript of Advances in Local Anesthetics - dentalcetoday.com · Advances in Local Anesthetics: ... Handbook of...
Continuing Education
Advances in Local Anesthetics: pH Buffering and Dissolved CO2
Authored by Stanley F. Malamed, DDS, and Mic Falkel, DDS
Course Number: 149
Upon successful completion of this CE activity 2 CE credit hours may be awarded
A Peer-Reviewed CE Activity by
Opinions expressed by CE authors are their own and may not reflect those of Dentistry Today. Mention of
specific product names does not infer endorsement by Dentistry Today. Information contained in CE articles and
courses is not a substitute for sound clinical judgment and accepted standards of care. Participants are urged to
contact their state dental boards for continuing education requirements.
Dentistry Today, Inc, is an ADA CERP Recognized Provider. ADA CERP isa service of the American Dental Association to assist dental professionalsin indentifying quality providers of continuing dental education. ADA CERPdoes not approve or endorse individual courses or instructors, nor does itimply acceptance of credit hours by boards of dentistry. Concerns orcomplaints about a CE provider may be directed to the provider or to ADA CERP at ada.org/goto/cerp.
Approved PACE Program ProviderFAGD/MAGD Credit Approvaldoes not imply acceptanceby a state or provincial board ofdentistry or AGD endorsement.June 1, 2009 to May 31, 2012AGD Pace approval number: 309062
LEARNING OBJECTIVESAfter participating in this CE activity, the individual will learn: • An overview of local anesthetic pharmacology.• The benefits of ex vivo buffering of local anesthetic.
ABOUT THE AUTHORSDr. Malamed is professor of anesthesiaand medicine at the Ostrow School ofDentistry of the University of SouthernCalifornia, in Los Angeles. He is aDiplomate of the American Dental Board ofAnesthesiology as well as a recipient of the
1996 Heidebrink Award from the American Dental Society ofAnesthesi ology and the 1997 Horace Wells Award from theInternational Federation of Dental An esthesia Societies. He isan expert in dental anesthesia and pain control, the effects ofdrugs on oral health, and emergency medicine. He hasauthored more than 140 scientific papers and 17 chapters invarious medical and dental journals and textbooks in the areasof physical evaluation, emergency medicine, local anesthesia,sedation, and general anesthesia. He is the author of 3 widelyused textbooks, published by CV Mosby—Handbook ofMedical Emergencies in the Dental Office (6th edition, 2007);Handbook of Local Anesthesia (6th edition, 2012); andSedation—A Guide to Patient Management (5th edition2010)—and 2 interactive DVDs: Emergency Medicine (2ndedition, 2008) and Malamed’s Local Anesthetic Technique(2004). He can be contacted at [email protected].
Disclosure: Dr. Malamed is a paid consultant to Onpharma.
Dr. Falkel is a practicing general dentistwith offices in Monterey, California. He isa partner in the Monterey PeninsulaDental Group and serves as an adjunctprofessor of restorative dentistry at theUniversity of the Pacific’s Aurthur A.
Dugoni School of Dentistry. He was on the board ofdirectors of the Monterey Bay Dental Society from 1998 to 2005 and served as its president from 2002 to 2004. He lectures on dental anesthetic technique and the science of local anesthesia. He can be contacted [email protected].
Disclosure: Dr. Falkel is a co-founder and chief medicalofficer of Onpharma.
INTRODUCTIONLocal anesthetics are the safest and most effective drugs inmedicine for the prevention and management of pain. Thefirst widely used dental anesthetic was cocaine, thenprocaine and lidocaine. All of these local anestheticsolutions have been formulated with vasoconstrictors, most often with epinephrine, but in some cases withlevonordefrin. Other anesthetics with properties useful inspecific circumstances have been used in dentistry assubstitutes for lidocaine, such as bupivacaine (long durationof analgesia), mepivacaine (effective for a short durationwithout a vasoconstrictor), and prilocaine (effective withouta vasoconstrictor). Articaine has been available in theUnited States since 2000 and generally has the sameanesthetic characteristics as lidocaine.
Recent technical advances have made it practical toalkalinize dental anesthetic cartridges at chairside,immediately prior to injection. Alkalinization hastens theonset of analgesia and reduces injection pain, making thescience of buffering local anesthetic worthy of considerationby dentists interested in anesthesia that is more rapid, moreefficient, and more predictable, as well as being morecomfortable for the patient.
This article provides an overview of local anestheticpharmacology, with an emphasis on pH, dissociation constant(pKa), bicarbonate buffering, and the collateral benefitsprovided by the dissolved CO2 that is created in the bolus ofthe injection as a result of the bicarbonate buffering process.
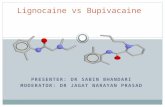
ANESTHETIC FORMULATIONS The molecular structures of typical local anesthetics are shownin Figure 1. All local anesthetics are amphipathic; that is, theypossess both lipophilic and hydrophilic characteristics,
Continuing Education
1
Advances in Local Anesthetics:pH Buffering and Dissolved CO2Effective Date: 05/1/2012 Expiration Date: 05/1/2015
generally at opposite ends of themolecule. The largest portion of themolecule is the lipophilic portion. At theother end of the molecule is the hydro -philic portion. The anesthetic structureis completed by an intermediatehydrocarbon chain containing either anester or an amide linkage.
Local anesthetics are classifiedas either amino esters or aminoamides by their intermediate chain.The nature of this intermediatelinkage is important in definingseveral properties of the local anesthetic, including thebasic mode of biotransformation. Ester-linked localanesthetics (Figure 1) such as procaine and tetracaine arereadily hydrolyzed in aqueous solution. Amide-linked local anesthetics (Figure 1) such as lidocaine, articaine,mepivacaine, prilocaine, and bupivacaine are relativelyresistant to hydrolysis and, as prepared in the laboratory,are poorly soluble in water. Amides are also unstable onexposure to air. Being weakly basic, these anestheticsreadily combine with acids to form local anesthetic salts, inwhich form they are quite soluble in water andcomparatively stable.
