Using the Periodic Table 6 Carbon C 12.011 amu Atomic number- always a whole number, increases in...
-
Upload
gervase-joseph -
Category
Documents
-
view
221 -
download
0
Transcript of Using the Periodic Table 6 Carbon C 12.011 amu Atomic number- always a whole number, increases in...

Using the Periodic Table
6Carbon
C12.011 amu
• Atomic number- always a whole number, increases in order, represents the number of protons in each atom
• Atomic name, often in Latin, sometimes uses a common name, sometimes there are disagreements
• Atomic symbol – often, but not always, matches the name; Always capitalize the first (only the first ) letter
• Atomic mass- the mass of the atoms based on the average of all the most common isotopes

Atoms
Chapter 6

• Aristotle, the famous Greek philosopher and scientist, argued that a substance could be divided again and again, forever getting smaller and smaller pieces
• During his time people believed the universe was made of four elements; fire, water, earth and air.

• The idea of “uncuttable” particles was first suggested by Democritus around 440 B.C.
• “Atomos” means indivisible

Section 1 Atomic Theory
• Atomic Theory (by John Dalton) published in 1803– All substances are made
from atoms. Atoms cannot be created, divided or destroyed.
– Atoms of the same element are exactly alike.
– Atoms can join together to form new substances.

• JJ Thomson discovered the negatively charged electrons inside the atom.
• Ernest Rutherford discovered that the atom is mostly empty space with a tiny positive nucleus and orbiting electrons.
• Neils Bohr determined that electrons traveled along paths or energy levels.
• Schrodinger & Heisenberg

Pictures of Atoms

Atomic Size• Atoms are extremely small…• Aluminum foil is 100,000 atoms thick.
• A Penny has 20,000,000,000,000,000,000,000 atoms!

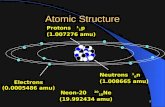
What’s Inside an Atom?Protons (+):
Positively Charged Particles
Mass=1amu
Location: Nucleus
Neutrons (0):
Particles with No Charge
Mass=1amu
Location: Nucleus
Electrons (-):
Negatively Charged Particles
Mass= almost zero
Location: Electron Clouds

Parts of the Atom
Particle Charge Location Size
Proton Positive In the nucleus 1 a.m.u.
Neutron No charge (neutral)
In the nucleusAlmost 1 a.m.u.
Electron NegativeOutside the nucleus, orbiting at nearly the
speed of light
Very tiny, 1/1000th a.m.u.

Structure of the Atom
• Protons– Positively charged– Found in the nucleus of the
atom (center)– Mass of 1 amu (atomic mass
unit)– Gives the element its atomic
number on the periodic table• #1 (hydrogen) has 1 proton
• #4 (beryllium) has 4 protons
+
Protrons were discovered by Ernest Rutherford in 1911 and he discovered the empty space within an atom in 1909

• Neutrons– Neutral charge ( no
charge)– Found in the nucleus
(center) – Mass slightly smaller
than a proton, still considered 1 amu
– Protons + Neutrons atomic mass (rounded to the nearest whole number)
N

• Isotope– Atoms can often be found
with different numbers of neutrons
• Some atoms have only one stable isotope, others have several
• Averaging the masses of the isotopes in their correct percentages gives the exact atomic mass for each element
• Unstable isotopes break down (radioactive decay)
Hydrogen (H-1), most common 99.9%
tritium (H-3), least common, radioactive
deuterium (H-2),

• Electrons– Negatively charged
particles– Found outside the nucleus,
moving at nearly the speed of light, in specific levels
– Most commonly atoms are neutral particles having equal numbers of protons and electrons
– Very small ( about 1/1000th of a proton)
Proton
Neutron
Electron
Electrons were discovered by J.J. Thompson in 1897

* In 1913 Niels Bohr suggested that electrons travel in specific paths called Electron Shells
• Each level can only contain a certain number of electrons before it is full
• Each level will fill completely before electrons go to the next level
• Lower levels fill first
1832