mrsbirtwistle.weebly.com · Web viewExperiments were done using cathode ray tubes (CRT) in which...
Transcript of mrsbirtwistle.weebly.com · Web viewExperiments were done using cathode ray tubes (CRT) in which...
Sci 10 Name:Unit 2a Radioactivity Notebook
Unit VocabularyWord DefinitionRadioactivity
Natural Background RadiationRadiation
Light
Isotope
Mass Number
Nuclear Symbol
Radioactive Decay
Radioisotope
Alpha Particle
Alpha Decay
Beta particle
Beta Decay
Gamma Radiation
Nuclear Equation
Half Life
Radiocarbon Dating
Decay Curve
Parent Isoptope
Daughter Isotope
Nuclear Fission
Nuclear Reaction
Nuclear Fusion
HISTORY OF THE DISCOVERY OF RADIOACTIVITY
1 | P a g e
Radioactivity is the spontaneous emission of radiation from the nucleus of an atom and in the late 1800’s it was determined that there are different types of radiation that are either positive, negative or neutral. Each type of radiation exhibits its own set of properties.
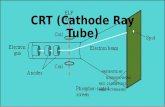
Experiments were done using cathode ray tubes (CRT) in which a high voltage is applied to two electrodes inside a glass tube containing a gas. The inside of this tube has most of the air removed by a vacuum and an electric current passes through the tube from the negative (cathode) end to the positive (anode) end and a ray is produced that causes a fluorescent screen at the end of the tube to glow. If an electric current or a magnet is placed near the cathode ray tube, the ray can be deflected.
What can you conclude about the charge of the cathode ray based on the
diagram above? It is negative as it is repelled from the negative plate and attracted to the positive plate
It was from these types of experiment that J.J. Thomson identified the negatively charged particles as electrons. Cathode ray tubes were commonly used in television sets before plasma and liquid crystal screens were discovered.
Further studies were conducted with cathode ray tubes and in 1895 Wilhelm Röntgen discovered that there were rays outside of the cathode ray tube and these rays caused fluorescent minerals to glow and would cause exposure to occur on photo paper. He did not know what these rays were and he referred to them as X rays. Further study showed that these X rays were high energy components of the electromagnetic spectrum that would penetrate some materials but not others. X rays are produced by converting the kinetic energy of the high speed electrons directly into electromagnetic radiation when they strike a metal plate.
Henri Becquerel was born into a family of scientists. His grandfather had made important contributions in the field of electrochemistry while his father had investigated the phenomena of fluorescence and phosphorescence. Becquerel not only inherited their interest in science, he also inherited the minerals and compounds studied by his father. And so, upon learning how Wilhelm Röntgen discovered X rays by observing the fluorescence they produced, Becquerel had a ready source of fluorescent materials with which to pursue his own investigations of these mysterious rays. The material Becquerel chose to work with was a double sulfate of uranium and potassium which he exposed to sunlight and placed on photographic plates wrapped in black paper. When developed, the plates revealed an image of the uranium crystals. Becquerel concluded "that the phosphorescent substance in question emits radiation which penetrates paper opaque to light." Initially he believed that the sun's energy was being absorbed by the uranium which then emitted X rays. Further investigation, on the 26th and 27 of February, 1896, was delayed because the skies over Paris were overcast and the uranium-covered plates Becquerel intended to expose to the sun were returned to a drawer. On the first of March, he developed the photographic plates expecting only faint images to appear. To his surprise, the images were clear and strong. This meant that the uranium emitted radiation without an external source of energy such as the 2 | P a g e
sun. Becquerel had discovered radioactivity, the spontaneous emission of radiation by a material. Later, Becquerel demonstrated that the radiation emitted by uranium shared certain characteristics with X rays but, unlike X rays, could be deflected by a magnetic field and therefore must consist of charged particles.
Marie Curie, intrigued by what Becquerel had discovered, examined the mineral pitchblende (uranium oxide) and found it to be more radioactive than could be accounted for by just uranium. She chemically separated the mineral into its component elements (of which there are about 30) and found that two separated samples were much more radioactive than uranium. A sample of barium contained a new element that was about a million times more radioactive than uranium. It came through with barium because both have similar chemical properties. She named this element radium, and its existence was confirmed through spectrographic analysis. A sample of bismuth contained a second element that was also highly radioactive, this she called Polonium, named after Poland, where she was born. She was never successful at separating polonium from bismuth, but she did separate the radium from the barium. She discovered that it had some interesting properties:
1) Radium emits particles, some of which are affected by a magnetic field. These must be charged particles. One traveled very fast and was of negative charge, this was an electron (beta particle). Another was larger and positive, a helium nucleus (alpha particle). Another was not affected by the field, and penetrated through lead. This was the gamma ray.
2) Radium emits a radioactive gas when it decays. This is now known as radon, which gained notoriety in the last couple of decades as a source of potential lung cancer. It may collect in basements of houses built in areas of high pitchblende (uranium oxide) concentration.
3) Radium causes glow-in-the-dark (phosphorescent and fluorescent) materials to glow.
a) Fluorescent: glows only as long as a source of radiation is present….like stuff that glows when a black-light hits it. Take away the black-light and the glow disappears.
b) Phosphorescent: Glows after source of radiation is taken away….like paste-on stars that you might put on the ceiling. Light energizes the material, and after the light is taken away the material continues to glow, the glow slowly disappearing as the night goes on.
Radium gives these materials a constant source of energy through radiation, so the glow is continuous. This property was used to make glow-in-the-dark clock dials so you could see what time it is at night. The dials were hand-painted, and the painters kept their brushes sharp by licking the tips. Over time, they began to grow tumors on their tongues, an indication of the health effects of radiation. Marie Curie herself died of leukemia, possibly caused by the work she was doing with radiation.
4) Radium gives off enough heat to melt its weight in ice in an hour.
5) Radium causes electroscopes to become charged. She reasoned that this is because of the charged particles that it gives off. This is called “ionizing” the air. Ionized air 3 | P a g e
conducts electricity. This property is useful in detecting radiation with a device called a “Geiger-Mueller Tube”, sometimes called a Geiger Counter.
Marie worked with her husband Pierre Currie and made several significant discoveries in the field of radiation. One of these included the discovery that radiation comes from the core of the atom.
Electromagnetic SpectrumThe electromagnetic (EM) spectrum is the name given to a group of different types of radiation. Radiation is energy that travels and spreads out as it goes. Electromagnetic radiation includes visible light, radio waves, microwaves, infrared and ultraviolet light, X-rays and gamma-rays. Types of radiation in the EM spectrum, in order from lowest energy to highest:
Radio: The kind of energy that radio stations emit into the air to broadcast to your radio. Radio waves are also emitted by stars and gases in space.
Microwaves:
Infrared:
Visible:
Ultraviolet:
X-rays:
Gamma-rays:
4 | P a g e
A review of Atomic StructureAtoms are the smallest pieces an element can be broken into and still retain the properties of that element. It comes from the Greek word atomos, meaning “indivisible” (unbreakable). Atoms are so tiny that they can’t be seen directly. They can be detected through X-ray crystallography or atomic force microscopes, but only indirectly. It takes 602 000 000 000 000 000 000 000 atoms of hydrogen to weigh 1 gram (the mass of a small paper clip).