THE pH OF COMMERCIAL LOCAL ANESTHETICS FOR DENTISTRYIn compounding local anesthetics for dental injection,manufacturers first combine the local anesthetics withhydrochloric acid (HCl) to form their hydrochloride salts (eg,lidocaine HCl), and then these salts are dissolved in sterilewater. These “plain” local anesthetic solutions typically havea pH of about 5.5.
However, vasopressors (such as epinephrine) are addedto the most commonly used dental anesthetics in order toinhibit circulation in the area of the injection, thereby limitingsystemic uptake, decreasing toxicity, reducing bleeding, andextending the duration of analgesia. Epinephrine oxidizesreadily at more alkaline pH levels and accordingly, lidocainewith epinephrine in anesthetic cartridges has beencommonly distributed with sufficient HCl to achieve a pHbetween 3.3 and 5.5, per the United States Pharm acopeia
(USP) Com pendia of Standards.1
This range is relatively acidic, anddental anesthetic buffering studieshave measured cartridges toward thebottom of the range, at pH 3.5,2 aswell as below the USP range at 2.92.3
As oxidation occurs in commercialanesthetic cartridges during storage,either at the manufacturer or on ashelf in a dental office, the cartridgesbecome more acidic and presumablylose some of their epinephrine todegradation over time, primarily
through oxidation.4 Hondrum and Ezell4 studied thechanges that occur in dental anesthetic cartridges afterthey are manufactured and found that, among 9 lots ofcartridges of lidocaine with epinephrine they tested (all ofwhich were before their expiration dates), one measuredbelow the USP minimum at 2.86.4 The loss of epinephrineover time through oxidation may account for theobservation by some practitioners that their anestheticsbecome less effective toward their expiration dates.
DISSOCIATION OF LOCAL ANESTHETICS Local anesthetic salts (eg, lidocaine HCl) are dissolved ineither sterile water or saline and are both water soluble andstable. In solution, these anesthetics exist simultaneouslyas uncharged or deionized (RN) molecules and charged orcationic (RNH+) molecules. The deionized lipo philic base issometimes called the active form5 of the anesthetic. Therelative proportion of each ionic form in the solution varieswith the pH of the solution or surrounding tissues.
In the presence of a high concentration of hydrogen ions(at a lower more acidic pH), the equilibrium shifts to the left andmost of the anesthetic solution exists in the charged form:
RNH+ RN + H+
As hydrogen ion (H+) concentration decreases (at ahigher or more alkaline pH), the equilibrium shifts righttoward the active or free base form:
RNH+ RN + H+
The relative proportion of ionic forms also depends on thepKa, or dissociation constant, of the specific local anesthetic.The pKa is a measure of a molecule’s affinity for hydrogen ion
Continuing Education
2
Advances in Local Anesthetics: pH Buffering and Dissolved CO2
Figure 1. Molecular structures of injectable localanesthetics for dentistry.
COOR1R
ESTERS:
AMIDES:
Lipophilicpart
Intermediatechain
Hydrophilicpart
R R2
R2
N
R3
NHCO
R3
N
R4
(H+). When the pH of the solution hasthe same value as the pKa of the localanesthetic, exactly 50% of the drugexists in the RNH+ form and 50% inthe RN form. The percentage of drugexisting in either form can bedetermined from the Henderson-Has selbalch equation. The roomtemperature pKa of lidocaine is 7.9,Articaine is 7.8, and mepivacaine is7.6 (Table 1).
LIPID SOLUBILITY ANDDIFFUSIONLipid solubility of a local anesthetic appears to be related toits intrinsic potency. The active form (RN) of local anestheticis 4,000 times more lipid soluble than the cationic form(RNH+).6 Higher lipid solubility permits the active RN form ofanesthetic to penetrate tissues and the nerve membrane(which itself is 90% lipid) more easily, while the nervemembrane presents a barrier to the cationic RNH+ form.
PHYSIOLOGY, ANATOMY, AND THE KINETICS OFLOCAL ANESTHETICSThe Diffusion Process—After depositing the local anestheticas close to the nerve as possible, solution will diffuse in alldirections according to prevailing concentration gradients. Aportion of the injected local anesthetic diffuses toward thenerve and may cross the axonal membrane. However, asignificant portion of the injected drug also diffuses awayfrom the nerve. The following reactions occur after depositionof anesthetic:
l Some of the drug is absorbed by nonneural tissues(eg, muscle and fat)
l Some is diluted by interstitial fluidl Some is removed by capillaries and lymphatics from
the injection site (uptake)l Some reaches the nerve.The sum total of the first 3 factors is to decrease the local
anesthetic concentration outside the nerve; however, theconcentration of local anesthetic within the nerve continues torise over time as diffusion into the nerve progresses.
