1. Polyalveolar lobe with emphysema
-
Upload
nguyencong -
Category
Documents
-
view
215 -
download
0
Transcript of 1. Polyalveolar lobe with emphysema

Thorax (1970), 25, 682.
New pathological findings in emphysema ofchildhood: 1. Polyalveolar lobe with emphysema
ALISON HISLOP and LYNNE REID
Department of Experimental Pathology, Institute of Diseases of the Chest,Brompton Hospital, London, S.W.3
A new pathological entity is here described-a polyalveolar lobe with or without emphysema-giving rise to the clinical features of childhood lobar emphysema.A detailed and quantitative study of the airways, alveoli and arteries was carried out on the
left upper lobe removed because of shortness of breath, thought to be due to 'childhood lobaremphysema'. The child was 17 days old and the radiograph showed hypertransradiancy of theleft lung. The alveolar number was increased five-fold. Alveolar size was normal, so it was
found that emphysema, accepted today as a structural diagnosis, was not present. The increasein alveolar number seemed confined to the apical, posterior, and anterior segments, the lingulabeing unaffected. By contrast, the airways and arteries were normal for age in number, sizeand structure, suggesting that the condition was a 'giantism' of the alveolar region. The bloodflow was probably too low for the lobar volume; certainly the excessive alveolar number couldnot be due to increase in blood flow.
In two further specimens, previously dissected, a similar polyalveolar condition was found,associated with emphysema. The patients were older at the time of surgical resection and theemphysema may have developed post-natally. In all three cases the radiographic features hadsuggested emphysema. It is suggested that the condition be called 'polyalveolar lobe', 'withemphysema' or 'without emphysema' being added as a separate item to the description.
Infantile lobar emphysema (also called congenitallobar emphysema and congenital obstructiveemphysema) usually presents clinically in earlychildhood because of acute dyspnoea. The radio-logical appearance is of a hypertransradiant lobewith compression of the remainder of the lungand contralateral displacement of the heart.
It has been suggested that the condition arisesfrom (1) infection damaging alveoli (Leape andLongino, 1964), (2) developmental abnormality ofalveoli (Bolande, Schneider, and Boggs, 1956), (3)an obstruction to the lobar bronchus perhaps frominspissated secretion (Thomson and Forfar, 1958),or (4) from abnormal collapsibility of the airwaydue to absence of cartilage (Bolande et al., 1956)or abnormality of cartilage (Stovin, 1959).
It has recently been emphasized that clinicalfeatures of infantile lobar emphysema may arisefrom several different pathological entities (Reid,1967a). For example, atresia of the apico-posteriorbronchus of the left upper lobe, as reported in theadult (Simon and Reid, 1963; Waddell, Simon,and Reid, 1965), has also been seen by one of us
as the cause of this clinical presentation in thechild.The purpose of this paper is to report in detail
on the operation specimen from a patient present-ing clinically as infantile lobar emphysema, andto refer to two additional cases, all arising froma lung abnormality not previously described-alocalized gross increase in alveolar number repre-senting virtually 'giantism' of the alveolar region.The term we suggest to describe this condition is'polyalveolar lung', with or without emphysema.This finding is based on a detailed study of theairways, arteries and alveoli. Methods of examina-tion not previously applied in this condition havebeen used, and the way in which these have beencombined to give the maximum amount of infor-mation is demonstrated.
MATERIAL AND METHODS
Study in detail of the left upper lobe from a patientwith congenital lobar emphysema (case 1) stimulatedre-examination of two other left upper lobe specimens,
682

Polyalveolar lobe with emphysema
though in less detail, since they had already beenfixed and cut (cases 2 and 3).
Planimetry was used on the antero-posterior andlateral radiographs of case 1 to assess the increase inlobar volume in life by comparison with the expectedvolume of the lobe.