Parts of the Atom
A) Nucleons (Particles in the Nucleus)
1) Protons: have a mass of 1 atomic mass unit and a charge of +1. They are found in the nucleus of the atom, and the number of protons in the atom is the atomic number, which identifies what element the atom is. Oxygen (O) has an atomic number of 8, which means there are 8 protons in the nucleus. Since protons are the only particle in the nucleus to have a charge, the charge of the nucleus is + (# of protons). Since oxygen has 8 protons in the nucleus, oxygen has a nuclear charge of +8.
2) Neutrons: have a mass of 1 atomic mass unit, and no charge. They are found in the nucleus of the atom, and the number of neutrons added to the number of protons gives you the mass number of the atom. The number of neutrons does not affect the identity of the element. Oxygen’s most common form has a mass number of 16. Since there are 8 protons in the nucleus of oxygen, this means there must also be 8 neutrons to give a combined mass of 16. The number of protons and neutrons does NOT have to be equal. In addition, atoms of any given element can have differing numbers of neutrons. Atoms of the same element with different numbers of neutrons in their nuclei are called ISOTOPES of one another. The most common isotope is the one who’s mass equals the average atomic mass given on the periodic table rounded to the nearest whole number. Since O has a given average mass of 15.9994, the most common isotope of O is O-16, or Oxygen with a mass number of 16. The chemical properties of different isotopes of the same element have the same chemical properties as they all have the same number of electrons. Physical properties of the different isotopes can vary from one isotope to another for the same element. Isotopes for the same element have the same name (the name of the element) but a different number which indicates the mass of the isotope.
B) Particles Outside The Nucleus
3) Electrons: have a mass of 1/1836 amu and a charge of -1. They are found orbiting the nucleus in energy levels. Atoms gain, lose or share electrons when they form chemical bonds. If electrons are gained and lost, an ionic bond is formed. If electrons are shared, a covalent bond is formed. The number of electrons in the atom equals the number of protons. Atoms are neutrally charged, so the + charged protons and the – charged electrons must be equal in number to give a neutral charge. Oxygen has 8 protons in its nucleus, so there must be 8 electrons zipping around outside the nucleus in energy levels. 5 | P a g e
Rutherford`s Gold Foil ExperimentErnest Rutherford carried out a series of experiment that involved shooting alpha particles at a thin sheet of gold foil. At the time J J Thomson`s plum pudding model was the readily accepted model of the atom. This model suggested that the atom was a dense positive sphere with negative charges embedded in it. Based on this model Rutherford expected that the alpha particles he shot at the gold foil would be stopped or deflected straight back by the dense gold atoms making up the thin sheet of gold foil. He was surprised at the results he obtained from his experiments. The diagram below shows the set up for his gold foil experiments.
Set Up Results
When he shot a stream of alpha particles at the thin sheet of gold foil he observed that:1.
2.
3.
From these result he concluded that the most of the atom must be empty space and that most of the mass of the atoms in concentrated in the center or the nucleus. From these results Rutherford proposed the planetary model of the atom which resembles the solar system with protons in the nucleus and electrons orbiting around the nucleus. The electrons are held in place by electric forces between the negative force of the electron and the positive force of the proton. Bohr carried out further experiments and later proposed a model where electrons are located in specific orbits, with each orbit being able to hold a maximum number of electrons.
1st 2nd 3rd
6 | P a g e
1) How many protons does an atom of iron (Fe) have?
2) What is the nuclear charge of an atom of Fe?
3) How many atomic mass units (amu) do the protons in an atom of Fe weigh?
4) How many electrons does Fe have around its nucleus?
5) What is the most common isotope of Fe?
6) How many neutrons are in the nucleus of the most common isotope of Fe?
Quick Check #1Name of Ion Symbol Number of Protons Number of Electrons Ion
ChargeLithium ion
19 18 1+
Mg2+
Chloride ion
9 1-
O2-
I-
7 | P a g e
OK, what about these isotopes? Why does Fe have an AVERAGE atomic mass of 55.9099? What are the other isotopes of iron? There are four common naturally occurring isotopes of iron:Mass ofIsotope(amu)
Notation 1Symbol – mass #
Notation 2Mass #Symbol
# protons(atomic #)
# neutrons(mass # - atomic #)
# electrons(atomic #)
%Abundancein Nature
54 Fe-54 54Fe 26 54-26 = 28
26 5.845%
56 Fe-56 56Fe 26 56-26 = 30
26 91.754%
57 Fe-57 57Fe 26 57-26 = 31
26 2.119%
58 Fe-58 58Fe 26 58-26 = 32
26 0.282%
The most common (abundant) isotope of iron really is Fe-56! It makes up more than 90% of all iron atoms. But that average looks kind of suspicious! If you average 54, 56, 57 and 58, you get 56.25, not 55.9099. Apparently the average mass is not a straight average. The average mass is based on the percentage of each isotope found in a naturally occurring sample of an element.
Quick Check #2i. What do two isotopes of an element have?
A. the same number of electrons but a different number of protonsB. the same number of neutrons but a different number of protonsC. the same number of protons, electrons, and neutronsD. the same number of protons but a different number of neutrons
ii. What does a sodium-23 isotope contain?
A. 11 protons and 12 neutronsB. 11 protons and 23 neutronsC. 12 protons and 11 neutronsD. 23 protons and 23 neutrons
iii. Isotopes are two atoms of the same element that do which of the following?A. become electrically chargedB. differ in mass but are chemically alikeC. give away electrons to become positively charged
D. have the same mass but different chemical properties
iv. Which of the following describes the isotope 4020Ca ?A. 40 protons and 20 neutronsB. 20 protons and 40 neutronsC. 20 protons and 20 neutronsD. 40 electrons and 20 neutrons
Use the following information to answer the next two questions.The nuclear notations for four mystery elements are shown below.
56 6026AA 28 CC
60 5827BB 28 DD
v. How many subatomic particles does mystery element AA have?
A. 26 protons, 26 neutrons, and 26 electrons
8 | P a g e
B. 26 protons, 30 neutrons, and 26 electronsC. 30 protons, 26 neutrons, and 30 electronsD. 26 protons, 56 neutrons, and 30 electrons
vi. Which mystery elements are isotopes of the same element?
A. AA and BB B. BB and CCC. CC and DD D. AA and DD
Read page 275-282 Workbook Questions 1-3
Natural RadioactivityAn unstable nucleus that emits radiation is undergoing radioactive decay.
Nuclear Stability: the larger (more massive) a nucleus is, the harder it is for it to stay together.
When a nucleus is radioactive, it gives off decay particles and changes from one element to another. This is also known as natural decay or natural transmutation. Atoms with an atomic number of 1 through 83 have at least one stable (nonradioactive) isotope, but all isotopes of elements with an atomic number of 84 or more are radioactive.
The Three Types Of Natural Decay Decay Type(symbol)
Radiation
Notion
Mass Charge What happens to the atom when it undergoes thistype of decay
Characteristics
Alpha(α)
Alpha particle
The nucleus loses 2 protons (atomic mass decreases by 2) and 4 total particles (mass decreases by 4). It turns into a different element.238 4 234 92U 2He + 90Th
The alpha particles released by uranium in the Earth’s crust build up underground in porous rock, where they gain electrons and turn into actual atoms of pure helium. This is where we get the helium that is in balloons!
