S8P1 b. Describe the difference between pure substances (elements and compounds) and mixtures.
-
Upload
matthew-palmer -
Category
Documents
-
view
214 -
download
0
Transcript of S8P1 b. Describe the difference between pure substances (elements and compounds) and mixtures.


S8P1 b. Describe the difference between pure substances (elements and compounds) and mixtures.

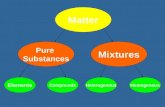
Matter can be classified as elements, compounds, or mixtures.
Elements and compounds are examples of pure substances.
http://www.docstoc.com/docs/51158457/NOTES-22-23-Elements-Compounds-and-Mixtures

A substance in which there is only one type of particle.
Each element contains only one type of particle and these particles are called atoms.

An Element is a substance that cannot be broken down into any other substances by chemical or physical means.
There over 100 known elements. All matter is made up of elements.

92 Elements are found in nature The other 17 are made by
scientists under special laboratory conditions.
Most elements are solids at room temperature.

Examples of solid elements: iron, tin, lead, silver, gold, calcium, and copper.
Elements that are gasses: Oxygen, hydrogen, and nitrogen.
www.livetradingnews.com

Some types of matter are made up of only one element.
Examples: iron nail only contains the element iron.
Aluminum foil is made up of only the element aluminum.
http://www.thereadystore.com/cooking-and-fuel/5077/10-awesome-uses-for-tin-foil/

Physical property can be observed or measures without changing the matter’s identity.
Each element can be identified by its unique set of properties.

Boiling point Melting point Density Malleability Ductility State of matter


Chemical properties describe matter based on it ability to change into new matter that has different properties.
Reactivity with i.e. acid, oxygen Flammability

Other substances are made up of more than one element and are known as compounds.
Examples: › Water – hydrogen and oxygen› Table salt – sodium and chlorine› Sugar – carbon, oxygen, and hydrogen

A Compound is a pure substance made of two or more elements chemically combined.
Basically, a compound is formed as a result of a chemical change.
Examples: H2O, H2SO4 (Sulfuric acid)

Most of the matter making up the earth is composed of compounds.
Sugar is a compound – it is made up of carbon, hydrogen, and oxygen.
Table salt – sodium and chlorine.

A formula is a combination of symbols that shows the ratio of elements in a compound.
Example: C9H8O4 otherwise known as aspirin
C12H22O11 – Sugar!

Water is a familiar compound. Water is made up of two elements – hydrogen and oxygen. The chemical formula is H2O.
The 2 in the formula is a subscript. It tells the number of atoms of that element. So Hydrogen has how many atoms? How many oxygen?

The suffix “–ide” is often used in chemistry to form the names of compounds
Example: Iron Sulfide – iron and sulfur Sodium Chloride – table salt

A mixture consists of two or more substances that are in the same place together but are not chemically combined into a new substance.
Example: Garden soil

A homogeneous mixture has the same uniform appearance and composition throughout. Many homogeneous mixtures are commonly referred to as solutions.
A heterogeneous mixture consists of visibly different substances or phases. The three phases or states of matter are gas, liquid, and solid.

Air Vinegar Blood plasma Sugar water Salt in water
(dissolved)
Sand in water Trail mix Vinegar in oil Milk Chicken Soup

A solution is a type of mixture. Individual components are mixed
evenly throughout the mixture.
Example: saltwaterhttp://thepracticalmama.com/?p=437

A solution is a mixture that appears to be a single substance.
At least two substances must be mixed in order to have a solution.
The SOLUTE is the substance being dissolved.
The SOLVENT is the substance in which the solute is dissolved.


If you add too much sugar to a glass of lemonade, not all of the sugar can dissolve. Some of it sinks to the bottom. To find the maximum amount of sugar that can dissolve, you would need to know the solubility of sugar.
Solubility of a solute is the ability of the solute to dissolve in the solvent under certain conditions: temperature of the solution, chemical nature of the solvent and volume of the solvent.

Solubility of a salt in a liquid increases with an increasing temperature.
Solubility of a gas in a liquid increases with decreasing temperature.

More solvent volume means more solute can dissolve.

Mass of solute that can dissolve in a mass or volume of solvent at a certain temperature.

SUPERSATURATED SOLUTIONSSUPERSATURATED SOLUTIONS contain more solute than is contain more solute than is possible to be dissolvedpossible to be dissolved
Supersaturated solutions are Supersaturated solutions are unstable. The supersaturation is unstable. The supersaturation is only temporary, and usually only temporary, and usually accomplished in one of two accomplished in one of two ways:ways:
1.1. Warm the solvent so that it will Warm the solvent so that it will dissolve more, then cool the dissolve more, then cool the solution solution
2. Evaporate some of the solvent carefully so that the solute does not solidify and come out of solution.

Saturated
Unsaturated
Supersaturated

Any point on a line represents a saturated solution.
In a saturated solution, the solvent contains the maximum amount of solute.
Example At 90oC, 40 g of NaCl(s)
in 100g H2O(l) represent a saturated solution.

Any point below a line represents an unsaturated solution.
In an unsaturated solution, the solvent contains less than the maximum amount of solute.
Example At 90oC, 30 g of NaCl(s)
in 100g H2O(l) represent an unsaturated solution. 10 g of NaCl(s) have to be added to make the solution saturated.

Any point above a line represents a supersaturated solution.
In a supersaturated solution, the solvent contains more than the maximum amount of solute. A supersaturated solution is very unstable and the amount in excess can precipitate or crystallize.
Example At 90oC, 50 g of NaCl(s)
in 100g H2O(l) represent a supersaturated solution. Eventually, 10 g of NaCl(s) will precipitate.

Mixtures are:› Made of two or more substances
mixed together› Substances keep their own
properties› Can be separated by physical
means› Have no definite chemical
composition

Made of two or more substances chemically combined
Substances lose their own properties Can be separated only by chemical
means Have a definite chemical composition

When a certain poisonous gas is combined with a flammable metal, a fine white powder results. The powder is neither flammable nor poisonous. Is the powder a mixture or a compound? How do you know?

Compound The substances lost their properties.