Analysis of the airways and arteries demands someidea of their three-dimensional branching pattern andof their size and structures. Methods of alveolar quan-tification after lung sampling, based on the methodsof Chalkley (1943) and recently popularized by Weibeland Gomez (1962) and Dunnill (1962a), are com-plementary to this analysis.
In the first specimen examined the artery wasinjected by hand, with a Micropaque gelatin suspen-sion, according to the method of Elliott (see Reid,1967a). The lung was then inflated and fixed by intra-bronchial injection of buffered formol saline. Thespecimen was radiographed, and the arteriogramrevealed the overall pattern of arterial branching andcould be used to measure lumen size both at thehilum and progressively along its course (Millard,1965; Davies, 1969).The volume of the freshly inflated specimen was
recorded and also the volume after fixation and beforeslicing. This specimen was fixed for six months beforeit was sliced, although one week's fixation suffices.For quantitation the lung is usually sliced throughits largest plane, i.e., roughly parallel to the medi-astinal surface. On this occasion the specimen wassliced into 1 0-cm. slices in a transverre plane so thatthe axial artery and airway of the posterior segmentpassed roughly at right angles to the plane of section,which facilitated taking blocks throughout the lengthof these structures. Random selection of blocks wasmade but additional blocks were taken so that thewhole of the axial pathway from the posterior seg-ment of the upper lobe and tissue from each of theother segments was included.
Before any blocks were taken, macroscopic quan-tification of the following lung structures was madceby a point-counting technique (Dunnill, 1962a): (1)'alveolar region' (that is, that containing no structureover 1 mm. in diameter); (2) arteries; (3) bronchi;(4) pleura and septa.The airways were traced and, in addition to
the number of their branches, the distal extent ofcartilage along axial airways was established. If thespecimen is large enough, certain airways can betraced by macroscopic dissection to within the lastfew branches; for these, microscopic sections arenecessary. This gave the proportion of the total air-ways that were bronchi, i.e., proximal to the mostdistal plate of cartilage, and bronchioli, i.e., distalto the ultimate cartilage. The staining charac-teristics of cartilage were also checked to see, inparticular, whether acid glycoprotein had formed.The arterial branching pattern was reconstructed by
using step or serial sections. Wall structure and thick-ness and external diameter of the arteries weremeasured over a range of arterial size. These results
could be analysed to give the population distributionof small arteries by structure (Elliott, 1964) and theextent of muscle in the arterial tree, estimated bothby reference to external diameter and to the airwayaccompanying the given artery.The percentage of the 'alveolar region' (see macro-
scopic point-counting above) that is made up ofalveolar air is determined by microscopic point-counting of the randomly taken lung sections. Thenumber of alveoli and arteries per unit area were alsocounted. From the analysis of these results the totalalveolar number was estimated, the alveoli per milli-litre (Davies, 1969; Davies and Reid, 1970) and thealveoli/blood vessel ratio.Emery and Mithal (1960) described a 'radial
alveolar count'. This figure represents the number ofalveoli transected by a perpendicular from therespiratory bronchiolus to the perimeter of the acinus,as indicated by 'the nearest and definite connectivetissue septum'.
