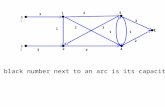
2/19/2016 3:18 PMSkip Lists1 S0S0 S1S1 S2S2 S3S3 103623 15 2315.
What happens to the absorbed energy?. Energy soso s1s1 t1t1.
-
Upload
patricia-townsend -
Category
Documents
-
view
222 -
download
0
Transcript of What happens to the absorbed energy?. Energy soso s1s1 t1t1.

What happens to the absorbed energy?

Energy
so
s1
t1



EDTA Titrations

Outline What is EDTA?What is EDTA? Metal-Chelate ComplexesMetal-Chelate Complexes
ATP4- with Mg2+
Fe(NTA)23-
Fe(DTPA)2-
Chelate EffectChelate Effect EDTAEDTA
Acid Base Properties Y nomenclature Conditional Formation Constants
EDTA TitrationEDTA Titration

Metal-Chelate Complexes
Lewis Acid/Base ChemistryMonodentate
Multidentate and Chelates

Review:
What is a Lewis Acid? Examples?
And a Lewis Base? Examples?

Transition Metal with ligand
Central Metal ion is a Lewis Acid
Ligand – All ligands are Lewis Bases

Multidentate
Multidentate or chelating ligand attaches to a metal ion through
more than one atom is said to be multidentate, or a chelating
ligand.Examples?


ATP4- can also form complexes with metals

Complex of Iron and NTA
Fe3+ +
Fe(NTA)23-
2

Medical ApplicationsMedical Applications
The Thalassemia Story




The Chelate EffectThe Chelate Effect
Question: Describe in your own words, the “chelate
effect”.

The Chelate Effect!
Cd(H2O)62+ + 2
Cd(H2O)62+ + 4CH3NH2
H2N NH2
H2N
NH2
Cd
H2N
NH2
OH2
OH2
2+
+ 4 H2O
K = B2 = 8 x 109
H3CH2N
H3CH2N
Cd
NH2CH3
NH2CH3
OH2
OH2
2+
+ 4 H2O
K = B2 = 4 x 106

13-2 EDTA
“EDTA is by far, the most widely used chelator in analytical chemistry. By direct titration or through indirect series of reactions, virtually every element of the periodic table can be
measured with EDTA.” - Daniel Harris

Acid/Base Properties
H
H
(H6Y2+)

Acid/Base Properties
H
(H5Y+)
pKa = 0.0

Acid/Base Properties
(H4Y)
pKa = 0.0
pKa = 1.5

Acid/Base Properties
(H3Y-)
pKa = 0.0
pKa = 1.5
-pKa = 2.0

Acid/Base Properties
(H2Y-2)
pKa = 0.0
pKa = 1.5
-pKa = 2.0
- pKa = 2.7

Acid/Base Properties
(HY-3)
pKa = 0.0
pKa = 1.5
-pKa = 2.0
- pKa = 2.7
pKa = 6.16

Acid/Base Properties
(Y-4)
pKa = 0.0
pKa = 1.5
-pKa = 2.0
- pKa = 2.7
pKa = 6.16pKa = 10.24

Fraction as Y4-
The fraction of EDTA in form Y4- is given as 4-
][][ 44 EDTAY
Y (13-3)
Concentration in the form Y4-
Total Concentration of EDTA
Fraction of EDTA ion the form YFraction of EDTA ion the form Y4-4-

Fraction as Y4-
Equation 13-4 in text
654321543211
43212
3213
214
156
654321
][][][][][][4
KKKKKKKKKKKHKKKKHKKKHKKHKHH
KKKKKKY


Example
You make a solution of 0.10 M EDTA and you buffer the pH to (a) 10.0. What is Y4-
? (b) What is Y4- if the pH of the
solution is buffered to 11.0?

][][ 44 EDTAY
Y
)10.0(36.0][ 4 MY
MY pH 036.0][ 0.104
)10.0(85.0][ 4 MY
MY pH 085.0][ 0.114

EDTA reactions with Metals
Silver – Ag+
Mercury - Hg2+
Iron (III) – Fe3+

EDTA
ethylenediaminetetraacetate anion => EDTA-4 => Y-4
+1 cationAg+ + Y-4 AgY-3

EDTA
ethylenediaminetetraacetate anion => EDTA-4 => Y-4
+2 cation Hg+2 + Y-4 HgY-2

EDTA
ethylenediaminetetraacetate anion => EDTA-4 => Y-4
+3 cationFe+3 + Y-4 FeY-1

EDTA
ethylenediaminetetraacetate anion => EDTA-4 => Y-4
+n ionM+n + Y-4 MY(n-4)+

EDTA [MY(n-4)+]
KMY = -------------- [M][Y-4]
[MY(n-4)+]KMY = -------------------
[M+n] * 4 * [EDTA]
[MY(n-4)+]K'MY = KMY x 4 = -------------------
[M+n] [EDTA]
][][ 44 EDTAY
Y
Conditional formation constant!Conditional formation constant!


