The Puzzle of the Atom Protons and electrons are attracted to each other because of opposite...
-
Upload
clyde-walters -
Category
Documents
-
view
216 -
download
0
Transcript of The Puzzle of the Atom Protons and electrons are attracted to each other because of opposite...

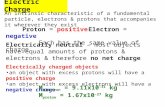
The Puzzle of the AtomThe Puzzle of the Atom Protons and electrons are attracted to each other because of opposite charges
Electrically charged particles moving in a curved path give off energy
Despite these facts, atoms don’t collapse

Wave-Particle DualityWave-Particle DualityJJ Thomson won the Nobel prize for describing the electron as a particle.
His son, George Thomson won the Nobel prize for describing the wave-like nature of the electron.
The electron
is a particle!
The electron is an energy
wave!

The Wave-like ElectronThe Wave-like Electron
Louis deBroglie
The electron propagates through space as an energy
wave. To understand the atom, one must
understand the behavior of
electromagnetic waves.

c = C = speed of light, a constant (3.00 x 108 m/s)
= frequency, in units of hertz (hz, sec-1)
= wavelength, in meters
Electromagnetic radiation Electromagnetic radiation propagates through space as a wave propagates through space as a wave moving at the speed of light.moving at the speed of light.

Electromagnetic radiation.

Electromagnetic RadiationElectromagnetic RadiationElectromagnetic RadiationElectromagnetic Radiation• Most subatomic particles behave Most subatomic particles behave
as PARTICLES and obey the as PARTICLES and obey the physics of waves.physics of waves.

• Waves have a frequencyWaves have a frequency• Use the Greek letter “nu”, Use the Greek letter “nu”, , for , for
frequency, and units are “cycles per frequency, and units are “cycles per sec”sec”
• All radiation: All radiation: • • = c = cwhere c = velocity of light = 3.00 x where c = velocity of light = 3.00 x 101088 m/sec m/sec
Electromagnetic RadiationElectromagnetic RadiationElectromagnetic RadiationElectromagnetic Radiation

Types of electromagnetic radiation:Types of electromagnetic radiation:

The Electromagnetic Spectrum
AM radio
Short waveradio
Television channels
FM radio
RadarMicrowave
Radio Waves Gamma Rays
X- Raysinfrared
Increasing photon energy
Increasing frequency
Decreasing wavelength
Red Orange Yellow Green Blue Indigo Violet
UV Rays
Visible
Light
R O Y G B I V

E = h EE = Energy, in units of Joules (kg·m= Energy, in units of Joules (kg·m22/s/s22))
hh = Planck’s constant (6.626 x 10= Planck’s constant (6.626 x 10-34-34 J·s) J·s)
= frequency, in units of hertz (hz, sec= frequency, in units of hertz (hz, sec-1-1))
The energy (The energy (E E ) of electromagnetic ) of electromagnetic radiation is directly proportional to radiation is directly proportional to the frequency (the frequency () of the radiation.) of the radiation.

Long Wavelength
= Low Frequency
= Low ENERGY
Short Wavelength
= High Frequency
= High ENERGY
Wavelength TableWavelength Table

Relating Frequency, Relating Frequency, Wavelength and EnergyWavelength and Energy
c hE
hc
E
Common re-arrangements:
E
hc

Modern model of the atom
Every element has a unique chemical behavior: carbon acts like carbon; iron like iron. In 1913, the Danish physicist Niels Bohr proposed a model of the atom that explained these differences, as well as other observations known at the time.

Line Emission Spectra Line Emission Spectra of Excited Atomsof Excited AtomsLine Emission Spectra Line Emission Spectra of Excited Atomsof Excited Atoms
• Excited atoms emit light of only certain wavelengths
• The wavelengths of emitted light depend on the element.

…produces all of the colors in a continuous spectrum
Spectroscopic analysis of the visible Spectroscopic analysis of the visible spectrum…spectrum…

Spectrum of Spectrum of Excited Hydrogen GasExcited Hydrogen Gas

Line Spectra of Other ElementsLine Spectra of Other Elements

What did Bohr’s model propose?
Bohr's central insight was that the electrons in an atom can have only certain, specific energies: Their energy is quantized.
• The higher an electron's energy, the farther the electron is from the nucleus. For this reason, he called the energy levels shells.

The Bohr Model of the AtomThe Bohr Model of the Atom
Neils Bohr
I pictured electrons orbiting the nucleus much like planets orbiting the sun.But I was wrong! They’re more like bees around a hive.
WRONG!!!

Physics and Chemistry
QuantumPhysics of atomic
and subatomic particlesObjects can have only certain energies
Classical: objects can have any energy
Physics

Quantum MechanicalQuantum MechanicalModel of the AtomModel of the Atom
Mathematical laws can identify the regions outside of the nucleus where electrons are most likely to be found.
These laws are beyond the scope of this class…

The closer the electron is to the nucleus the lower the electron’s energy and the more stable the atom is.