In summary, in addition to a general macroscopicand microscopic examination, the use of the abovetechniques, in combination, allowed quantification ofthe following features of lung structure:
ARTERIES
FROM THE ARTERIOGRAM Arterial lumen diameter atthe hilum and its reduction to the penriphery
FROM MICROSCOPIC EXAMINATION Number of branchesalong an axial arterial pathwayArterial wall thickness related to external diameterDistribution of the types of small pulmonaryartery-an arterial 'population count'Arterial level to which muscle penetrates, estab-lished (a) by arterial diameter, and (b) by the struc-ture of the accompanying airway, i.e., terminal orrespiratory bronchiolus
BRONCHI
FROM MACROSCOPIC DISSECTION Number of bronchialbranches from the axial pathway
FROM MICROSCOPIC EXAMINATION Proportion ofbronchi to bronchioliDiameter of airwaysDistal extent of cartilage assessed by airwaygeneration
ALVEOLI
FROM COMBINED MACROSCOPIC AND MICROSCOPIC POINT-COUNTING Proportion of alveolar and large-structure regions
FROM MICROSCOPIC EXAMINATION Total alveolarnumberNumber of alveoli/millilitreAlveoli/blood vessel ratioRadial alveolar count
683

Alison Hislop and Lynne Reid
RESULTS
CASE 1
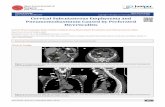
Case 1 was a male child born at term after anormal pregnancy and weighing 7 5 lb. (3 4 kg.)at birth. The immediate neonatal period wasnormal, but at seven days he became short ofbreath with marked sternocostal recession andcentral cyanosis. There was reduced air entry overthe left hemithorax; some crepitations were heardover the right lung. No other abnormalities weredetected and, in particular, there was no evidenceof heart failure.The chest radiograph (Fig. 1) showed the left
lung to be enlarged with downward displacementof the left hemi-diaphragm and deviation of theheart and trachea to the right. The left hemi-thorax was hypertransradiant; hilar and proximallung vessels were seen with a virtually normalarrangement.A provisional diagnosis of severe infantile
lobar emphysema was made. There was gradualdeterioration in the baby's condition, and at 17days a left lateral thoracotomy was performedthrough the 6th interspace. A 'hyper-inflated'upper lobe occupied almost the whole of thehemithorax, but the surgeon commented that thelingula appeared normal. The left lower lobe wasaerated and of normal appearance save that itwas small. The left upper lobe and lingula were
removed and, although the lower lobe expandedsatisfactorily, it did not completely fill the lefthemithorax. The left lower lobe has graduallyexpanded to fill the left chest and for 12 monthssince the child has remained well.
MEASUREMENTS FROM CHEST RADIOGRAPH In theapico-posterior view the area occupied by the leftupper lobe was roughly 46 cm.2; as the expectedarea in this child's chest would be only 15 cm.2,this represented a three-fold increase. The leftlower lobe was airless. The right lung had an areaof only 11 cm.2, due to the shift of the heart tothis side, although normally in this child it shouldbe about 15 cm.2. In the lateral view the area ofthe upper lobe was approximately 60 cm.2, com-pared with a probable normal of 20 cm.2-againa three-fold increase.
This three-fold increase in area represents a3:2 change in volume; that is an increase involume of the order of five times.
MACROSCOPIC EXAMINATION AND ARTERIAL INJECTION
OF SPECIMEN When received at the laboratorythe lobe was mostly aerated, although the lingulawas represented by a collapsed region below thehilum with a torn tag of tissue attached. Thespecimen showed no evidence of obstruction tothe airways. The left upper lobe was roughly8 x 5 5 cm. in area by 2 5 cm. thick. The arteriesto the apical and posterior segments were injected
I
FIG. 1. Case 1. Pre-operative radiograph, showing enlarged, trans-radiant, avascular left upper lobe. The left hemi-diaphragm is flattenedand the heart and trachea are displaced to the right.
684

Polyalveolar lobe with emphysema
by hand with Micropaque gelatin solution(Fig. 2); the lingular region could not be inflatednor injected because of tearing and of the diffi-culty in identifying its major structures.
After inflation the volume of the left upper lobewas 125 ml. Some months after fixation, whenthe specimen was studied, the volume was againmeasured and found to have fallen to 90 ml.the normal at this age being about 30 ml.Through the pleura the lobular arrangementseemed clear over most of the lobar surface, butthere was little evidence of emphysema and thiswas confirmed when the lobe was cut, there beinglittle retraction below the cut surface.
Macroscopic point-counting of the sliced lungshowed that the 'alveolar region' as defined abovewas 85% of the total lobar volume, within anormal range.
Alveolar region Microscopically the alveolarwalls appeared normal in thickness and containeda normal amount of collagen and reticulin.The estimated total alveolar number in the lobe
was 27-259 x IO', the normal value for the agebeing 5 x 106 (Dunnill, 1962b). This represented afive-fold increase (see Table).
