1 Carbon! Why it’s cool, and what it can do. 2 ORGANIC = FROM A LIVING SOURCE, OR CONTAINING...
-
Upload
coleen-lynn-cole -
Category
Documents
-
view
214 -
download
0
Transcript of 1 Carbon! Why it’s cool, and what it can do. 2 ORGANIC = FROM A LIVING SOURCE, OR CONTAINING...

11
Carbon!Carbon!Why it’s cool, and Why it’s cool, and
what it can dowhat it can do

22
ORGANIC = FROM A LIVING ORGANIC = FROM A LIVING SOURCE, OR CONTAINING CARBONSOURCE, OR CONTAINING CARBON
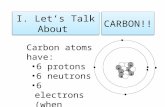
• The fact that Carbon needs 4 electrons to complete its valence shell means it has 4 “connection spots”
• It can also do neat things like:– form long chains or rings– form especially strong & stable bonds
(covalent)– Can be the “anchor piece” for very large
molecules

33
Carbon Ring
Carbon Chains

44
Monomers & Polymers: Monomers & Polymers: when carbons get togetherwhen carbons get together
• Monomer: small, simple molecule (beads)
• Polymer: larger, more complex molecules made from monomers strung together. (necklace)
monomer + monomer + monomer =
Polymer

55
Very large polymers made from Carbon-based chains are called
MACROMOLECULES“Macro” = giant
(so, “giant molecule”)

66
How do monomers come together How do monomers come together to form a polymer?to form a polymer?
Dehydration Synthesis:
This is a chemical reaction where two monomers join
together and release water.
Dehydration synthesis is a “building up” or anabolic
reaction.

77
How do polymers break down into How do polymers break down into monomers?monomers?
Hydrolysis:This is a chemical reaction in which water
is added and splits a polymer back into monomers.
Hydrolysis is a “breaking down” or catabolic reaction.
Dehydration Synthesis-Hydrolysis

88
MACROMOLECULES come in MACROMOLECULES come in different varietiesdifferent varieties
4 important groups:
–Carbohydrates–Proteins–Lipids–Nucleic Acids

99
What type of macromolecule is shown here?

1100
CarbohydratesCarbohydrates• Monomer = monosaccharide
– Ex: glucose, sucrose, fructose• Function: energy, structure, fuel
storage, strength • Example: Sugar, • cellulose Polysaccharide• (crunchy part of plants), starch (plant
energy storage), glycogen (animal muscle energy)

1111

1122
What type is shown here? (Okay, there a
few “hints”.)

1133
ProteinsProteins• Monomer = amino acid• Function
– Structure (bones, muscles, etc.)– Fight disease– Control rates of reaction– Transport substances in & out of the cellExample: Insulin, hormones (chemical
messengers)

1144

1155
What type of macromolecule is shown here?

1166
LipidsLipids• Monomer: fatty acids on a “glycerol
backbone”• Function:
– Chemical messengers (steroids)– Insulation and cushioning– Compact, long-lasting energy source– Make up cell membrane
• Examples:– Triglycerides (fats)– Phospholipids (cell membrane fats)– Wax– Oils– Steroids

1177
Saturated fats have only Saturated fats have only singlesingle bonds in their fatty acid bonds in their fatty acid “tail”.“tail”.
UnUnsaturated fats have one or more saturated fats have one or more doubledouble bonds in their bonds in their fatty acid tail.fatty acid tail.

1188
Can you identify this type of macromolecule?

1199
Nucleic AcidsNucleic Acids
• Monomer = nucleotide• Function:
– Store & transmit Hereditary information– Direct growth & development– Use to construct proteins (RNA) Examples: DNA and RNA