9 | P a g e
Beta(β−)
Beta particle
- A neutron in the nucleus decays to form a proton (atomic number increases by 1, but mass stays the same) and an electron (the beta particle) which leaves the nucleus at high speeds.42 4219K -1e + 20Ca
Gamma(γ)
Gamma ray
This takes the form of a high energy particle of light that is given off as the nucleus becomes more stable. It does not change the identity of the element. It has no mass or charge, and is so energetic that it can only be stopped by a 30-cm thick layer of concrete. Gamma can be given off by itself, or it can be given off with any of the other types of decay.
When a nucleus emits a particle and the identity of the element changes the process of transmutation has occurred. Transmutation takes place in alpha and beta decay when the parent nucleus changes into the daughter nucleus. Transmutation does not occur in gamma decay because the charge on the nucleus does not change, it is only some energy that is lost.
This diagram shows the path taken by radioactive particles as they pass through an electric field. On the left is a shielded container that holds a sample of radioactive substance, with a small hole at the end to allow decay particles to stream out in a straight line. Above and below the stream are electrically charged plates, which deflect (change the path of) the beams as they come out. Positive-charged particles (like alpha) are attracted to the negatively charged plate. Negative-charged particles (like beta) are attracted to the positively charged place. The gamma rays, having no charge, pass through undeflected. On the right side is a screen that has been coated with zinc sulfide (ZnS), which is a phosphor (material that glows when energized particles hit it). ZnS is used as a phosphor coating on television sets with picture tubes and CRT (cathode ray tube) computer monitors.
10 | P a g e
Alpha DecayAn alpha particle is essentially a Helium nucleus as it is made up of two protons and two neutrons. When a radioactive atom emits an alpha particle it releases the two protons and two neutrons (helium nucleus). This alpha particle is slowed down by crashing into other atoms and eventually it will acquire two electrons from other atoms and become a helium atom.
When writing a balanced equation for alpha decay the electric charge (atomic number) and the total number of protons and neutrons must be conserved.
11 | P a g e
Write the nuclear equation for the alpha decay of americium-241. What element is it transmuted into?
24195Am 23793 Np + 42 He Write the nuclear equation for the alpha decay of Radium-226. What element is it transmuted into? 22688 Ra 22286 Rn + 42 He
A= Z = X = Y=
Write the nuclear equation for the alpha decay of americium-241. What element is it transmuted into?
Write the nuclear equation for the alpha decay of Radium-226. What element is it transmuted into?
Beta Decay
When the nucleus of a radioactive element emits a Beta particle (an electron) the atom has undergone beta decay. The neutrons that are contained in an unstable nucleus can decay into a proton, neutron and a neutrino (subatomic particle with high energy but no mass or electric charge). The decay of the neutron produces the electron for beta decay. A neutrino is also emitted but is generally left off the beta decay equation. The neutrons in an atom undergoing beta decay change into a proton and an electron. As a neutron is lost but a proton is produced the mass number stays the same for the daughter nucleus. The atomic number increases because one proton is added to the nucleus.
Write the nuclear equation for the beta decay of the isotope Cesium 137
Gamma DecayWhen a nucleus has emitted an alpha and beta particle, the nucleus has a surplus of energy and it is referred to as being in the excited state. The energy level is lowered by emitting a gamma ray, which is high energy electromagnetic radiation. The gamma ray has no mass or energy so it does not change the isotope itself, only the energy level.
12 | P a g e
Decay SeriesA decay series is a series of radioactive decays where the parent nuclei becomes a daughter nuclei and then that daughter nuclei becomes a parent itself. The number of reactions is different for different decay series but they always end with a stable isotope. Uranium Decay: U-238 is unstable and decays into more stable nuclei. It takes 14 decay steps until a stable, non-radioactive nucleus is finally reached. The daughter nuclide of one step becomes the parent nuclide of the next:
13 | P a g e
Read page 284 – 288Nuclear EnergyRadioactive decay gives off energy. In fact, the energy given off by the radioactive isotopes in the Earth’s core has kept the core molten for the last 4.5 billion years. The molten core gives us our planet’s magnetic field, which shields us from high-energy particles given off by the sun The energy that is given off during a nuclear change comes from the MASS DEFECT...a little bit of mass that is destroyed (so little that you would hardly even miss it) and converted into huge amounts of energy. This would appear to be in direct violation of the Law of Conservation of Energy, but that only covers physical and chemical changes, not nuclear changes. The equation that is used to calculate the energy given off during a nuclear change is E=mc2, discovered by Albert Einstein, but used by Lise Meitner to make nuclear power possible. E is energy, in joules. m is the mass that was destroyed by the nuclear change, in kg. c2 is the speed of light squared, a huge number (9.00 X 1016 m2/sec2). You are not responsible for doing any calculations involving energy formed from the destruction of mass, what you need to really focus on is this fact:A HUGE amount of energy can be created by destroying a TINY amount of mass.Later, you’ll see how you can use this fact to make nuclear power plants to generate electricity!
How Can Radioactivity Be Detected? Cloud ChamberA cloud chamber is a small plastic chamber that is sealed and contains a sponge soaked in alcohol. The bottom of the cylinder is placed on a piece of dry ice and this causes the air in the chamber to become supersaturated with alcohol. When radioactive material is placed in the chamber it emits charged particles that remove some electrons from the atoms in the air and covert these atoms into positive ions. The positive ions cause the vapour to condense and create a visible track in the chamber.
14 | P a g e
Bubble ChamberA bubble chamber contains a super heated liquid that forms bubbles around the tracks of charged particles that pass through it. An electromagnet causes the charged particles to be deflected. As alpha and beta particles have opposite charges they are deflected in opposite directions.
Geiger CounterA Geiger counter is a made up of a cylinder that contains a gas and a wire. A high voltage is passed between the cylinder case and the wire and as charged particles enter through a window they ionize the gas in the cylinder. The positive ions are attracted to the negative wire and this produces a current that is detected and read. Alpha particles are not able to penetrate the window and are there for not able to enter the Geiger counter. It is only beta and gamma particles that can be detected.
Half LifeRadioactive Decay is a random process. It is not possible to predict when a particular nucleus will decay, but we can make fairly accurate predictions regarding how many nuclei in a large sample will decay in a given period of time. With a large sample there will initially be a large portion of parent nuclei and a small proportion of daughter nuclei and the rate of decay will be high. Over time the rate of decay will decrease and the 15 | P a g e
proportion of parent nuclei will decrease and the proportion of daughter nuclei will increase.
Rate = number of decays per second1 Bq = 1 decay per second
The half-life of a radioactive isotope is defined as the period of time that must go by for half of the nuclei in the sample to undergo decay.
During one half-life period:
Half of the radioactive nuclei in the sample decay into new, more stable nucleiIf a sample contains 1000 nuclei of a radioactive isotope now, 500 will undergo decay over the course of one half-life.Half the mass of the radioactive isotope is converted into a new, more stable isotopeIf a sample contains 4.0 grams of a radioactive isotope now, after one half-life, 2.0 grams will remain undecayed, the other 2.0 grams will be made up of a new, more stable isotope.
A Geiger-Müeller counter’s count-per-time period will be half of what it started atIf a Geiger-Müeller counter is showing 400 counts per minute now, after one half-life, the counter will show 200 counts per minute.
After one half-life, half (50%) of the original amount of the sample will have undergone radioactive decay.
After a second half-life, one quarter (25%) of the original sample will remain undecayed.
After a third half-life, one eighth (12.5%) of the original sample will remain undecayed.
NOTE: The actual half-life time is constant. If the half-life is 10 days, then 2 half-lives would take 20 days, 3 half-lives take 30 days, 4 half-lives take 40 days and so on.