TABLEALVEOLAR RESULTS IN THREE CASES OF CHILDHOODLOBAR EMPHYSEMA (POLYALVEOLAR EMPHYSEMA)
MeanLobar Total Alveoli/ Radial
Case Age Volume Alveolar ml Acinar(fixed) No. x 0) Alveolar(ml.) (x 10') (x10) Wall
Count
1 17/7 90 27-259 0 356 9-25(30) (5)' (0$336) (5 5)
2 3/12 75 27-65 0-512 10-16(50) (18)' (0-785)2 (5-5)
3 8/12 0-377 10-16(0 785)' (6-6)
The normal value at the appropriate age is shown in parentheses1 Dunnill (1962b)' Davies & Reid (1970)
The number of alveoli per millilitre offixed alveolar tissue was 0-356 x 106. Comparablefigures for a normal lung of this age are not avail-able; for a two-day-old lung it has been estimatedto be 0X336 x 106, for a four-month lung 0 785 x 106.This rapid decrease in alveolar size doubtlessreflects the great increase in alveolar number justbefore the eighth week (Boyden and Tompsett,1965). Boyden has emphasized that before thistime the alveolar region is represented by airspaces larger and more simple than adult typealveoli, which he has called 'primitive saccules'.Even at 17 days these are still the main type ofairspace in the alveolar region, which suggests that
in the present specimen when fixed there was noevidence of emphysema, that is, of alveolar airspaces too large for age. While lung volume duringlife must be taken into account before denyingthe presence of clinical emphysema, it can be saidthat emphysema was not apparent after inflationat the pressures usual in our laboratory.The radial acinar alveolar count confirmed that
there was an increase in the number of alveoliwithin an acinus. In the apical, posterior andanterior segments (henceforth referred to as theupper three segments) a mean number of 9-25alveoli lay between the respiratory bronchiolusand the pleura, the normal number for this agebeing 5-5. In the lingula, on the other hand, thecount was within the normal range. It would seemthat it is only in the upper three segments thatthere is an excess of alveoli.
In the upper three segments the number ofalveoli was also increased in relation to thenumber of arteries per unit area of lung. Themean ratio of alveolar/artery number in a micro-scopic field was 21 8 in the diseased lobe, and is 8-8in the normal. The number of arteries per squaremillimetre was also reduced, there being 5-35 inthe normal and 2 85 in the abnormal diseasedsegments. The arteries were reduced per unitnumber of alveoli and per unit volume of lung.In other respects the arterial system was normal,emphasizing that it is the alveolar increase thatis abnormal, that is, the 'emphysematous' leftupper lobe showed a five-fold increase in thenumber of alveoli, probably localized to the threeupper segments. The specimen did not show anyincrease in alveolar size.
Bronchi Reconstruction from serial sections ofthe axial pathway of the posterior segment of theupper lobe showed that the bronchial branchingpattern was normal as was the size of bronchi.The number of generations between the segmentalhilum and terminal bronchiolus was 16, withinthe normal range, and cartilage extended tobetween the 7th and 8th generations; thereforethe proportion of bronchi to bronchioli was alsonormal.The cartilage plates in the posterior segment
were thin and elongated; in the anterior andlingular segments they appeared normal. Alcianblue staining of the cartilage plates showed thatacid glycoprotein was present.The cartilage plates were normal in number
although in the posterior segment they appearedsparser because of the 'stretching' of the airway.Individual plates in this region were thinner but
685

Alison Hislop and Lynne Reid
FIG. 2. Case 1. Arteriogram of left upper lobe. The posterior segment isinjected with a radio-opaque medium. The arteries appear sparse because ofthe large size of the lobe. Part of the lingula (L) can be seen as a tag of tissue.(x1.)
covered a larger area of bronchial wall-perhapsbecause of compensatory overgrowth. It seemsthat the bronchi were not affected except that thelength between branches was increased because ofthe excessive lobar volume.