The Impulse Blocking Process—The function of a nerveis to carry messages from one part of the body to another.In dentistry, the key concern is to temporarily disable thetransmission or propagation of pain im pulses to the brainfrom the area of treatment. The pain messages the clinicianwishes to block are carried by nerves in a complexelectrochemical process that is itself worth separate study,but cannot be addressed in detail here. For the purposes ofthis discussion, the key understanding is that nerveimpulses propagate along myelinated nerves by jumpingfrom node of Ranvier to node of Ranvier (Figure 2). Asanesthetic reaches the nerve, active RN molecules diffuseacross the nerve sheath at the nodes of Ranvier and enterthe axoplasm. Be cause the axoplasm is more acidic thanthe surrounding tissue, some of the RN molecules that crossthe nerve membrane will obtain a charge (H+) in theaxoplasm and will become RNH+ molecules, which arecapable of binding to receptor cites in the sodium channels,inhibiting impulse propagation. The diffusion processcontinues until an equilibrium results between theintraneural and extraneural concentrations of anestheticsolution. If sufficient anesthetic enters the nerve, theimpulses at a node of Ranvier may be completely blocked.Because the nerve impulses jump from node of Ran vier tonode of Ranvier and be cause these impulses are capable ofjumping more than one node, complete analgesia is said torequire blockade of at least 3 nodes of Ranvier,7 or aminimum of 8 mm of the nerve.8
Continuing Education
3
Advances in Local Anesthetics: pH Buffering and Dissolved CO2
Table 1. Dissociation Constants (pKa)Local Anesthetics Used in Dentistry
Local Percent Base FormAnesthetic or “Active Form”Agent pKa (RN) at pH 7.4
Mepivacaine 7.6 39%
Articaine 7.8 28%
Lidocaine 7.9 24%
Prilocaine 7.9 24%
Bupivacaine 8.1 17%
INCREASES IN pH AND THE ACTIVERN FORM OF LOCAL ANESTHETICAs discussed in the dissociation sectionabove, the pH of the anesthetic solutionaffects the relative amount of the active RNform of anesthetic available in the bolus of theinjection. Because local anesthetic solutionsexist in a dynamic equilibrium, some of themolecules will be in the active RN form andsome of the molecules will be in the inactiveRNH+ form. The ratio of RN and RNH+
molecules in a given volume of localanesthetic is dependent on the pH of thesolution per the Henderson-Hasselbalchequation; at a typical pH of commerciallidocaine solutions containing epinephrine(3.5), there will be approximately onemolecule of active RN anesthetic for every 25,000 moleculesof the inactive cationic RNH+ form. At a physiologic pH of 7.4,the ratio of active anesthetic (RN) is vastly im proved toapproximately one molecule of active RN form for every 3molecules of the cationic RNH+ form.
ONSET TIME AND THE pH OF LOCAL ANESTHETICINJECTIONS Onset time for a peripheral block is the period of time fromdeposition of the anesthetic solution to complete analgesiaof the treatment area. The pH of the anesthetic solutiondetermines the initial availability of the active RN form of theanesthetic in a given injection. Since the body’s mechanismfor raising the pH of injected anesthetic depends upon thebicarbonate found in the tissues and fluids at the injectionsite, the rate at which buffering occurs after a traditionallocal anesthetic injection will be dependent upon theindividual’s physiology, as well as the state of the tissues inthe area of the injection. Notably, infected tissue may havea pH as low as 5.0,5,9 which may explain why infected teethare sometimes reported to be more difficult or evenimpossible to numb.
OPTIMIZING ACTIVE ANESTHETIC BEFOREINJECTION: pH BUFFERINGIn clinical situations, the pH of the anesthetic at the time of
injection has been shown to be a strong determinant ofanesthetic latency. Raising the pH of local anesthetic (alsocalled “anesthetic buffering” or “alkalinization”) immediatelyprior to injection using a bicarbonate solution represents analternative to relying on the body to accomplish theequivalent pH change after injection. The ex vivo process(outside the body) uses the same chemical mechanism andmolecule (NaHCO3) as the body will use to buffer theanesthetic after the injection in vivo (inside the body), butthe ex vivo process represents a way to accomplish the pHchange instantaneously and more dependably.
Alkalinization has been part of the local anestheticliterature for more than 100 years. The first clinical report ofimproved onset time by combining sodium bicarbonatesolution with procaine with epinehrine was by Gros in1910.10 The first anesthesia textbook reference toanesthetic buffering (adding sodium bicarbonate toprocaine with epinephrine) was made by Gwathmey andBaskerville in 1914.11
Today, leading textbooks in medical anesthesia12-16 andlocal anesthesia for dentistry17 teach that alkalinization isthe most effective method for improving anesthetic onsettime. Among anesthesiologists, local anesthetic buffering isconsidered a sufficiently fundamental skill that it is taught inpreparation for the An esthesiology Boards.18-22 Until recentadvances in technology made it expedient to buffer dental
Continuing Education
4
Advances in Local Anesthetics: pH Buffering and Dissolved CO2
Figure 2. Structure of a myelinated nerve and nodes of Ranvier, with impulse propagation.(Redrawn from Malamed SF. Handbook of Local Anesthetics. 5th edition. Elsevier Mosby.2004;7.)
anesthetic cartridges at chairside, buffering local anestheticin dentistry had not been considered practical.
To illustrate the impact that ex vivo buffering can haveon latency, following are 2 examples of anesthetic diffusioninto the axoplasm using lidocaine with epinephrine bufferedto physiologic pH (Example A), and nonbuffered standardlidocaine with einephrine (Example B).
EXAMPLE A (BUFFERED INJECTION) 1. Presume the injection contains 100,000 molecules oflidocaine ap proximately at physiologic pH (7.4), and that theinjection is made at or near the inferior alveolar nerve (IAN).
2. From the Henderson-Hassel balch equation, we cancalculate that 25% (25,000) molecules will be in the activeRN lipid soluble form, and 75% (75,000 molecules) will bein the cationic RNH+ form. Theoretic ally, all 25,000 RNmolecules could diffuse through the nerve sheath to reachthe interior (axoplasm) of the neuron and block nerveconduction.
