This type of data may also be presented in graphical form from a mass spectrometer.
description
Transcript of This type of data may also be presented in graphical form from a mass spectrometer.


http://www.rsc.org/learn-chemistry/resource/res00001041/spectroscopy-videos#!
cmpid=CMP00001768

The mass spectrometer can give information about the relative isotopic abundances of
different elements

Antimony has two isotopes 121-Sb and 123-Sb. In a bullet at a crime scene there was a sample of antimony containing 57.3% 121-Sb and 42.7% 123-Sb.
Calculate the relative atomic mass of the sample of antimony from the bullet.

This type of data may also be presented in graphical form from a mass spectrometer.

Tungsten
182 183 184 185 186
M/Z
Rela
tive
abun
danc
e
1
2
3
4

Chlorine
34 35 36 37 38
Relative atomic mass
Rela
tive
abun
danc
e
1
2
3
4

Complete Question 1 and 2 on page 171

Complete the isotopes exam questions
Finish for prep

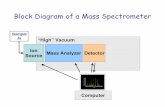
What do you remember about mass spectrometry?

Make a spider chart on the uses of mass spectrometry

Identifying organic compounds• Organic molecules do not hold charge well.
• The ions break up or fragment.
• They break into smaller ions and neutral fragments.
• They do this in regular patterns.i.e. the same molecule always fragments in the same way.• This allows us to analyse them.

Mass spectrum of propan-1-ol

Mass Spectrum of ethanol
Molecular ion: tells you the molecular mass of the whole compound.

Mass Spectrum of ethanol: Identify the fragments responsible for the following
peaks:
46
45
31
29
15

+
Possible fragments
+
+ +
+M+
46
29
45
15
31
++

Identify the fragments responsible for the peaks at 15, 29 and 44 and thus identify this alkane.

Mass Spectrum of an alcohol. Identify the alcohol and draw structures for the fragment ions represented by the peaks at: 60,
59, 43, 31 and 29.

Possible fragments
+ +
+ +
+M+
60
43
59
29
31
++


IR spectroscopy
All molecules absorb Infra-red radiation
The absorbed energy makes covalent bonds vibrate (bending or stretching)

All molecules absorb infrared radiation; absorbing energy makes the bonds vibrate.
Every bond vibrates at its own frequency, dependant upon bond strength, bond length and the mass of atoms involved in bond.
STRETCHING
BENDING

http://www.rsc.org/learn-chemistry/resource/res00001041/spectroscopy-videos#!cmpid=CMP00001771

How IR spectroscopy works…
•Shine a range of IR frequencies, one at a time through a sample of organic compound and at some of the frequencies the energy will be absorbed. •A detector on the other side of the sample would show that some frequencies are absorbed whilst others are not.

• If a particular frequency is being absorbed as it passes through the compound being investigated, it must mean that its energy is being transferred to the compound.
• Energies in infra-red radiation correspond to the energies involved in bond vibrations.

Bend and Stretch
• In covalent bonds, atoms aren't joined by rigid links;
• the two atoms are held together because both nuclei are attracted to the same pair of electrons.
• The two nuclei can vibrate backwards and forwards - towards and away from each other - around an average position.

Fingerprint regions
• You must be able to identify the following peaks.– C-H in alkanes/alkenes/aldehydes– O-H in alcohols/carboxylic acids– N-H in amines– C=O in aldehydes and ketones– C-X in halogenoalkanes
• You will have data books in exam, if required.

http://www.rsc.org/learn-chemistry/resource/res00000059/infra-red-spectrometer

C-O in alcohols, esters & carboxylic acids 1000-1300
C=O in aldehydes and ketones 1640-1750
C-H in alkanes/alkenes/aldehydes 2850-3100
O-H in carboxylic acids 2500-3300 (very broad)
O-H in alcohols/carboxylic acids 3200-3550 (broad)
Identify the group next to the yellow box using the key below.

C-O in alcohols, esters & carboxylic acids 1000-1300
C=O in aldehydes and ketones 1640-1750
C-H in alkanes/alkenes/aldehydes 2850-3100
O-H in carboxylic acids 2500-3300 (very broad)
O-H in alcohols/carboxylic acids 3200-3550 (broad)
Identify the groups next to the yellow boxes using the key below.

C-O in alcohols, esters & carboxylic acids 1000-1300
C=O in aldehydes, ketones & carboxylic acids 1640-1750
C-H in alkanes/alkenes/aldehydes 2850-3100
O-H in carboxylic acids 2500-3300 (very broad)
N-H in amines/amides 3200-3500
O-H in alcohols/carboxylic acids 3200-3550 (broad)


Whiteboard quiz

Quiz
1) Identify the species responsible for the peak at m/z = 29

2) Structural formula of 1-chlorobutane

3) What is the mass of the molecular ion?

4) Skeletal formula of ethanol

5) How do you get from an alcohol to a carboxylic acid?

6) What is missing?

C-O in alcohols, esters & carboxylic acids 1000-1300
C=O in aldehydes and ketones 1640-1750
C-H in alkanes/alkenes/aldehydes 2850-3100
O-H in carboxylic acids 2500-3300 (very broad)
O-H in alcohols/carboxylic acids 3200-3550 (broad)
7) Identify the group next to the yellow box.

8) Draw the mechanism for the reaction of ethene with Hydrogen Bromide