It would thus seem that even in the segmentswith too many alveoli the bronchial tree wasnormal, suggesting that it was not involved in thedisturbance to development. Clustered at the endof the pathways studied, the normal number ofterminal bronchioli was found. This, together withthe normal branching pattern, suggests that thetotal number of terminal bronchioli in the lobewas normal, that is, the acinar number wasnormal. This means that each acinus containedtoo many alveoli.
Arteries In the arteriogram (Fig. 2) the arteriesappeared sparse because of the increase in thevolume of the lobe. Lumen diameter along theaxial pulmonary artery of the posterior segmentwas measured at levels from the hilum to theperiphery. The diameter at each level was normal
for the child's age, but since there was an increasein lobar volume it was small in proportion to thelung it supplied.The arterial branching pattern along the axial
pathway of the posterior segment, when tracedmicroscopically, was found to be normal both innumber of branches and in the proportion ofsupernumerary to conventional type arteries(Elliott, 1964; Elliott and Reid, 1965). It might beexpected that the increase in alveolar numberwithin an acinus would be associated with anincrease in arterial blood supply, but there wasno increase in the supernumeraries which supplythe acinar periphery, nor was there any increase inblood flow to the acinus since there was noincrease in the size of the arterial lumen.The wall structure of the 1,500 um. segmental
artery was normal-that of a muscular artery:an elastic arterial media has not been found below1,700 ,um. at this age (Hislop, 1970).Random sections showed that throughout the
lobe muscle penetrated to a normal level whetherjudged by the size of the artery or by the structure
686

Polyalveolar lobe with emphysema
of the airway it accompanied. At this age somewholly muscular arteries are found with terminalbronchioli, but not beyond. The size above whichall arteries are muscular is 150 /um. It thus followedthat the external diameter of arteries running withterminal and respiratory bronchioli was normal.
'rhe distribution by size, or 'population count',of the three types of small artery-muscular, par-tially muscular and non-muscular-was normal.For arteries below 200 ,um. external diameter thewall thickness of the arteries related to externaldiameter was normal. For larger sizes the meanwall thickness was slightly increased. This is ofdoubtful significance since the injection was doneby hand so that distension may have been lesscomplete than usual, and other signs of increasedmuscularity such as extension of muscle to theperiphery were absent.No cross-filling from the pulmonary artery to
the bronchial arteries was seen, though in thenormal lung at this age with our technique thereis a considerable cross-filling. This evidentlyreduces with age since, after early childhood, noneis seen: we have seen considerable cross-filling atfour months but not at 18 months or older.Though not injected, it appeared that the veinswere normal. There was no arterio-venous cross-filling.
The pulmonary arterial circulation was thusrelatively normal in structure. The branchingpattern and diameter were normal, suggesting thatflow and pressure were normal for age. The formerwas reduced when related to alveolar number andlobar volume, and there were fewer arteries peralveolus in the intra-acinar region. It can bepresumed, therefore, that the blood flow per unitvolume of lung was less than in the normal.The striking abnormality in this case was a five-
fold increase in lobar volume associated with asimilar increase in alveolar number, but withvirtually no increase in alveolar size, and there-fore no emphysema. The airways were normal andso were the arteries for age, although this meansthat the arteries were too few and too small inrelation to acinar volume and alveolar number.
In two cases (one described as case 10 in Reid(1967a)) further quantitative examination of thematerial still available from specimens was made.
CASE 2
From one patient (female) the left upperlobe had been removed at the age of 3 months,after several weeks of shortness of breath. Atoperation the lobe was found to be large, theapical segments being most affected (Fig. 3); even
FIG. 3. Case 2. The left upper lobe shows that the apical segments are more inflated thanthe lingula (L), to produce a groove mimicking the appearance ofa fissure.