3. After the RN molecules cross the nerve sheath intothe more acidic environment of the axoplasm, they readilytake on a hydrogen ion (H+), becoming RNH+ moleculeswhich are unable to diffuse back out across the nervemembrane. Thus, most of these 25,000 molecules aretrapped in the axon, a process that is called “ion trapping.”As the following steps show, ion trapping is a process thathelps anesthetic molecules accumulate in the axoplasm,and is a key to ultimately achieving an anestheticconcentration inside the axon sufficient to create andmaintain a block.
4. Meanwhile, outside the neuron, during this first cycle,the equilibrium between RNH+ and RN molecules has beendisrupted by the passage of the 25,000 active RN moleculesinto the neuron. The remaining 75,000 extracellular RNH+
molecules now re-equilibrate per the Henderson-Hasselbalch equation. The calculation to re-achieve the75%:25% ratio dictated by the tissue’s pH of 7.4 is:
RNH+ (57,000 molecules) RN (18,000 molecules) + H+
5. The 18,000 newly created active RN moleculesdiffuse into the cell, and 57,000 remain outside the axon,where the re-equilibration process described in Step 3eventually occurs again as follows:
RNH+ (42,750 molecules) RN (14,250 molecules) + H+
6. After the 14,250 newly created RN molecules enter theaxon, a third re-equilibration cycle occurs among the 42,750RNH+ molecules, as follows:
RNH+ (32,062 molecules) RN (10,688 molecules) + H+
7. To summarize, after 3 re-equilibration cycles, about70,000 molecules of lidocaine are trapped in the axon.Theoretically, these cycles continue until all local anestheticmolecules diffuse into the axoplasm. Of course, in reality, notevery anesthetic molecule reaches the interior of the neuronthrough diffusion. Some are not in contact with the membrane,and some of the extra-axonal RN and RNH+ molecules will beabsorbed into local blood vessels and carried off before theycan cross the nerve membrane (preventing this “uptake” is akey benefit of the vasoconstrictor). In any case, it is clear thatgiven enough time, much of the injected anesthetic willeventually be converted to the active RN form and will enterthe nerve axoplasm.
8. Ultimately, when 3 successive nodes of Ranvier areblocked, analgesia is achieved.
EXAMPLE B (STANDARD INJECTION)1. Presume the injection of 100,000 molecules of lidocaineat commercial pH (3.5) at or near the IAN.
2. From the Henderson-Hassel balch equation, we cancalculate that 0.004% (400) molecules will be in the activeRN lipid soluble form, and 99.996% (99,600 molecules) willbe in the inactive RNH+ form. Theoretic ally, all 400 of the RNmolecules could diffuse through the nerve sheath to reachthe interior (axoplasm) of the neuron.
3. After 3 re-equilibration cycles, about 1,600 RNmolecules have been ion trapped, compared to the 70,000molecules that were ion trapped in the buffered anestheticexample. At this rate, in order to achieve the same 70,000RN molecules in the axon, it would require approximatelyanother 175 re-equilibration cycles.
4. However, even as these re-equilibration cycles areoccurring, 2 other things are happening: (a) The body’stissues and fluids are gradually raising the pH of theextracellular anesthetic molecules—a process that isdependent upon the patient’s physiology; and (b) Thevasculature in the area of the injection is carrying some of theanesthetic away in the uptake process, which is dependentupon the patient’s anatomy.
Continuing Education
5
Advances in Local Anesthetics: pH Buffering and Dissolved CO2
5. At this point it becomes very difficult to estimate therate of ion trapping, which like latency, varies widely frompatient to patient. Elimi nating this variability, and therebyincreasing predictability, is one of the most attractive
features of buffering anesthetic outside the body.6. In the second example, sufficient anesthetic may be
eventually created via re-equilibration and in vivo bufferingsuch that a successful block is created. According to
Continuing Education
6
Advances in Local Anesthetics: pH Buffering and Dissolved CO2
Glossary of Key Dental Anesthetic Terms
Alkalinization The process of raising the pH of an acid anesthetic solution toward physiologic pH (7.4)
Analgesia The state of complete absence of the sensation of pain
Anatomical Miss When anesthetic is deposited in a place where the desired nerve does not exist
Anesthetics Substances used to achieve analgesia
Anesthetize Create a state of analgesia
Anesthesia The process of achieving analgesia
Anesthetic Block Analgesia created by disrupting nerve impulses in 3 consecutive nodes of Ranvier
Anesthetic Success Instances in which the patient has achieved analgesia by the time the dentist is ready to proceed
Anesthetic Failure Instances in which the patient has not achieved analgesia when the dentist is ready to proceed
Extra-Axonal Outside the nerve sheath
EPT Electric Pulp Tester
IANB (or IAN Block) Inferior Alveolar Nerve Block (also sometimes “Mandibular Block”)
Incomplete Anesthesia A reduction in the sense of pain without complete analgesia
Infiltration Anesthesia Injection of anesthetic directly into the soft tissues to be anesthetized
Injection Pain (3 types) Needle Insertion Pain The first phase of injection pain—caused by initial penetration of tissue
Needle Advancement Pain The second phase—caused by advancing the needle to the injection site
Anesthetic Deposition Pain The third phase—caused by the delivery of anesthetic solution into tissue
Lipsign Achieving soft-tissue analgesia of the lower lip after an IANB
Onset [of Analgesia] The moment that complete analgesia is achieved
Onset Time (also “Latency”) The amount of time between injection, and onset of analgesia
Pulpal Analgesia Absence of sensation of pain in all tooth structures
Regional Block Blocking a nerve bundle that serves a specific anatomical area or structure
Soft-Tissue Analgesia Absence of pain in all tissues except tooth structures
Visual Analog Scale A linear measurement of patient-reported pain ranging from “none” to “worst imaginable”
Fer nandez and colleagues,23 for any one patient, this willtake between 5 and 45 minutes and, if the practitioner waitsthe full 45 minutes, success will be achieved 95% of the time.