16 | P a g e
IsotopeParent Daughter Half-Life of Parent (years)Carbon - 14 Nitrogen – 14 5730
Uranium – 235 Lead – 207 710 million
Potassium – 40 Argon – 40 1.3 billion
Uranium -238 Lead - 206 4.5 billion
Thorium – 235 Lead - 208 14billion
Rubidium - 87 Strontium - 87 47 billion
Number of half-lives
1 2 3 4 5 n
Fraction remainingExponential notation
Number of Half-lives
1 2 3 4 5
Percent Remaining
Quick Check #3When radioactive isotopes (parent – P) decay, they produce daughter products (D) at a constant rate, called the half-life (T). Example: if we start with 100 atoms of the parent, after one half-life, there will be 50 parent atoms remaining and 50 daughter atoms newly made. After another half-life (two half-lives), there will be 25 parent atoms remaining and now 75 daughter atoms. Each parent-daughter isotope pair has its own half-life. To achieve the above example with U-238 takes 9 billion years (two half-lives). To achieve the above example with C-14 takes 11400 years (two half-lives). In the geologic environment, we use a mass spectrometer to count the number of Parent and Daughter atoms in a closed-system (like minerals crystallizing from magmas), and use the relative proportions to find out how old the closed-system is. .Assuming we start with only parent isotopes (no daughter), after one half-life has passed, there should be ½ parent remaining and ½ daughter newly formed. The ratio of P:D is ½ : ½ or 1:1. Complete the rest of this table, as in the first example:
# Half lives Fraction of original Parentremaining
Fraction of original parentturned into daughter
Parent: Daughter ratio
1 ½ ½ 1:123456
SOLVING HALF – LIFE PROBLEMS
17 | P a g e
1) You know how much of the isotope you have now, you want to find out how much will be left after a certain amount of time (going into the future).
Step 1: Determine how many half-lives have gone by. Take how much time has gone by and divide it by the duration of the half-life.Step 2: Cut the amount (mass, percent, fraction, number of nuclei) in half as many times as there are half-lives.
The half-life of Rn-222 (a carcinogenic house pollutant) is 3.8 days. If today your basement contains 20.0 grams of Rn-222, how much will remain after 19 days assuming no more leaks in?
A laboratory sample of 32P triggers 400 clicks per minute in a Geiger-Mueller counter. How many days will it take for the 32P to decay enough so that there are only 50 clicks per minute?
A cylinder contains 5.0 L of pure radioactive 19Ne. If the cylinder is left to sit for 103.2 seconds, what percent of our original sample of 19Ne will remain? The half-life of N is 17.2 seconds.
2) You know how much of the isotope you have now, you want to find out how there was a certain amount of time ago (going into the past).
Step 1: Determine how many half-lives have gone by. Take how much time has gone and divide it by the duration of the half-life.Step 2: Double the amount (mass, percent, fraction, number of nuclei) as many times as there are half-lives.
The half-life of Tc-99m* (used to locate brain tumors) is 6.0 hours. If 10 micrograms are left after 24 hours, how much Tc-99m was administered originally?
18 | P a g e
A laboratory sample of 32P triggers 100 clicks per minute in a Geiger-Mueller counter. How many days ago did the 32P to decay enough to produce 1600 clicks per minute? The half-life for 32P is 14.3 days.
3) You want to find out how long the half-life is, knowing how much a sample has decayed over a given amount of time.
Step 1: Determine how many times you can cut your original amount in half in order to get to your final amount. This is the number of half-lives that have gone by.Step 2: Divide the time that has elapsed by the number of half-lives that have passed.
A radioactive sample is placed next to a Geiger counter and monitored. In 20.0 hours, the counter’s reading goes from 500 counts per minute to 125 counts per minute. How long is the half-life?
A sample of pure radioactive isotope is left to decay. After 40.0 days, the sample is placed in a mass spectrometer, and it is determined that the sample only 25% of the original isotope remains. How long is the half-life?
19 | P a g e
4) Radioactive Dating is used to determine the age of a substance that contains a radioactive isotope of known half-life.Step 1: Determine how many times you can cut your original amount in half in order to get to your final amount. This is the number of half-lives that have gone by.Step 2: Multiply the number of half-lives by the duration of a half-life
The oldest rocks on Earth have been found to contain 50% U-238 and 50%Pb-206 (what U-238 ultimate decays into). What is the age of these rocks?
An ancient scroll is discovered, and it is found that only 25% percent of the original concentration of C-14 (a radioactive isotope found in equal concentration in all living beings) remains. How old is the scroll?
Consider the following graph showing the decay curve for uranium-235.
20 | P a g e
a) How long is one half-life of uranium-235?
b) What percentage of uranium-235 remains after 2000 million years?
c) How much time passes before only 10% of the original sample of uranium-235 remains?
Radioactive DatingRadioactive isotopes can be used to date material because they decay according to their half life.
USES OF RADIOACTIVE ISOTOPESMany radioactive isotopes are very useful to us! Here is a sampling of isotopes that we have put to good use:
Radioactive Isotope Use
C-14 Used to determine the age of biological remains (archaeology)I-131 Used to detect and cure hyperthyroidism (overactive thyroid)Co-60 Used as a source of radiation for radiotherapy of cancerTc-99m Used to image blood vessels, especially in the brain, to detect tumorsPu-239 Used as a highly fissionable fuel source for nuclear power or nuclear weaponsAm-241 Used in tiny amounts in smoke detectors as a source of ions to make a currentU-235 Used as fissionable fuel source for nuclear power or nuclear weaponsU-238 Used to determine the age of uranium-containing rock formations (geology)
Irradiation of food: kills bacteria, allowing it to be stored for a longer time without having to pasteurize. Pasteurizing involves heating the food to kill bacteria, which can change the flavor of the food. Irradiation does not change the flavor.Radioactive isotopes are often used medically in the body to either treat cancer or to detect potential problems. Since radioactivity itself can cause cancer with exposure, any isotopes administered to a person should have a short half- life and be quickly eliminated from the body (usually via urination).
Read page 290 – 295Nuclear Reactions
Artificial RadiationIn 1934, Irene and Frederic Joliot found that they could create radioactive isotopes by combining the non-radioactive particle with an alpha particle.
Eg. Aluminum 27 + alpha particle phosphorus 30P-30 does not occur naturally and spontaneously decomposes to produce artificial radiation. In order to use an alpha particle to bombard a nucleus, the particle must be 21 | P a g e
travelling at a high enough speed to overcome the repulsion of the positively charged target nucleus. Enrico Fermi realized that neutrons could be used to bombard nuclei and because they have no charge and there was no issue of repulsion. Fermi produced several new elements with atomic numbers greater than uranium.
Artificial Transmutation: Changing one element into another one Artificial Radiation
The Sun contains 92 naturally occurring elements, from hydrogen to uranium. All of the elements more massive than uranium were produced using artificial transmutation. From Neptunium (93) to Ununoctium (118), these “transuranium” elements were made by people!So, how can you make an element? You need three ingredients:1) A sample of target nuclei, usually very heavy ones.2) A particle “bullet” that has a charge (almost always a positive charge, like an alpha particle or another nucleus, but electrons can be accelerated, too)3) A particle accelerator to make the bullet move fast enough (close to the speed of light) to collide with the nuclei and change them into different elements.The particle accelerator uses electromagnetic fields to accelerate charged particles and change stable isotopes into radioactive ones. These particle accelerators can be circular or linear. Neutral particles, such as neutrons or gamma rays, cannot be accelerated in a particle accelerator. Particle accelerators need to be HUGE in order to get the bullet particles up to speed. Here is a list of a few currently operating particle accelerators and their sizes:
1) U.C. Berkeley Cyclotron: round, 153 cm in diameter. Element 97, Berkelium (Bk) is named in honor of this place where so many new isotopes were made.2) LEP at CERN, ring-shaped, 27 kilometers in diameter. CERN is located northwest of Geneva, Switzerland, on the French/ Swiss border.3) Large Hadron Collider at CERN, multiple-rings, 27 kilometers in diameter.