687

Alison Hislop and Lynne Reid
after removal from the body this region remainedlarge and showed air trapping, while the lingularand posterior segments collapsed normally. Herealso the number of airway generations wasnormal, when the airway was dissected macro-scopically. The cartilage in the anterior and apicalsegments showed thin prolongations, as describedby Stovin (1959), while elsewhere the plates ofcartilage appeared normal.
Although the lobar volume is smaller than incase 1, it was doubtless considerably larger at thetime of resection since blocks had been cut fromit, thereby causing deflation. By quantitation onfurther blocks considerable increase in alveolarnumber could still be shown-27 65 x 106 insteadof the normal 18 x 106 for this age (see Table).The methods of calculation are based on totallung volume which largely corrects for deflation,but not for the tissue removed earlier. The radialacinar alveolar count confirmed an increase inalveolar number since in this case it was 10-16,the normal being 5 5. Some degree of emphysemais present, the number of alveoli per milli-litre being 0 512 x 106; the normal would be0 785 x 106. It would seem, however, that excessivealveoli in a localized region within the lobe is themain abnormality.
CASE 3
In the third case the left upper lobe wasremoved from a male child aged 8 months, inwhom shortness of breath of moderate severityhad been the presenting symptom. In this speci-men also, deflation since resection and blocktaking had affected precise estimation of thealveolar number. The method of radial acinaralveolar count showed that, within the acinus,there was considerable increase of alveoli, themean number being 10-16 instead of the normal6 6 at this age. In addition to the increased totalalveolar number, slight emphysema was found-0 377 x 106 alveoli/ml., the normal being0 785 x 106 alveoli/ml. (see Table).
It seems then that, although clinically andradiologically the cases were typical of childhoodlobar emphysema, the main abnormality is exces-sive multiplication of alveoli, a condition notpreviously reported. In case 1 one region of thelobe was affected, and in the other two also itis probable that it was localized. In case 1 firmevidence of emphysema could not be establishedfrom examination of the specimen. In cases 2 and3 emphysema was shown even in the fixed speci-men. It is possible that this had developed in thepostnatal months.
DISCUSSION
The detailed methods for assessing pulmonarygrowth described here make it possible to analyseits nature and to suggest the time of onset of anyabnormality.From the Laws of Lung Development (Reid,
1967b) the following general conclusions follow.Reduction in the number of airways below normalpoints to abnormal growth in the first half ofintra-uterine life. A total alveolar number lessthan the figure found at birth also indicates ante-natal disturbance, though probably in the latermonths; a figure between the normal at birth andthat appropriate to a given age suggests postnataland, perhaps, antenatal slowing. The pre-acinararterial development is normally in line withbronchial development, the intra-acinar withalveolar (Hislop, 1970). They are not interdepen-dent, however, as is shown by case 1 reportedhere, where the arteries are normal for age butthe alveoli greatly exceed the normal.
In case 1 the airways and pre-acinar arterieswere normal, suggesting that development duringthe first half of intra-uterine life was not disturbed.The lobe was removed so soon after birth that theovergrowth could not have occurred in these fewdays-particularly as in the normal lung alveolido not multiply in the first post-natal weeks(Boyden, 1965; Boyden and Tompsett, 1965). Itis likely then that the abnormal developmentstarted in late fetal life after the canalicularstage.
POLYALVEOLAR LUNG
The abnormality is in the alveolar number, anestimated five-fold increase for the whole lobe,probably a nine-fold increase for the affectedupper three segments. The excess alveoli do notform a separate mass, but since the number ofterminal bronchioli is normal they are evenlydistributed throughout the acini of the affectedregion and in normal communication with air-ways. The lobe is thus 'polyalveolar' with eachacinus a 'giant'. In spite of this, all methodsshowed that the intra-acinar arterial developmentwas normal for the age of the lung but not forits size. This is an instance where blood supplyhas not followed demand. It suggests that beforebirth respiratory and circulatory development areindependent of each other. 'Polyalveolar lung'would thus seem the best term to describe thiscondition.
