Lesson Overview Lesson Overview Properties of Water Lesson Overview 2.2 Properties of Water.
The properties of water
description
Transcript of The properties of water

The properties of water
Life depends on them!

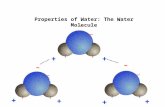
Water is polar
Covalent bond
Covalent bond

Polar molecules
• Molecules are electrically neutral.
• Portions of a molecule can act as though they have an electrical charge if the components have different attractions for electrons.

Polar molecules
• In water, the oxygen acts negative and the hydrogens act postive.
• In effect, a water molecule has a positive and a negative pole, or end.
• As in magnets and ions, opposites attract.

Hydrogen bonds
• Polar molecules with hydrogen atoms are very strongly attracted to the negative regions of other polar molecules.

Cohesion
• Hydrogen bonds forming between water molecules cause them to stick together. This is cohesion.
• Cohesion creates surface tension.

Hydrogen bonds are responsible

Adhesion
• Water molecules are also attracted to other substances, especially if they carry an electrical charge.

High specific heat
• The hydrogen bonds between water molecules mean that a great deal of energy must be added or subtracted to cause a state change: solid to liquid or liquid to gas.
• http://www.bgfl.org/bgfl/custom/resources_ftp/client_ftp/ks3/science/changing_matter/index.htm

High specific heat
• This also means that water helps to prevent large rapid temperature changes in the environment.

Ice is an insulator
• Also, ice can act as an insulator since it floats: ice is less dense than water!
• Again, hydrogen bonds are responsible.

Ice and water

Solutions
• Water is the universal solvent.
• This means that it can dissolve many solutes, especially polar molecules.
• Water also causes ionic compounds to dissociate (separate into ions.)

Sodium chloride dissociates
salt http://programs.northlandcollege.edu/biology/Biology1111/animations/dissolve.html

Dissociation
• The “positive” hydrogens in water are attracted to negative chloride ions.
• The “negative” oxygen in water is attracted to positive sodium ions.
• A shell of water molecules around the ions keeps ionic bonds from reforming.

Water dissolves molecules
• Water molecules will also surround polar molecules.
• Even fairly large molecules with charged regions can be surrounded with water and dissolved.

Water dissolves molecules

Suspensions
• Particles that are too large or are hydrophobic nonpolar molecules will not dissolve.
• If enough energy is added, the particles may be temporarily surrounded by water molecules, but they eventually separate into a distinct layer.

Liquid mixtures in biology
• What are solutions containing dissociated ions good for?
• Dissolved substances can be transported easily, and moved across membranes.
• What is a biological suspension?

Acids and bases
• A small number of water molecules (1 in 550 million or so) will dissociate spontaneously.
H20 H+ + OH-
• Usually the concentrations of H+
and OH- are balanced and the solution is neutral.

Acids
• If a solution contains an excess of H+ ions, the solution is said to be an acid.
• The concentration of H+ ions is measured by the pH scale.
• A higher concentration means a lower pH value.

The pH scale
• The pH (power of Hydrogen) scale runs from 0 to 14.
• Water, with equal concentrations of H+ and OH- ions, has a pH of 7.

Bases
• If there is an excess of OH- ions, the solution is said to be a base, or an alkali.
• A basic solution has a very low concentration of H+ ions and a pH value above 7.

The pH scale
• http://www.johnkyrk.com/pH.html
• What types of materials are bases?
• What types are acids?
• What is the ideal pH of intravenous solutions?

Acid rain
• Increasing acidification of rain has severe environmental consequences.

Effects of acid rain.
http://www.partnersinair.org/en/images/curr_unit2a_bkgd_figure22.jpg



![The International Association for the Properties of Water ... · The International Association for the Properties of Water ... for the Thermodynamic Properties of Water and ... [2].](https://static.fdocuments.us/doc/165x107/5b8f203709d3f2103e8bebc7/the-international-association-for-the-properties-of-water-the-international.jpg)