file · Web viewPolymers. A polymer molecule is a long chain molecule (sometimes called a...
Transcript of file · Web viewPolymers. A polymer molecule is a long chain molecule (sometimes called a...

Polymers
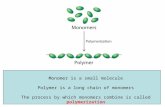
A polymer molecule is a long chain molecule (sometimes called a macromolecule) made from small molecules called monomers.
For AS we focus on addition polymerisation where the monomer molecules contain a C=C bond (alkenes).
n = a large number typically 5000-10000 monomer units per chain.
If a mixture of monomers are polymerised the resulting polymer is called a copolymer
What influences the properties of polymers?
Chain length – longer = stronger Side groups – side groups which can attract other side groups by intermolecular forces
increase polymer tensile strength Branching – more branched chains cannot pack as closely – reducing IMF – not as strong Chain flexibility – rigid chains are stronger Cross linking – covalently linking chains makes them harder and more difficult to melt
Classifying polymer properties
Elastomers – return to their original shape after a force is applied and removed
Plastics – do not return to their original shape after force is removed
Fibres – strong polymer chains that can be arranged into strands/threads.
Thermoplastics – when heated can be melted and cools to give a new shape. Forces between polymer chains are relatively weak and easily overcome by heating – upon cooling the forces are re-established.
Thermoset – shape can’t be changed by heating – do not melt – all the polymer chains are joined by permanent covalent bonds.
(figure 1 page 108 CI)
HDPE – chains have no branches – they pack very closely increasing IMF and hence strength
LDPE – chains have branches – polymer chains cannot pack very closely thus reducing IMF and hence strength
Solubility of polyethenol
Polyethenol contains OH side groups which can hydrogen bond with water – therefore it can be made to be water soluble.

The solubility depends upon the % of OH groups on the chain. Strangely when the % of OH exceeds 99% the polymer becomes insoluble - the hydrogen bonding between polymer chains becomes so strong that water molecules cannot hydrogen bond to the polymer.