688

Polyalveolar lobe with emphysema
EMPHYSEMA
The emphysema embodied in the term 'childhoodlobar emphysema' must be separately assessed;its presence may be established from the specimen,and perhaps from the radiograph during life.
In case 1 the radiograph showed transradiancy,avascularity and an increased volume-all signsof air trapping. In general terms, transradiancyarises from a change in air/tissue ratio. Thealveolar walls were of normal thickness and therewas no significant increase in alveolar size. Areduction in blood increases transradiancy (Reid,1967a; Reid, Simon, Zorab, and Seidelin, 1967)and the transradiancy in case 1 probably reflectsreduction in blood flow per unit volume of lung.The number of blood vessels per unit volume oflung was reduced, i.e., the amount of blood inthe alveolar wall.The evidence of air trapping was the depression
of the left hemi-diaphragm, the bow chest andthe displacement of the heart and trachea. Thereason for the air trapping is not clear; there mayhave been premature collapse of the airways or
the volume increase with shift of mediastinalstructures may have caused kinking of the leftmain or upper lobar bronchus. Air trapping is a
condition usually associated with emphysema, butwithout further evidence we cannot equate thetwo because the radiographic features are nottruly diagnostic. Emphysema was demonstratedpathologically in cases 2 and 3 even in the fixedspecimen, and would certainly have been more
severe during life. In these two cases the large airspaces may have been due to interference withalveolar multiplication after birth (Reid, 1967b,1970). In the specimen of case 1 emphysema was
not demonstrated but might have developed if thelobe had not been removed. Shortness of breathand air trapping are sufficient clinical evidencefor the diagnosis of emphysema and it wouldcertainly seem justified, perhaps even in case 1, todescribe the clinical condition as a 'polyalveolarlung probably with emphysema'. Certainty of thediagnosis of emphysema must rest on pathologicalexamination.
PATHOGENESIS
The excessive number of alveoli suggests a
'giantism' or hypertrophy of the affected part ofthe lung. It is reminiscent of the enlarged limbsometimes associated with a systemic arterio-venous fistula, and possibly brought about by an
excessive blood flow. In the present case, how-ever, the diameter of the lobar artery, its pattern
of branching and its reduction in size toward theperiphery were all normal, suggesting that therehad been no significant increase in flow. No cross-filling occurred between the pulmonary andbronchial arteries. Within the alveolar regionfewer arteries per alveolus, or per unit of lungarea were seen. This points to a reduced flow perunit volume of tissue: certainly it would not seempossible to suggest an increased blood flow as thecause of the condition.The fact that only part of the lobe seems to be
affected has also to be considered. This conditionhas long been called lobar emphysema, and, cer-tainly to the surgeon at operation and usually tothe pathologist in the laboratory, it seems that thewhole lobe is affected. There is evidence (Reid,1967a) that this is certainly not always the caseand that, even when the whole lobe appears uni-formly increased in volume, different parts of thelobe may behave differently or have sufferedunequally. For example, on removal from thebody the lung may deflate unevenly, one part ofthe lobe staying over-inflated while the rest deflatesand behaves as normal lung might. The resultsdescribed here for case 1 confirm that theabnormality is certainly worse in certain segmentsand may even be confined to one region only. Ithas not been possible to ascertain whether thesechanges are precisely localized to a given segment,or to one region independently of segmentalarrangement.
It is of interest that in all three cases describedhere it seemed that the apical three segments wereall affected, but the fact that the cartilage moreclosely resembled normal in the anterior segmentcould mean that even the apical segments were
not affected evenly.This abnormality of the newborn lung thus
represents a localized overgrowth of the alveolarregion. The fact that the alveoli are normallydifferentiated and in normal communication withairways probably distinguishes this condition fromhamartoma, in which anatomical derangement ismore severe.The growth achieved, whether in an organ or
a part of it, represents a balance between stimulusand inhibition to growth. A local over-action of'growth' organizer substance could be postulatedas the cause of the change described here. In thepresent state of our knowledge it would not seem
possible to go further.