Generally, the larger the collider, the faster the particle bullets can travel, so heavier and heavier elements can be made.
To determine the products of artificial transmutation:
Step 1: One of the products will be unknown. Add up the mass numbers on the side that all particles are known. The mass numbers of the other side should add up to the same thing.Step 2: Add up the atomic numbers (charges) on the side where all particles are known, The charges of the other side should add up to the same thing.Step 3: Look up the atomic number (if 3 or more) on the Periodic Table and identify what element you have. If the atomic number is 2 or less (including -1), then look up the identity of the particle on a reference table.
27 4 1 On the top: 27 + 4 = x + 1, so the mass is 30.13Al + 2He ---> X + 0 n On the bottom: 13 + 2 = x + 0, so the charge is 15.
30 Element 15 is PHOSPHOROUS (P).22 | P a g e
X is 15 P
These transmutations were first performed by Ernest Rutherford, the scientist who first identified alpha particles.
Mass and Energy in Nuclear ReactionsDuring nuclear reactions large amounts of energy are released. Einstein proposed that mass and energy are related with his equation E= mc2 (E = energy, m = mass, c= speed of light). In nuclear reactions the mass of the product is less than the mass of the reactants, the missing mass was released as kinetic energy of the emitted radiation and daughter nuclei. The kinetic energy spreads to other atoms and takes the form of thermal energy which is seen as a temperature increase. The thermal energy can be converted to other types of energy such as electrical energy.
So, what’s the difference between NATURAL DECAY and ARTIFICIAL TRANSMUTAION?Unique To Natural Decay234 0 23491 Pa -1e + 92 U
Common to both Unique to ArtificialTransmutation239 4 24294Pu + 2He 96Cm + X
Unstable nucleus undergoes decay all by itself, turning into a new element.
Both form new elements from old ones.
Stable nucleus is forced to change into a less stable nucleus of a new element.
The left side of the equation has only the unstable nucleus, the right side has both the decay particle and the new, more stable nucleus.
In both, the masses on top of each side add up to the same, and the charges on the bottom of each side add up to the same.
The left side of the equation has the target nucleus and the particle bullet, the right side shows the results of that collision.
Produces energy through the destruction of mass.
Both follow Einstein’s equation E=mc2. A tiny bit of mass (mass defect) is
Produces energy through the destruction of mass, however much more
23 | P a g e
destroyed and energy is created.
energy has to go into the process than comes out of it.
Effect on HumansRadiation is produced when radioactive isotopes decay and this radiation can interfere with the DNA in cells. Most of the damage to DNA can be repaired but double strand breaks can cause mutations which can be very harmful to the body. The body absorbs energy when exposed to radiation and the amount absorbed is measured in grays (Gy) (1 Gy = 1 J/kg). Equal amounts of different types of radiation does not cause the same damage. The dose unit for radiation is the Sievert (Sv).
To
minimize Radiation Effects on the body1. limit your exposure time to radiation2. keep far away from radioactive sources3. shield yourself from radioactive sources4. make sure that radioactive sources in your environment are contained
NUCLEAR FISSION
The nucleus of an atom contains protons and neutrons. The protons, which have a positive charge can exist side by side in the nucleus of an atom and do not repel each other. When nuclear fission occurs the nucleus is split into two smaller nuclei and the positively charged nuclei of the two new atoms repel each other. This repulsion results in the atoms being forced apart with great speed. This fast speed indicates that the nuclei have a large amount of kinetic energy (energy in motion). A few nuclei larger than Fe-56 can be split into smaller nuclei, destroying a tiny bit of mass and creating vast amounts of energy. Nuclear fission produces, gram for gram, thousands of times more energy than burning fossil fuels.
NUCLEAR FISSION REACTORS operate on the following reaction:Eg. UraniumUranium 235 absorbs a neutron to become Uranium 236
24 | P a g e
Whole body equivalent does (mSv)
Exposure
0.0010 Annual dose from nuclear power0.04 Dose during coast to coast flight
across Canada0.1 Typical chest X-ray1 Dose limit for general public above
natural background radiation3 Average annual dose from natural
radiation10 CT body scan12 Apollo astronaut on lunar mission20 Annual dose limit for radiation
workers averaged over a 5 year period
Uranium 236 is very unstable and exists very briefly and splits into two smaller nuclei and neutrons and release large amounts of energy
U-235 is the FISSIONABLE FUEL. It is found in the reactor in the form of uranium oxide pellets, sealed into FUEL RODS. When nuclei of U-235 are hit by a SLOW-MOVING NEUTRON, the nucleus absorbs the neutron and splits apart into TWO SMALLER NUCLEI (usually of different sizes). The split also releases 2 or 3 (depending on what the two smaller nuclei were) FAST-MOVING NEUTRONS, which, if you can slow them down, can be used to split even more U-235 nuclei. This process destroys a tiny bit of mass, producing huge amounts of energy. In order to slow the fast-moving neutrons down so that more U-235 nuclei can absorb them, the fuel rods are placed into a MODERATOR, a material that can slow the neutrons down without stopping them. In the United States, water is used as a moderator. This is convenient, because the water also acts as a coolant in the case of an emergency. The Chernobyl reactor used a moderator made of graphite, which does the job, but is a solid and has no cooling properties. Graphite is found in your pencil (it is crystalline carbon). As more and more U-235 nuclei are split, even more neutrons are released. This produces a CHAIN REACTION, which can get out of control and cause the U-235 to heat up enough to melt. Molten U-235 can melt its way right through the reactor’s containment structure, which is what happened in Chernobyl. In 1979, the Three Mile Island nuclear power plant near Harrisburg, Pennsylvania, had a partial meltdown, but the multiple levels of containment kept the radioactive material from escaping. To control the flow of neutrons, CONTROL RODS can be inserted between the fuel rods. These are made of steel alloys that have very high melting points, and can absorb neutrons without becoming radioactive themselves. To slow the chain reaction down, the rods are pushed further down between the fuel rods, and to speed the reaction up, the rods are raised until the desired temperature is reached. In the event of an emergency, or if the reactor needs to be shut down for maintenance (like refueling the reactor), the control rods drop all the way down, cutting off all of the neutrons flying between the fuel rods and stopping the reaction completely. This mechanism was defective in the Chernobyl plant, leading to a meltdown and a pressure-induced explosion.
How Uranium Is Obtained
25 | P a g e
U-235 exists only in tiny concentrations (0.72%) in naturally occurring uranium ore. To make it suitable for fuel rods, the uranium must be ENRICHED to a minimum of 3%. This is done using diffusion or centrifuge techniques. In a centrifuge, the uranium (which boils at 3818C) is vaporized into a gas. It is put into a centrifuge, which spins the sample and separates the isotopes out by mass. In this manner, uranium can be enriched to 3% for nuclear power plants, or beyond, up to 90% or more for nuclear fission bombs.