These examples show that there is definitely room forimproving the physiologic process of diffusion and bufferingthat must occur before an anesthetic injection createsanalgesia. Many of the leading textbooks in local anesthesiarecommend bicarbonate buffering to hasten onset ofanalgesia. In addition, 8 peer-reviewed published studiesinvolving a total of 620 patients have evaluated the efficacy ofbuffering commercially prepared lidocaine with epinephrine inorder to hasten the onset of analgesia, and all 8 concludedthat buffering significantly improved latency.2,3,24-29 InDecember 2010, the Cochrane Col laboration published theresults of its systematic review of buffering lidocainesolutions with sodium bicarbonate, concluding that ex vivo buf -fering is a safe and effective method for reducing localanesthetic injection pain.30
THE COLLATERAL BENEFITS OF BICARBONATEBUFFERING: CO2The process of buffering a local anesthetic containinghydrochloric acid using bicarbonate creates salt, water, andfree carbon dioxide as byproducts of the buffering reaction.Investigators have shown that creating dissolved CO2 in thelocal anesthetic has benefits in addition to raising the pH ofthe solution before injection. Catchlove31 demonstrated thatfree CO2 in lidocaine solution had an independentanesthetic effect, which was similar to the effect thatlidocaine had on peripheral nerves. He31 suggested thatwhere a solution contains both lidocaine and free CO2, it isthe CO2 that may cause the more immediate form ofanalgesia, writing:
“Since CO2 diffuses rapidly through the sheath and hasan independent anesthetic effect, it probably reaches theaxon before the local anesthetic, causing the earliest phaseof the block.”
In addition to the independent anesthetic effect identified byCatch love,31 Bokesch et al32 demonstrated that CO2 served asa catalyst for the anesthetic process using lidocaine. Their studyshowed a significantly more profound conduction block whenfree CO2 was present in lidocaine solution. Raymond and
colleagues33 reported that lidocaine was twice as potent in thepresence of free CO2. Condouris and Shakalis34 reported thatfree CO2 caused a tenfold in crease in procaine action.
SUMMARYThe physiology, anatomy, and the kinetics of the time courseof local anesthetics suggest that the body’s processes ofconverting the cationic RNH+ form of local anesthetic to theactive RN form are responsible for significant delay,uncertainty and inconsistency in anesthesia. Taking apatient’s physiology out of the latency equation by bufferingthe local anesthetic solution at chairside immediately prior toinjection is an attractive alternative to waiting for the body toaccomplish the same buffering process. Ex vivo bicarbonatebuffering has been studied and written about for more than100 years, it is a process recommended by the authors of theleading anesthesiology textbooks, and it is a process that hasbeen evaluated in a recently published Systematic Review bythe Cochrane Collabor ation,30 which concluded that thesodium bicarbonate buffering of lidocaine is safe andeffective for reducing injection pain.
Although it is clear that there are significant benefits tobuffering the lidocaine solutions that represent the goldstandard in dental anesthetics, the problem with adoption ofthis technique in dentistry (versus medical specialties suchas emergency medicine where preinjection anestheticbuffering is common) has been that dental anestheticcartridges are sealed containers that do not lendthemselves to conveniently and precisely adding sodiumbicarbonate solution prior to delivering the injection.35 Thescience of buffering and the need for more rapid andpredictable onset (as well as for more comfortableinjections) in dentistry has led to the development of atechnology for buffering lidocaine with epinephrine atchairside using the Onset Mixing Pen with Sodium Bi -carbonate Injection, 8.4% USP Neutralizing AdditiveSolution, both products by Onpharma (Figure 3).
CLINICAL RECOMMENDATIONS FOR PRACTITIONERSBuffer Cartridge Immediately Before Delivering theInjection—According to Catchlove,31 Bokesch et al,32 Con -douris and Shakalis,34 Ibusuki et al,36 and others as
Continuing Education
7
Advances in Local Anesthetics: pH Buffering and Dissolved CO2
referenced previously, the free CO2 in solutionthat is created during the anesthetic bufferingprocess plays a meaningful role in theimproved performance of buffered localanesthetics. Delay in administering thebuffered anesthetic can cause some of theCO2 to come out of solution and adhere to theglass of the cartridge, reducing the benefitsby eliminating the CO2 from the bolus of theinjection. Because of this, buffered anestheticshould be prepared chairside only when theclinician is ready to administer the injection.By no means should a practitioner or staffbuffer cartridges in advance of procedures.
Buffer Every Injection—Practition ersshould buffer each of their injections thatinclude lidocaine with epinephrine. Whether theinjection technique is an IANB, a palatalinjection, an intraligamentary injection, amaxillary infiltration, a buccal infiltration, oranother technique, raising the pH of theinjection will increase the active anesthetic availableimmediately at the time of the injection and will eliminate theneed for the body to buffer the anesthetic toward physiologicpH as part of the process of achieving analgesia. This will limitthe uncertainty that is always inherent in any process thatrelies upon the patient’s unique physiology. It will also raise thepH of the injection toward physiologic, reducing the “bee stingeffect” that some patients experience, even for thosepractitioners who generally deliver comfortable injections.