Example
Calculate the concentration of Ni2+ in a solution that was prepared by mixing 50.0 mL of 0.0300 M Ni2+ with 50.0 mL of 0.0500 M EDTA. The solution was buffered to pH of 3.00. Two Parts
1. Reaction2. Then equilibrium is established

EDTA Titrations


Figure 13-10 Theoretical titration curves

EXAMPLE:
Calculate the conditional constant:
Derive a curve (pCa as a function of volume of EDTA) for the titration of 50.0 mL of 0.0500 M Ca+2 with 0.1000 M EDTA in a solution buffered to a constant pH of 10.0.
pCa at Equivalence
Equivalence Volume
pCa at Pre-Equivalence Point
pCa at Post-Equivalence Point
pCa at Initial Point

Example
Derive a curve (pCa as a function of volume of EDTA) for the titration of 50.0 mL of 0.0500 M Ca+2 with 0.1000 M EDTA in a solution buffered to a constant pH of 10.0.
[CaY-2]K'CaY = KCaY * 4 = ----------------
[Ca+2] * [EDTA]
where Y4- = 0.36 at pH = 10.0
K'CaY = KCaY * 4 = 4.9 x 1010 * 0.36 = 1.8 x 1010
KCaY = 4.9 x 1010

EXAMPLE:
Derive a curve (pCa as a function of volume of EDTA) for the titration of 50.0 mL of 0.0500 M Ca+2 with 0.1000 M EDTA in a solution buffered to a constant pH of 10.0.Equivalence Volume 1 Mole of EDTA = 1 Mole of MetalM1V1 = M2V2 (Careful of Stoichiometry)
50.0 mL (0.0500 M) = 0.1000 M (V2)V2 = 25.0 mL

EXAMPLE:
K'CaY = 1.8 x 1010
0.00 mL EDTA added
pCa = - log[Ca+2]
Initial Point
= - log(0.00500 M) = 2.301
Derive a curve (pCa as a function of volume of EDTA) for the titration of 50.0 mL of 0.0500 M Ca+2 with 0.1000 M EDTA in a solution buffered to a constant pH of 10.0.

EXAMPLE:
Derive a curve (pCa as a function of volume of EDTA) for the titration of 50.0 mL of 0.0500 M Ca+2 with 0.1000 M EDTA in a solution buffered to a constant pH of 10.0.At 25.0 mL (Equivalence Point)At 25.0 mL (Equivalence Point)
Ca2+ + Y4- -> CaY2-
Before 0.0025 moles 0.0025 moles
-
After - - 0.0025 moles
What can contribute to Ca2+ “after” reaction?

EXAMPLE:
Derive a curve (pCa as a function of volume of EDTA) for the titration of 50.0 mL of 0.0500 M Ca+2 with 0.1000 M EDTA in a solution buffered to a constant pH of 10.0.
Ca2+ + Y4- CaY2-
I - - 0.0025 moles/V
C +x +x -x
E +x + x 0.0333 –x
]][[
][2
2'
CaEDTA
CaYK CaY
2' 0333.0
x
xK CaY
X = [Ca2+] = 1.4 x10-6
pX = p[Ca2+] = 5.866
0.0025moles/0.075 L0.0025moles/0.075 L

Pre-Equivalence Point
Let’s try 15 mL

EXAMPLE:
Derive a curve (pCa as a function of volume of EDTA) for the titration of 50.0 mL of 0.0500 M Ca+2 with 0.1000 M EDTA in a solution buffered to a constant pH of 10.0.At 15.0 mL Ca2+ + Y4- -> CaY2-
Before 0.0025 moles 0.0015 moles
-
After 0.0010 moles - 0.0015 moles
What can contribute to Ca2+ after reaction?
K’CaY = 1.8 x 1010
negligiblenegligible

EXAMPLE:
Derive a curve (pCa as a function of volume of EDTA) for the titration of 50.0 mL of 0.0500 M Ca+2 with 0.1000 M EDTA in a solution buffered to a constant pH of 10.0.At 15.0 mL
[Ca2+] = 0.0010 moles/0.065 L[Ca2+] = 0.015384 Mp [Ca2+] = 1.812

Post Equivalence Point
Let’s Try 28 ml

EXAMPLE:
Derive a curve (pCa as a function of volume of EDTA) for the titration of 50.0 mL of 0.0500 M Ca+2 with 0.1000 M EDTA in a solution buffered to a constant pH of 10.0.At 28.0 mL Ca2+ + Y4- -> CaY2-
Before 0.0025 moles 0.0028 moles
-
After - 0.0003 moles
0.0025 moles
What can contribute to Ca2+ after titration?

EXAMPLE:
Derive a curve (pCa as a function of volume of EDTA) for the titration of 50.0 mL of 0.0500 M Ca+2 with 0.1000 M EDTA in a solution buffered to a constant pH of 10.0.
Ca2+ + Y4- CaY2-
I - 0.0003 moles/V 0.0025 moles/V
C +x +x -x
E +x 0.003846 + x 0.03205 –x
]][[
][2
2'
CaEDTA
CaYK CaY
))(003846.0(
03205.0'
xx
xK CaY
X = [Ca2+] = 4.6 x10-10
pX = p[Ca2+] = 9.334
0.078 L


Experimental Considerations

EDTA Titration Techniques
Erichrome Black T
MgIn + EDTA MgEDTA + In
(red) (colorless) (blue)

Figure 13-13 Guide to EDTA titrations, light color, pH range for quantitative analysis, dark area where ammonia must be present