The primary structure of a protein is the sequence of amino acids in the peptide chain...
-
Upload
darlene-garrett -
Category
Documents
-
view
258 -
download
1
Transcript of The primary structure of a protein is the sequence of amino acids in the peptide chain...
The primary structure of a protein is the sequence of amino acids in the peptide chain
Ala-Leu-Cys-Met
19.6 Primary Structure
CH CH3
CH3
H3N CH C
O
N
H
CH C
O
N
H
CH C
O
N
H
CH C O-
OCH CH2
CH2
S
CH3
CH2
SH
CH3
Protein backbone
Insulin
Insulin: The first protein to have its
primary structure determined 51 residues 2 chains 2 disulfide bridges
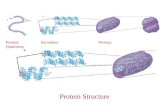
The secondary structure of a protein
indicates the arrangement of the
polypeptide chains in orderly patterns.
1. Alpha helix
2. Beta-pleated sheet
19.7 Secondary Structure
Alpha Helix
The -helix is a three-dimensional arrangement of the polypeptide
chain that gives a corkscrew shape like a coiled telephone cord
The coiled shape of the alpha helix is held in place by hydrogen bonds
between the amide groups and the carbonyl groups of the amino acids
along the chain
Beta-Pleated Sheet
The -pleated sheet: Holds proteins in a parallel arrangement with hydrogen bonds Has R groups that extend above and below the sheet Is typical of fibrous proteins such as silk
Indicate the type of structure as:
1) primary 2) -helix 3) -pleated sheet
A. Polypeptide chains held side by side by H bonds.
B. Sequence of amino acids in a polypeptide chain.
C. Corkscrew shape with H bonds between amino acids.
Learning Check
The tertiary structure: Gives a specific overall shape to a protein Involves interactions and cross-links between R
groups in different areas of the peptide chain Is stabilized by:
Hydrophobic and hydrophilic interactions Salt bridges (electrostatic interactions) Hydrogen bonds Disulfide bridges
19.8 Tertiary Structure
Tertiary Structure
The interactions of the R groups give a protein its specific three-dimensional tertiary structure
Select the type of tertiary interaction as:
1) disulfide 2) salt bridge 3) H-bonds 4) hydrophobic
A. Leucine and valine
B. Cysteine and cysteine
C. Aspartate and lysine
D. Serine and threonine
Learning Check
19.9 Quaternary Structure
The quaternary structure contains two or more tertiary subunits (protein chains)
Held together by same interactions as tertiary structure
Hemoglobin contains four chains
The heme group in each subunit picks up oxygen in the blood for transport to the tissues
Identify the level of protein structure:
A. Beta-pleated sheet
B. Order of amino acids in a protein
C. A protein with two or more peptide chains
D. The shape of a globular protein
E. Disulfide bonds between R groups
Learning Check
Learning Check
In myoglobin, about one-half of the 153 amino acids have nonpolar
side chains.
A. Where would you expect those amino acids to be located in the tertiary
structure?
B. Where would you expect the polar side chains to be?
C. Why is myoglobin more soluble in water than silk or wool?
Learning Check
State whether the following statements apply to primary, secondary, tertiary, or quaternary protein structure:
A. Side groups interact to form disulfide bonds or salt bridges.
B. Peptide bonds join amino acids in a polypeptide chain.
C. Hydrogen bonding between carbonyl oxygen atoms and nitrogen atoms of amide groups causes a polypeptide to coil.
D. Hydrophobic side chains seeking a nonpolar environment move toward the inside of the folded protein.
E. Protein chains of collagen form a triple helix.
F. A protein contains four tertiary subunits.
Amide hydrolysis (review section 16.8):
Protein hydrolysis:
Splits the peptide bonds to give smaller peptides and amino acids
Catalyzed by enzymes
Occurs in the digestion of proteins Occurs in cells when amino acids are needed to synthesize new proteins
and repair tissues
Hydrolysis of Amides
R C OH
O
+ N RR C N
O
+ H2OR
H H
H
acid
or base
Disruption of bonds in the native secondary, tertiary and quaternary protein structures
Covalent amide bonds (primary structure) are not affected
Loss of biological activity with loss of structure
Denaturation
Cooking food containing protein
Wiping the skin with alcohol
(denaturation of bacterial proteins)
Hair permanents
Applications of Denaturation