Set Aside 15 to 20 Minutes at the Start of the First Dayof Use to Train Staff on Buffering—The cartridge bufferingmethod and armamentarium de scribed above can be usedto buffer dental anesthetic cartridges in less than 5 secondsat chairside. However, dentists should spend time with staffthat may either buffer the anesthetic cartridges or assist thedentist in buffering the anesthetic, so that each personinvolved can review and discuss the package insert for theneutralizing solution, review the instructions for use, andreview the dentist’s aseptic technique. Key points of em -phasis in the discussion are confirming the volume ofbuffering solution to use (per table in the package insert)
and noting that buffering is only indicated for use withcartridges of lidocaine with epinephrine. Staff shouldpractice buffering a few cartridges to get the feel for theprocess of connecting the anesthetic cartridge to the pen,dialing the pen, buffering the anesthetic cartridge, and thenremoving the anesthetic cartridge from the pen to beloaded into the dental syringe or other delivery device.
Try Staying with the Patient—The improved onset timeallows practitioners to stay with the patient, as animprovement over the normal regime of leaving the patientafter administering the injection, handling other tasks outsidethe operatory for a period of time, and then returning,regloving, and beginning the procedure. Formerly, theduration of the latency period made it impractical to stay withthe patient until analgesia was achieved. Doctors who haveused chairside buffering long enough to become familiar withits faster onset are finding it practical to remain with thepatient and begin work without interrupting the process toleave the operatory. These practitioners have reported that bysimply staying with the patient and completing the procedurewithout leaving, they are able to move the activities normally
Continuing Education
8
Advances in Local Anesthetics: pH Buffering and Dissolved CO2
Figure 3. Onpharma’s Onset Mixing Pen and Sodium Bicarbonate Injection 8.4% USPNeutralizing Additive Solution. The pen accommodates cartridges of Onpharma’sbicarbonate solution and can be used to precisely buffer a dental anesthetic cartridgecontaining lidocaine/epi (shown). The buffering process takes 5 seconds at chairside andshould be done immediately before loading the dental syringe and delivering the anestheticusing the practitioner’s standard injection technique.
handled during the latency period (hygiene exams, checkinge-mail, etc) to the back end of the appointment, and that theyare ending up with a meaningful net gain in time, which isintuitive, considering the disruption in workflow inherent inleaving/returning to the operatory. They also report that theirnormal “interim tasks” are less hurried and/or stressful whena pa tient is not waiting for their return in the operatory chair.These are anecdotal reports, of course, but they may behelpful for practitioners who are wondering what practicalexperiences they may have if they evaluate buffering in theirpractices. Notably, practitioners report that patients arecommenting on the difference it makes in their perception ofthe care they are receiving when their dentists stay with themin the operatory after delivering their injection.
CONCLUSIONConsidering the central role that local anesthesia plays in atypical dental office, reducing latency and variability in thelocal anesthesia regime can improve patient care andreduce stress on the practitioner. Buffering local anestheticcan be an important tool for practitioners looking to improvetheir local anesthesia regime.
REFERENCES1. United States Pharmacopeial Con vention. The
Pharmacopeia of the United States. 26th ed. Rockville,MD: The United States Pharmacopeial Convention; 2003.
2. Al-Sultan FA, Fathie WK, Hamid RS. A clinicalevaluation on the alkalization of local anestheticsolution in periapical surgery. Al-Rafidain Dent J.2006;6:71-77.
3. Kim TH, Kim KW, Kim CH. A clinical study ofanesthetic efficacy of alkalinizing lidocaine in inferioralveolar nerve blocks. J Korean Assoc Maxillofac PlastReconstr Surg. 2005;27:276-282.
4. Hondrum SO, Ezell JH. The relationship between pHand concentrations of antioxidants andvasoconstrictors in local anesthetic solutions. AnesthProg. 1996;43:85-91.
5. Rood JP. The use of buffered lignocaine solution in thepresence of acute inflammation. J Dent. 1977;5:128-130.
6. Liepert DJ, Douglas MJ, McMorland GH, et al.Comparison of lidocaine CO2, two per cent lidocainehydrochloride and pH adjusted lidocaine hydrochloridefor caesarean section anaesthesia. Can J Anaesth.1990;37:333-336.
7. Hargreaves KM, Keiser K. Local anesthetic failure inendodontics: mechanisms and management.Endodontic Topics. 2002;1:26-39.
8. Franz DN, Perry RS. Mechanisms for differential blockamong single myelinated and non-myelinated axonsby procaine. J Physiol. 1974;236:193-210.
9. De Jong RH, Cullen SC. Buffer-demand and pH oflocal anesthetic solutions containing epinephrine.Anesthesiology. 1963;24:801-807.
10. Gros O. About anesthetics and local anesthetics [inGerman]. Arch Ecper Path Pharmakol. 1910;62:80-106.
11. Gwathmey JT, Baskerville C. Anesthesia. New York,NY: D. Appleton and Company; 1914.
12. Barash PG, Cullen BF, Stoelting RK. ClinicalAnesthesia. 4th ed. Philadelphia, PA: Lippincott,Williams & Wilkins; 2001.
13. De Jong RH. Local Anesthetics. St. Louis, MO: Mosby;1994.
14. Miller RD. Miller’s Anesthesia. 6th ed. New York, NY:Elsevier/Churchill Livingstone; 2005.
15. Stoelting RK, Miller RD. Basics of Anesthesia. 5th ed.Philadelphia, PA: Churchill Livingstone; 2007.
16. Vacanti CA, Sikka P, Urman R, et al. Essential ClinicalAnesthesia. New York, NY: Cambridge UniversityPress; 2011.
17. Malamed SF. Handbook of Local Anesthesia. 5th ed.St. Louis, MO: Mosby Elsevier; 2004.
18. Bhatt H, Powell KJ, Jean DA. First Aid for theAnesthesiology Boards: An Insider’s Guide. New York,NY: McGraw-Hill Medical; 2011.
19. Chu LF. Clinical Anesthesiology Board Review: A TestSimulation and Self-Assessment Tool. New York, NY:McGraw-Hill Medical; 2005.