Bohr’s shells
• He assigned principal quantum numbers to each shell (n)
• n=1 for the first• n=2 for the
second

Electron Energy Level (Shell)Electron Energy Level (Shell)Generally symbolized by n, it denotes the probable distance of the electron from the nucleus.
Number of electrons that can fit in a shell:
2n2

Maximum number of electrons
• The maximum number of electrons in each shell is 2n2
• Electrons in n=1 have the lowest energy and are the most stable

Main features of Bohr’s Model• 1.Orbits get larger as the orbit number
increases(the radius increases)
• 2.Electrons in n=1 orbit are the most stable with lowest energy
• 3.Each orbit hold a maximum of 2n2

Main features of Bohr’s Model
• 4. Orbits are filled with electrons from the innermost shell on out. Electrons are added to each orbit until filled to capacity before moving to next level.

Periodicity and line spectra


What is wrong with Bohr’s model?

Subshells and electron configuration
• Subshells are closely spaced in energy and size
• Letters are assigned to the subshells(s, p, d, f)
• n=2 has s and p subshells• Each shell has a different
maximum amount of electron capacity

Subshells and electron configuration
• Not every level contains all the subshells
• n=1 contains only subshell “s”• Every level gets an additional
subshell

Maximum number of electrons • s=2• p=6• d=10• f=14• Energy increases from spdf• Maximum electron capacity for a
shell is unchanged from Bohr’s original model

Subshells and electron configuration

4S4S
3S3S
2S2S
1S1S
3P3P
2P2P



• Electron configuration: exact way that the electrons are arranged in subshell.
• In the “d” block, n value for d subshell is 1 less than period number.
• In the “f” block, n value for f subshell is 2 less than period number.


Incr
easi
ng e
nerg
y
1s
2s
3s
4s
5s6s
7s
2p
3p
4p
5p
6p
3d
4d
5d
7p 6d
4f
5f

Partial electronic configurations for K through Kr

“f” block

Electron configuration for GadoliniumFrom 1st row: 1s2
From 2nd row: 2s2 2p6
From 3rd row: 3s2 3p6
From 4th row: 4s2 3d10 4p6
From 5th row: 5s2 4d10 5p6
From 6th row: 6s2 5d1 4f7
Gd is 1 deep in d block and 7 in f block

Diagonal Rule
ss
s 3p 3ds 3p 3d
s 2ps 2p
s 4p 4d 4fs 4p 4d 4f
s 5p 5d 5f 5g?s 5p 5d 5f 5g?
s 6p 6d 6f 6g? 6h?s 6p 6d 6f 6g? 6h?
s 7p 7d 7f 7g? 7h? 7i?s 7p 7d 7f 7g? 7h? 7i?
11
22
33
44
55
66
77
Steps:Steps:
1.1. Write the energy levels top to bottom.Write the energy levels top to bottom.
2.2. Write the orbitals in s, p, d, f order. Write Write the orbitals in s, p, d, f order. Write the same number of orbitals as the energy the same number of orbitals as the energy level.level.
3.3. Draw diagonal lines from the top right to the Draw diagonal lines from the top right to the bottom left.bottom left.
4.4. To get the correct order, To get the correct order,
follow the arrows!follow the arrows!
By this point, we are past By this point, we are past the current periodic table the current periodic table so we can stop.so we can stop.

neon's electron configuration (1s22s22p6)
Shorthand Configuration
[Ne] 3s1
third energy level
one electron in the s orbital
orbital shape
Na = [1s22s22p6] 3s1 electron configuration
AA
BB
CC
DD

Octet Rule
• Elements react to form compounds in such a way as to put 8 electrons in their outermost(valence)shell, giving them a valence electron configuration identical to a noble gas and making them exceptionally stable

Metal: element that tends to lose its valence electrons in chemical reactions (cation)
Nonmetal: element that tends to gain valence electrons in a reaction(anion)


Quantum Physicsuncertainty principle• Werner Heisenberg(1930s)• It is impossible to know exactly
where the electron is or even where it is going

Orbitals
An orbital is a region within an An orbital is a region within an atom where there is a probability atom where there is a probability of finding an electron. of finding an electron.

Orbitals of the same shape (s, for instance) grow larger as n increases…
Nodes are regions of low probability within an orbital.
Sizes of Sizes of ss orbitals orbitals

The s orbital has a spherical shape centered around the origin of the three axes in space.
s orbital shape

There are three dumbbell-shaped p orbitals in each energy level above n = 1, each assigned to its own axis (x, y and z) in space.
PP orbital shape orbital shape

Things get a bit more complicated with the five d orbitals that are found in the d sublevels beginning with n = 3. To remember the shapes, think of “double dumbbells”
…and a “dumbbell with a donut”!
d orbital shapes

Shape of f orbitalsShape of f orbitals