We thank Mr. Charles Drew for allowing us tostudy the specmens from cases 1 and 3, and Mr.Arthur Makey for case 2. To Dr. George Simon weare indebted for his opinion on the radiographs.
689

Alison Hislop and Lynne Reid
REFERENCES
Bolande, R. B., Schneider, A. F., and Boggs, J. D. (1956). Infantilelobar emphysema; an etiological concept. Arch. Path., 61, 289.
Boyden, E. A. (1965). The terminal air sacs and their blood supplyin a 37-day infant lung. Amer. J. Anat., 116, 413.and Tompsett, D. H. (1965). The changing patterns in thedeveloping lungs of infants. Acta anat. (Basel), 61, 164.
Chalkley, H. W. (1943). Method for the quantitative morphologicanalysis of tissues. J. nat. Cancer Inst., 4, 47.
Davies, G. (1969). The effect of chronic lung disease in childhoodon lung growth, including measurement of right ventricularweight, alveolar development and pulmonary artery structure.M.D. thesis, Cambridge.
-and Reid, Lynne (1970). Growth of the alveoli and pulmonaryarteries in childhood. Thorax, 25, 669.
Dunnill, M. S. (1962a). Quantitative methods in the study of pul-monary pathology. Thorax, 17, 320.
-(1962b). Postnatal growth of the lung. Thorax, 17, 329.Elliott, F. M. (1964). The pulmonary artery system in normal and
diseased lungs-structure in relation to pattern of branching.Ph.D. thesis, University of London.
and Reid, L. M. (1965). Some new facts about the pulmonaryartery and its branching pattern. Clin. Radiol., 16, 193.
Emery, J. L., and Mithal, A. (1960). The number of alveoli in theterminal respiratory unit of man during late intrauterine lifeand childhood. Arch. Dis. Childh., 35, 544.
Hislop, A. (1970). The non-muscular phase of the pulmonary circu-lation in the child. In Proceedings of the Fifth InternationalCystic Fibrosis Conference, Churchill College, Cambridge,22126 Sept. 1969, ed. David Lawson, p. 340. Cystic FibrosisResearch Trust, London.
Leape, L. L., and Longino, L. A. (1964). Infantile lobar emphysema.Pediatrics, 34, 246.
Millard, F. J. C. (1965). The development and the electrocardio-graphic diagnosis of right ventricular hypertrophy in chroniclung disease. M.D. thesis, University of London.
Reid, L. M. (1967a). The Pathway of Emphysema. Lloyd-Luke,London.(1967b). The embryology of the lung. In Ciba FoundationSymposium: Development of the Lung, 1966, p. 109, Edited byde Reuck, A. V. S., and Porter, Ruth. Churchill, London.
-(1970). The laws of lung development. In Proceedings of theFifth International Cystic Fibrosis Conference, Churchill College,Cambridge, 22/26 Sept. 1969, ed. David Lawson, p. 333. CysticFibrosis Research Trust, London.
- Simon, G., Zorab, P. A., and Seidelin, R. (1967). The develop-ment of unilateral hypertransradiancy of the lung. Brit. J.Dis. Chest, 61, 190.
Simon, G., and Reid, L. M. (1963). Atresia of an apical bronchusof the left upper lobe-report of three cases. Brit. J. Dis.Chest, 57, 126.
Stovin, P. G. I. (1959). Congenital lobar emphysema. Thorax, 14, 254.Thomson, J., and Forfar, J. 0. (1958). Regional obstructive emphy-
sema in infancy. Arch. Dis. Childh., 33, 97.Waddell, J. A., Simon, G., and Reid, L. (1965). Bronchial
atresia of the left upper lobe. Thorax, 20, 214.Weibel, E. R., and Gomez, D. M. (1962). A principle for counting
tissue structures on random sections. J. anpl. Physiol., 17, 343.
690