20. Hall BA, Chantigian RC; Mayo Foundation for MedicalEducation and Research. Anesthesia: AComprehensive Review. Maryland Heights, MO:Mosby Elsevier; 2010.
21. Lovich-Sapola JA, ed. Anesthesia Oral Board Review:Knocking Out the Boards. New York, NY: CambridgeUniversity Press; 2010.
22. Robertson KM, Lubarsky DA, Ranasinghe S.Anesthesiology Board Review. 2nd ed. New York, NY:McGraw-Hill Medical; 2006.
23. Fernandez C, Reader A, Beck M, et al. A prospective,randomized, double-blind comparison of bupivacaineand lidocaine for inferior alveolar nerve blocks. JEndod. 2005;31:499-503.
24. DiFazio CA, Carron H, Grosslight KR, et al.Comparison of pH-adjusted lidocaine solutions forepidural anesthesia. Anesth Analg. 1986;65:760-764.
Continuing Education
9
Advances in Local Anesthetics: pH Buffering and Dissolved CO2
25. Kanaya N, Imaizumi H, Matsumoto M, et al.Evaluation of alkalinized lidocaine solution in brachialplexus blockade. J Anesth. 1991;5:128-131.
26. Benzon HT, Toleikis JR, Dixit P, et al. Onset, intensityof blockade and somatosensory evoked potentialchanges of the lumbosacral dermatomes afterepidural anesthesia with alkalinized lidocaine. AnesthAnalg. 1993;76:328-332.
27. Mehta R, Verma DD, Gupta V, et al. To study the effect ofalkalinization of lignocaine hydrochloride on brachialplexus block. Indian J Anaesth. 2003;47:283-286.
28. Al-Sultan FA. Effectiveness of pH adjusted lidocaineversus commercial lidocaine for maxillary infiltrationanesthesia. Al-Rafidain Dent J. 2004;4:34-39.
29. Gormley WP, Hill DA, Murray JM, et al. The effect ofalkalinisation of lignocaine on axillary brachial plexusanaesthesia. Anaesthesia. 1996;51:185-188.
30. Cepeda MS, Tzortzopoulou A, Thackrey M, et al.Adjusting the pH of lidocaine for reducing pain oninjection. Cochrane Database Syst Rev. 2010 Dec8:CD006581.
31. Catchlove RF. The influence of CO2 and pH on localanesthetic action. J Pharmacol Exp Ther.1972;181:298-309.
32. Bokesch PM, Raymond SA, Strichartz GR.Dependence of lidocaine potency on pH and pCO2.Anesth Analg. 1987;66:9-17.
33. Raymond S, Wong K, Strichartz G. Mechanisms forpotentiation of local anesthetic action by CO2:bicarbonate solutions. Anesthesiology.1989;71(suppl):A711.
34. Condouris GA, Shakalis A. Potentiation of the nerve-depressant effect of local anaesthetics by carbondioxide. Nature. 1964;204:57-58.
35. Meit SS, Yasek V, Shannon CK, et al. Techniques forreducing anesthetic injection pain: an interdisciplinarysurvey of knowledge and application. J Am DentAssoc. 2004;135:1243-1250.
36. Ibusuki S, Katsuki H, Takasaki M. The effects ofextracellular pH with and without bicarbonate onintracellular procaine concentrations and anestheticeffects in crayfish giant axons. Anesthes iology.1998;88:1549-1557.
Continuing Education
10
Advances in Local Anesthetics: pH Buffering and Dissolved CO2
POST EXAMINATION INFORMATION
To receive continuing education credit for participation inthis educational activity you must complete the programpost examination and receive a score of 70% or better.
Traditional Completion Option:You may fax or mail your answers with payment to DentistryToday (see Traditional Completion Information on followingpage). All information requested must be provided in orderto process the program for credit. Be sure to complete your“Payment,” “Personal Certification Information,” “Answers,”and “Evaluation” forms. Your exam will be graded within 72hours of receipt. Upon successful completion of the post-exam (70% or higher), a letter of completion will be mailedto the address provided.
Online Completion Option:Use this page to review the questions and mark youranswers. Return to dentalcetoday.com and sign in. If youhave not previously purchased the program, select it fromthe “Online Courses” listing and complete the onlinepurchase process. Once purchased the program will beadded to your User History page where a Take Exam linkwill be provided directly across from the program title.Select the Take Exam link, complete all the programquestions and Submit your answers. An immediate gradereport will be provided. Upon receiving a passing grade,complete the online evaluation form. Upon submitting the form, your Letter Of Completion will be providedimmediately for printing.
General Program Information:Online users may log in to dentalcetoday.com any time inthe future to access previously purchased programs andview or print letters of completion and results.
POST EXAMINATION QUESTIONS
1. Local anesthetics are classified as:
a. Amino esters.
b. Amino amides.
c. Hydrochloride salts.
d. Both a and b.
2. Procaine and tetracaine are readily hydrolyzed inaqueous solution. Lidocaine and articaine are poorly
soluble in water.
a. The first statement is true, the second is false.
b. The first statement is false, the second is true.
c. Both statements are true.
d. Both statements are false.
3. The active form of a local anesthetic is known as:
a. RN.
b. RNH+.
c. HR.
d. HR+.
4. The dissociation constant of a specific localanesthetic is known as its:
a. KPA.
b. Kp.
c. pKa.
d. pK.
5. The active form of local anesthetic is _____timesmore lipid soluble than the cationic form.
a. 1,000.
b. 2,000.
c. 3,000.
d. 4,000.
6. The USP standards for the pH of cartridges oflidcaine with epinephrine is from 3.3 to 5.5. Studiesmeasuring the pH change that occurs via oxidationduring the shelf life of cartridges measured the pHas low as:
a. 3.15.
b. 3.00.
c. 2.86.
d. 1.86.