Nuclear Fission BombsA nuclear fission bomb releases thousands of times more energy than chemical explosives. The design is nearly identical to a fission reactor, except that a bomb has 90% or more enriched U-235, and no control rods. A typical fission bomb can produce an explosion with destructive energy equivalent to many thousands of tons (kilotons) of TNT, one of the most powerful chemical explosives. The bomb “Little Boy”, dropped on Hiroshima, Japan on August 6th, 1945, exploded with approximately 15 kilotons of force. This explosion produced a fireball 1200 feet across, which vaporized everything in its path. The heat caused a firestorm with a two-mile diameter that destroyed everything in that area. This bomb killed about 140,000 people. And yet, nuclear fission bombs are far from the most powerful weapon humans have created.
NUCLEAR FUSION
Nuclear fusion reactions occur in the sun and supply the energy needed to sustain life on Earth. In a nuclear fusion reaction two small nuclei combine together to form a single larger nucleus with slightly less mass than the nuclei that combined. The mass of the product nucleus is less than the sum of the masses of the original nuclei. The difference in mass, the mass defect, is converted into massive amounts of energy. In order for the two nuclei to join together, their natural electrostatic repulsion needs to be overcome and the nuclei must collide at very high speeds to do this. This requires a large amount of energy which is obtained from very high temperatures. The energy output of fusion reactions is even greater than the energy output of fission reactions. There are no harmful waste products in the form of long lived radioactive isotopes with nuclear fusion, but a major drawback is that extremely high temperatures are required. Whereas fission reactions can happen at any temperature, fusion can only occur at temperatures of millions of degrees. There are a few scientists who have claimed to have discovered “cold fusion” (at room temperature), but none of their experiments have been able to be duplicated. Because of technical issues, we have not been able to create a sustained fusion reaction. Our current technology uses more energy to make the fusion happen than we get out of it. Until that hurdle is overcome, fusion will be of limited use to us. Once we have broken the technological barrier, we will have at our disposal limitless energy, and the only fuel is the most common element in the universe: hydrogen.
26 | P a g e
Nuclear Fusion BombsIn order to reach the extremely high temperatures required for fusion, nuclear fusion bombs contain in them a nuclear fission bomb. The fission bomb generates the heat needed to get the fusion going. These bombs are thousands of times more powerful than plain old fission bombs. Fusion bombs are also known as “H-Bombs” or “Thermonuclear Weapons”. The United States has a total of over 4000 active nuclear weapons, combining both fission and fusion devices. Russia has nearly 6000. No other nation has more than 200 active nuclear weapons at the current time. The United Kingdom, France, China, India, Pakistan, North Korea and Israel all have an active nuclear weapons program, and Iran and Syria are suspected to have ones themselves. The most powerful thermonuclear bomb that has ever been exploded was the 50 megaton “Tsar Bomba”, which was detonated in a test on October 31st, 1961. There is a testing ban on nuclear weapons in force around the globe, which North Korea broke in 2006. Since then, there have been no (known) nuclear explosions on earth. An idea was floated to seal the 2010 Deep Horizon oil rig leak with a nuclear bomb a mile below the ocean’s surface, but that idea was never considered to be viable due to nuclear test ban treaties.
So, what’s the difference between NUCLEAR FISSION and NUCLEAR FUSION?
Unique To Nuclear Fission235 1 92 141 192U + 0n 36Kr +56Ba +3 0n + energy
COMMON TO BOTH Unique to Nuclear Fusion2 2 41H + 1H 2He + energy
Reaction splits a large nucleus apart to form two smaller ones.
Both generate their energy the same way by converting small amounts of mass (MASS DEFECT) into extraordinary amounts of energy.
Reaction combines two small nuclei together to form one larger one.
Reaction is unknown in the naturalworld, is a form of artificial
All stars are powered by nuclearfusion
27 | P a g e
transmutationReaction can take place at anytemperature or pressure
Reaction requires temperatures ofmillions of degrees and vastpressures
Reaction is currently being used toproduce electricity for our use
Reaction has not been made energy efficient enough for use
Requires mining to extract uraniumore
Hydrogen is the most abundantelement in the universe
Produces THOUSANDS of timesmore energy than conventionalchemical explosives
Produces MILLIONS of times moreenergy than conventional chemicalexplosives
Produces radioactive wastes Produces essentially no radioactivewaste
Nuclear Power GenerationThe energy produced from individual nuclear fission reactions is very small. To produce a useful amount of energy, many fission reactions need to occur simultaneously. Physicists realized that the clue lay in the neutrons emitted as part of a nuclear fission reaction. When a neutron hits a uranium-235 atom to produce a uranium-236 atom, the uranium-236 undergoes fission and produces two or three neutrons. Those neutrons could then cause other nuclear fission reactions and continue the process in a chain reaction.
The diagram below shows how a chain reaction could produce a large number of nuclear fission reactions. A single neutron begins the chain reaction. One event can cause three events, which can then cause nine events and so on. This is exactly what is needed to produce a large number of reactions required to produce usable energy from nuclear fission, but the rapid increase in the number of fissions also clearly gives it the potential to run out of control.
28 | P a g e
After the discovery of nuclear fission in 1939, Enrico Fermi began designing a way to create a self sustaining chain reaction. Fermi and his team built the first nuclear fission reactor in Chicago.
Before building the nuclear fission reactor, Fermi and his team of scientists had to solve five major problems. First, they had to determine which material would be used. Not every neutron produced as a result of fission is aimed at a uranium-235 atom. Rocks containing uranium are relatively common on Earth’s surface but do not form a chain reaction since the uranium atoms are spread relatively far apart. Even if the uranium is separated from the ore, over 99 % of the naturally occurring uranium is uranium-238, which absorbs neutrons without undergoing fission. The uranium had to be enriched to increase the proportion of uranium-235 atoms. The second problem was that the neutrons that are emitted in a nuclear fission reaction have high speed. However, the uranium-235 nucleus will only absorb a slow-moving neutron. To slow the neutrons down, scientists needed to find a moderator, which is a material that slows neutrons down as they move through it. Possible moderators considered were water (H2O), heavy water (D2O), and carbon. The hydrogen atoms in water tended to absorb the neutrons, so water could not be used. Heavy water is chemically identical to normal water except that the molecule is formed with deuterium atoms (21 H) rather than hydrogen atoms (11H). It is called “heavy water” because deuterium atoms are twice as heavy as hydrogen atoms. Heavy water was scarce and expensive to produce, so graphite, a form of carbon, was used as a moderator in the first nuclear fission reactor. The third problem was that the neutrons would simply escape if there was not enough uranium fuel. The minimum mass of fuel needed to produce a chain reaction is called the critical mass. The critical mass depends on the isotope used. For example, the critical mass for uranium-235 is only several kilograms. The fourth problem involved controlling the fission reaction. An uncontrolled chain reaction results in an explosion. To produce usable energy, the reaction must be controlled. Moveable control rods made of cadmium or boron were used to absorb neutrons. The rods were inserted near the uranium-235 fuel to slow down, or withdrawn to speed up, the rate of nuclear fission reactions.The fifth and final problem was dealing with the large amount of heat energy generated during the reaction. A coolant was used to remove the heat energy, which was then converted into electrical energy. Typically, the heat energy is used to boil water and produce steam that drives a turbine. The turbine is connected to a generator, which produces electricity.
Nuclear Fission ReactorsThere are over 440 nuclear reactors worldwide supplying 17 % of the world’s electricity. There are different designs for nuclear fission reactors. They differ in the substances used for the moderator and the coolant.
The CANDU ReactorsCANDU reactors have been supplying electricity to Canadian consumers since 1962. Today there are 32 CANDU reactors around the world, including 18 in Canada (Figure 3). The name CANDU comes from three words: CANadian, Deuterium, and Uranium. The reactor was designed in Canada, uses heavy water (dideuterium oxide) as both a 29 | P a g e
moderator and coolant, and natural uranium as the fuel (Figure 4). Natural uranium is cheaper, more easily available, and does not need enrichment facilities. Natural uranium only contains about 0.7 % uranium-235; the rest is uranium-238, which does not undergo fission readily. To increase the probability of fission, an excellent moderator, heavy water, is used to slow down the neutrons. The CANDU reactor is known as a pressurized heavy water reactor (PHWR). Heavy water is an expensive moderator and coolant. Other types of reactors use cheaper moderators, such as water or graphite. However, cheaper moderators require the use of expensive enriched uranium. The CANDU reactor also has control rods made of cadmium. By adjusting the amount the control rods are inserted, the rate of fissions and, therefore, the heat output is controlled. Although the fuel rods themselves are not radioactive, some of the fission products created are radioactive isotopes, which decay by producing alpha and beta particles, and gamma rays. While the alpha and beta particles produced can be easily stopped by solid materials, about a metre of concrete is required to stop the gamma rays. Since neutrons have no charge, they can penetrate solid walls. Neutrons are stopped by materials similar to those used for moderators and materials that contain hydrocarbons. For the safety of people working near the reactor, the core is contained in a series of shells that contain the radioactive by-products and protect workers from the radiation. In addition to shielding some of the operations that need to be done near the reactor, for added protection to workers, some operations are performed by remote control. At the reactor, the nuclear energy is converted into heat energy. The heat energy still needs to be converted into electrical energy. The heat is transported away from the reactor core by the heavy water coolant and transferred to fresh water. The fresh water boils and the steam turns a turbine, which causes a generator to produce electricity. The voltage of the electricity is raised by a transformer and transmitted down high voltage lines to consumers.
Pressurized Water ReactorThe most common nuclear fission reactor in the world is the pressurized water reactor (PWR). The PWR was originally developed to power nuclear submarines. This type of reactor uses normal water for the moderator and coolant. Since normal water absorbs neutrons, PWRs must use uranium fuel that has been enriched so that the percentage of uranium-235 in natural uranium is raised from 0.7 % to about 3 %. The coolant loop in the reactor is at high pressure and requires the use of high-strength materials, which increases the cost of the system. A disadvantage of the PWR is that it must be shut down every 12 to 18 months for refuelling. There are no PWRs in Canada.
Nuclear PowerRegardless of the type of reactor used, there are both advantages and disadvantages of nuclear power. Using nuclear energy to generate electricity does not produce air pollution. However, there are safety concerns associated with reactors. Although reactors have high levels of safety built into them, there is an inherent risk associated with the possibility of an accident. As reactors age, there is an increasing danger of minor failures of materials that may allow the release of radioactive materials into the environment (Figure 6). The advantages and disadvantages of nuclear power are summarized below.
30 | P a g e
Advantages: requires inexpensive fuel low levels of carbon dioxide produced produces large amounts of energy in a single plant fuel is easy to transport uranium is widely distributed around the world does not create acid precipitation or air pollution
Disadvantages requires large, expensive containment and waste storage facilities risk of accidents (although it is low) produces radioactive waste that requires long-term storage potential for waste products to be used in nuclear weapons limited supply of uranium on Earth perceived by the public as undesirable or too risky
A major concern with nuclear reactors is the possibility of a meltdown where the heat generated by the nuclear process increases uncontrollably and melts the materials containing the reactor. This would result in an explosion or fire that releases large amounts of radioactive materials into the environment. Nuclear power plants generate waste heat. In a nuclear power plant, the hot water used to drive the turbines often is dumped into a nearby lake or river. This can be avoided by recirculating water through large cooling towers. While these problems are of significant concern, the problem of nuclear waste products is both an immediate and long-term problem. Since the fission of uranium (or other nuclear fuel) produces a wide variety of fragments, there is a wide assortment of isotopes, many of which are radioactive. Some of the isotopes have short half-lives. For example, the half-life of iodine-131 is 8 days, while the half-life of krypton-85 is 11 years. However, the spent fuel rods also contain radioactive wastes that have very long half-lives. For example, the half-life of technetium-99 is 210 000 years. To deal with the short half-lives, the spent fuel rods are stored in water for several years. However, isotopes with long half-lives will be a problem for a very long time. There is an increasing amount of nuclear waste currently stored in temporary facilities around the world waiting for a permanent solution (Figure 7). One solution is to bury the radioactive waste deep in the ground in storage containers. This solution requires that the ground be geologically stable. The Canadian Shield has been proposed as a suitable area. However, people may be opposed to living near areas where radioactive wastes are stored.
Read page 307 – 322Workbook Questions1. Complete the following tableIsotope # Protons #Neutrons # Electrons Nuclear Charge39 K1942 Ca2056 Fe26
31 | P a g e
232 U92
2. What is the average atomic mass for thallium, Tl, if there are two isotopes with the following masses and abundances?
Tl-203 has a mass of 203 amu with an abundance of 29.5%Tl-205 has a mass of 205 amu with an abundance of 70.5%
3. A meteor crashes to Earth, is collected and analyzed. To everyone’s surprise, a new element is discovered with an atomic number of 120. Calculate the weight-average atomic mass for this new element, Ubn (Unbinnulium).
4. Given the unstable isotope symbol, mass and decay type, determine the daughter nuclide (decay product) by writing the complete nuclear equation (use atomic numbers and symbols from the periodic table.Example: 104 0 104
47Ag ----> -1e + 48Cd
a. beta decay of Kr-87b. alpha decay of Po-212c. beta decay of C-14d. alpha decay of u-240e. beta decay of F-20f. beta decay of Tl-208
5. Balance the following Nuclear Equations
a. 210 Po 206 Pb + f. 226 Ra + 4He84 82 88 2
b. 222 Rn + 4 He g. 234 Th + 4He86 2 90 2
c. 230 Th 226 Ra + h. 234 Th 234 Np + 90 88 92 93
d. 214 Pb + 0 e i. 206 Pb + 4 He82 -1 82 2
6. Write an equation to describe the alpha decay of a radium-226 nucleus to form a radon nucleus.
7. Write an equation to describe the alpha decay of a radon nucleus to form a polonium-218 nucleus.
8. Write an equation to describe the beta decay of a lead-214 nucleus to form a bismuth-214 nucleus.
9. Write the series of equations involving three alpha decays and two beta decays that transmutes a uranium-238 nucleus into a radium-226 nucleus. (The order is α, β, β, α, α)32 | P a g e
10. Neutron bombardment of plutonium-239 yields americium-240 and another particle. Write the nuclear equation and identify the other particle produced.
11) What is the half-life of a radioactive isotope if 25% of the original mass of the isotope remains after 20. days?
12) A Geiger counter is used to monitor the radioactivity level of a certain isotope. During a 30 .hour period, the count rate dropped from 600. counts/minute to 150. counts/minute. What is its half-life?