7. According to the Henderson-Hasselbalch equation, ata pH of 3.5 the ratio of ionized anesthetic moleculesto active de-ionized anesthetic molecules is:
a. 25:1.
b. 15:1.
c. 8,000:1.
d. 25,000:1.
Continuing Education
11
Advances in Local Anesthetics: pH Buffering and Dissolved CO2
8. The following reaction occurs after deposition oflocal anesthetic:
a. Some of the drug is diluted by interstitial fluid.
b. Some of the drug absorbed by muscle and fat.
c. Some of the drug is removed from the injection site bycapillaries.
d. All of the above.
9. Complete analgesia is said to require blockade of:
a. One node of Ranvier.
b. Two nodes of Ranvier.
c. Three nodes of Ranvier.
d. Four nodes of Ranvier.
10. Infected tissues may have a pH as low as:
a. 2.5.
b. 3.5.
c. 4.0.
d. 5.0.
11. In December 2010, the Cochrane Collaborationconcluded that:
a. In vivo buffering of lidocaine solutions is the onlyeffective method for reducing local anesthetic injectionpain.
b. Ex vivo buffering of lidocaine solutions is a safe andeffective method for reducing local anesthetic injectionpain.
c. Ex vivo buffering of lidocaine solutions has noapparent effect on local anesthetic injection pain.
d. None of the above.
12. Both the in vivo and the ex vivo pH buffering of localanesthetic solution are accomplished using:
a. Hydrochloric acid.
b. Sodium bicarbonate.
c. Magnesium phosphate.
d. Carbon dioxide.
13. A byproduct of ex vivo buffering of local anestheticscontaining hydrochloric acid that plays a meaningfulrole in the improved performance of the anesthetic is:
a. Salt.
b. Water.
c. CO2.
d. All of the above.
14. Buffered lidocaine with epinephrine should beprepared chairside:
a. One hour prior to injection.
b. Up to 4 hours prior to injection.
c. Up to 8 hours prior to injection.
d. Only when the clinician is ready to administer theinjection.
15. The injection technique that can be improved bychairside buffering of lidocaine with epinephrinesolution is:
a. Inferior alveolar nerve block.
b. Palatal injection.
c. Intraligamentary injection.
d. All of the above.
16. Benefits of chairside buffering of lidocaine withepinephrine solution include:
a. Reducing the “bee sting effect.”
b. Inducing more rapid onset of analgesia.
c. Eliminating the need for the body to buffer theanesthetic.
d. All of the above.
Continuing Education
12
Advances in Local Anesthetics: pH Buffering and Dissolved CO2
PROGRAM COMPLETION INFORMATION
If you wish to purchase and complete this activitytraditionally (mail or fax) rather than online, you mustprovide the information requested below. Please be sure toselect your answers carefully and complete the evaluationinformation. To receive credit you must answer at least 12 ofthe 16 questions correctly.
Complete online at: dentalcetoday.com
TRADITIONAL COMPLETION INFORMATION:Mail or fax this completed form with payment to:
Dentistry TodayDepartment of Continuing Education100 Passaic AvenueFairfield, NJ 07004
Fax: 973-882-3622
PAYMENT & CREDIT INFORMATION:
Examination Fee: $40.00 Credit Hours: 2.0
Note: There is a $10 surcharge to process a check drawn on any bank other than a US bank. Should you have additionalquestions, please contact us at (973) 882-4700.
� I have enclosed a check or money order.
� I am using a credit card.
My Credit Card information is provided below.
� American Express � Visa � MC � Discover
Please provide the following (please print clearly):
Exact Name on Credit Card
Credit Card # Expiration Date
Signature
PROGRAM EVAUATION FORMPlease complete the following activity evaluation questions.
Rating Scale: Excellent = 5 and Poor = 0
Course objectives were achieved. Content was useful and benefited your clinical practice. Review questions were clear and relevant to the editorial. Illustrations and photographs were clear and relevant.Written presentation was informative and concise.How much time did you spend reading the activity and completing the test?
Continuing Education
Advances in Local Anesthetics: pH Buffering and Dissolved CO2
ANSWER FORM: COURSE #: 149Please check the correct box for each question below.
1. � a � b � c � d 9. � a � b � c � d
2. � a � b � c � d 10. � a � b � c � d
3. � a � b � c � d 11. � a � b � c � d
4. � a � b � c � d 12. � a � b � c � d
5. � a � b � c � d 13. � a � b � c � d
6. � a � b � c � d 14. � a � b � c � d
7. � a � b � c � d 15. � a � b � c � d
8. � a � b � c � d 16. � a � b � c � d
PERSONAL CERTIFICATION INFORMATION:
Last Name (PLEASE PRINT CLEARLY OR TYPE)
First Name
Profession / Credentials License Number
Street Address
Suite or Apartment Number
City State Zip Code
Daytime Telephone Number With Area Code
Fax Number With Area Code
E-mail Address
/
Dentistry Today, Inc, is an ADA CERP RecognizedProvider. ADA CERP is a service of the AmericanDental Association to assist dental professionals inindentifying quality providers of continuing dentaleducation. ADA CERP does not approve or endorseindividual courses or instructors, nor does it implyacceptance of credit hours by boards of dentistry.Concerns or complaints about a CE provider may bedirected to the provider or to ADA CERP atada.org/goto/cerp.
Approved PACE Program ProviderFAGD/MAGD Credit Approvaldoes not imply acceptanceby a state or provincial board ofdentistry or AGD endorsement.June 1, 2009 to May 31, 2012AGD Pace approval number: 309062
13