13) The half-life of cesium-137 is 30. years. How much 137Cs was present originally if, after 120 years, 6.0 g remained?
14) The half-life of barium-131 is 12.0 days. How many grams of 131Ba remain after 60 days, if the initial sample weighed 10.0 g?
15) How much 32P was present originally if, after 71.5 days, 2.0 grams remain (half-life of 32P is 14.3 days)
16. Examine the graph showing the decay curve for carbon-14. The graph shows the amount ofradioactive carbon-14 that would be in a sample of organic material for 30 000 years after theorganism died.
(a) Define half-life.____________________________________________________________________________
(b) How long is one half-life for carbon-14? ____________________
(c) What percentage of carbon-14 remains (i) after one half-life? ____________________(ii) after two half-lives? ________________ (iii) after three half-lives? ________________
(d) Use the graph to estimate the percentage of carbon-14 remaining after(i) 5000 years __________ (ii) 10 000 years __________ (iii) 15 000 years __________.
(e) Use the graph to estimate the number of years that have passed since the organism died if33 | P a g e
the percentage of parent isotope that remains is(i) 40% ___________ (ii) 20% ____________ (iii) 5% ____________ .
(f) Explain why carbon-14 half-life measurements are not effective in dating an organismthat has been dead for more than 50 000 years.____________________________________________________________________________
17. Volcanic rocks can be dated using the potassium-40 clock, a dating method based on thedecay of the potassium-40 isotope into the argon-40 isotope. Potassium-40 can exist as hotmolten rock, whereas argon-40, the daughter isotope, escapes from the molten rock becauseit is a gas. When the molten rock solidifies, potassium-40 is present, but argon-40 is absent.The age of volcanic rock can be measured by comparing the amount of these two isotopespresent in the rock.
(a) Why is there no argon-40 present in the molten rock when it solidifies?________________________________________________________________________________________________________________________________________________________
(b) After many years, argon-40 is present in volcanic rock containing potassium-40, eventhough no argon-40 was there to begin with. How did the argon-40 get there?________________________________________________________________________________________________________________________________________________________(c) What is the length, in years, of one half-life of potassium-40? ____________________(d) Suppose a sample of volcanic rock contained 100 nanograms (a nanogram is a billionth ofa gram) of potassium-40 when the rock first formed. How many nanograms of potassium-40and of argon-40 would be present in the sample after
(i) 1 half-life? ________________(ii) 2 half-lives? ________________(iii) 3 half-lives? ________________
18. The table of parent-daughter isotopes shows three different isotope pairs that are used inradioisotope dating. Examine the chart and answer the following questions.
34 | P a g e
Num
ber
of
atom
s
(mill
ions
)
(a) Lead-207 is called the daughter of uranium-235. What does this mean?________________________________________________________________________________________________________________________________________________________(b) How old is a rock sample that contains uranium-235 and lead-207 in equal amounts?_________________________(c) The age of Earth was first established in 1953 when Claire C. Patterson of the CaliforniaInstitute of Technology used a uranium-lead clock to analyze rock. In comparing amounts ofuranium-235 with lead-207, he established that slightly less than 8 half-lives of uranium-207had passed since the rock formed. Using this data, estimate the age of Earth._________________________(d) The rocks that make up the Canadian shield are extremely old. They are estimated to bebetween 3.7 and 3.8 billion years. (A billion is a thousand million).(i) Estimate how many half-lives of uranium-235 would have passed in a sample of rockthis old. _________________________(ii) Estimate the percentage of original uranium that would remain in a sample of rock fromthe Canadian shield. _________________________(e) Suppose a sample of rock from the Canadian shield were analyzed using the potassium-40clock. What information could this give about the age of rock?____________________________________________________________________________(f) Could carbon-14 dating be used to estimate the age of rocks in the Canadian shield? Explain.________________________________________________________________________________________________________________________________________________________
19. Rhenium (Re)-184 has a half-life of 38 d. A sample containing Re-184 has an activity of 1 296 Bq.(a) What will the activity of the sample be in 152 days?(b) What was the activity of the sample 190 days ago?(c) After how many days will the activity level of the sample be 324 Bq?
20. The graph below shows the decay of a sample of platinum (Pt)-197.(a) What is the half-life of Pt-197?(b) Approximately how many atoms ofPt-197 will remain after 72 hours?
35 | P a g e
0 10 20 30 40 500
100
200
300
400
500
600
Time (hr)21. If you start off with 100 grams of Uranium-238, how much would you have left after 4 half-lives?
b. What if you started off with 200 grams of Uranium-238?c. What if you started with 500 grams of Uranium-238?
22. Carbon-14 has a half-life of 5,700 years and decays to produce the daughter element Nitrogen-14. If you start off with 100 grams of Carbon-14, how much Nitrogen-14 will exist after 17,100 years?
23. If a sample contains 50g of Carbon14 and 50g of Nitrogen14, how many half-lives has it undergone?
24. If a sample contains 25g of Carbon14 and 75g of Nitrogen14, how many half-lives has it undergone?
25. How old is a bone in which the Carbon14 in it has undergone 3 half-lives?
26. What percent of Carbon14 is left after 5 half-lives?
27. What happens to the amount of Nitrogen14 as the Carbon14 decays?
28. If a 20g of Carbon14 has a half-life of 5,700 years, what would be the half-life of a 40g sample?
29. An ancient scroll made of papyrus is analyzed, and it is found to contain only 25% of the steady-state concentration of C-14 found in living organisms. How old is the material that the scroll is made of?
30. Why would the 238U to 206Pb method be inappropriate for determining the age of a biological sample thought to be about 5000 years old? Explain, using the half-life duration of 238U to support your explanation.
31. 1. When a high-energy neutron strikes an atom of uranium-235, the uranium atom cansplit apart to form an atom of barium-143, an atom of krypton-91, and two neutrons.
a. What are the symbols for the starting materials?b. What are the symbols for the products?c. Write the nuclear equation for this reaction.
32. Boron-10 is used in some kinds of cancer therapies. When a neutron collides with a boron-10 atom, the products are an atom of lithium-7 and an atom of helium-4.
a. What are the symbols for the starting materials?b. What are the symbols for the products?c. Write the nuclear equation for this reaction.
36 | P a g e
0 10 20 30 40 500
100
200
300
400
500
600
d. Confirm that the total number of protons and the total number of protons plus neutrons is conserved. Show your work.
34 .During one type of nuclear fusion, an atom of helium-3 reacts with an atom of helium-4 to produce an atom of beryllium-7.
a. What are the symbols for the starting materials?b. What are the symbols for the products?c. Write the nuclear equation for this reaction.
35. One possible reaction that may occur in a nuclear fusion reactor is the reaction of an atom of hydrogen-2 with an atom of hydrogen-3 to form an atom of helium-4 and a neutron.
a. What are the symbols for the starting materials?b. What are the symbols for the products?c. Write the nuclear equation for this reaction.
37. Complete the following TableProperty Decay, Transmutation, Fission, Fusion?Requires temperatures of millions of degrees
Takes two small nuclei and combine them into a larger nucleus
Takes a stable nucleus and turns it into an unstable one
Releases millions of times more energy than chemical reactions
Takes a large nucleus and splits it into smaller nuclei
Happens all by itself because the nucleus is unstable
Can be detected with photographic film
Releases thousands of times more energy than chemical reactions
Requires a particle accelerator to carry out
Used to power nuclear submarines and aircraft carriers
37 | P a g e