The effects of serum cholesterol, LDL, and HDL levels on ...
THE LDL RECEPTOR AND THE REGULATION OF CELLULAR ... · cholesterol liberated from LDL regulates the...
Transcript of THE LDL RECEPTOR AND THE REGULATION OF CELLULAR ... · cholesterol liberated from LDL regulates the...

J. Cell Set. Suppl. 3, 131-137 (1985)Printed in Great Britain © The Company of Biologists Limited 1985
131
THE LDL RECEPTOR AND THE REGULATION OF CELLULAR CHOLESTEROL METABOLISMJO SEPH L. G O L D S T E IN * a n d M ICH AEL S. BROW NDepartments of Molecular Genetics and Internal Medicine, University of Texas Health Science Center at Dallas, Southwestern Medical School, 5323 Harry Hines Blvd, Dallas, TX 75235, U.SA.
I N T R O D U C T I O NCholesterol is a structural component of the plasma membrane that surrounds
animal cells. It is absolutely essential for cell growth and survival. Yet, excessive amounts of cholesterol can also be lethal - as when massive amounts of cholesterol deposit in cells of the artery wall producing atherosclerosis. Thus, animal cells have evolved a highly ordered mechanism for regulating their cholesterol content. In this article, we review some of the recent studies that have been carried out by our group on the cellular and molecular biology of the cell surface receptor for low density lipoprotein, or LDL, the major transport protein for cholesterol in human plasma.
The LDL receptor has turned out to be an exciting system in several ways. First, the LDL receptor has served as a prototype for the study of receptor-mediated endocytosis, a general biological process in which cells bind hormones and other macromolecules at the surface and take them into the cell for nutritional and regulatory processes. And second, the LDL receptor studies have taught us a great deal about how genes regulate cholesterol metabolism. Mutations occurring in the gene for the receptor disrupt the regulation of blood cholesterol levels, producing hypercholesterolaemia and atherosclerosis.
The LDL receptor is a member of a class of membrane glycoproteins called lipoprotein receptors (Goldstein & Brown, 1977). These receptors remove cholesterol-carrying lipoproteins from the circulation through the process of receptor- mediated endocytosis (Goldstein, Anderson & Brown, 1979). They are present on the surface of essentially all cultured mammalian cells, where they mediate the uptake of plasma LDL, thereby providing cells with the cholesterol that they need for growth. In the body, most LDL receptors are expressed in the liver, where they supply cholesterol for secretion into bile, for conversion to bile acids, and for resecretion into the plasma in newly synthesized lipoproteins (Brown & Goldstein, 1983; Mahley & Innerarity, 1983). LDL receptors are also present in high concentrations in the adrenal cortex and the ovarian corpus luteum, where they function to provide cholesterol for steroid hormone formation (Brown, Kovanen & Goldstein, 1979).
* Author for correspondence.

132 J . L. Goldstein and M. S. Brown
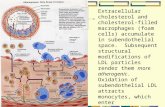
Fig. 1. The LD L receptor pathway in mammalian cells. The receptor undergoes a circuitous itinerary, beginning life as a newly synthesized protein on the endoplasmic reticulum. The remainder of the itinerary is discussed in the text. HMG CoA reductase denotes 3-hydroxy-3-methylglutaryl CoA reductase; AC AT denotes acyl-CoA: cholesterol acyltransferase. Vertical arrows indicate regulatory effects.
S T R U C TU RE AN D F U N C TI O NThe human LD L receptor is a glycoprotein of 839 amino acids that spans the
plasma membrane one time with its NH2 terminus facing the extracellular environment and its COOH terminus facing the cytoplasm (Schneider, Beisiegel, Goldstein & Brown, 1982; Schneider et al. 1983; Russell et al. 1984; Yamamoto et al. 1984). The external domain contains a cysteine-rich region that bears the binding site for LD L. It also contains amino acid sequences that serve as the sites of attachment for asparagine-linked (N-linked) and serine/threonine-linked (O-linked) carbohydrate chains. The cytoplasmic domain contains 50 amino acid residues that direct the receptor to its proper sites of function in the cell.
Fig. 1 illustrates the itinerary followed by the LD L receptor as it carries L D L into cells. The receptor is synthesized in the rough endoplasmic reticulum. Here the high mannose precursor of the N-linked carbohydrate is added, apparently co- translationally (Tolleshaug, Hobgood, Brown & Goldstein, 1983; Cummings et al. 1983). The receptor precursor also contains the core sugar (i.e. iV-acetylgalacto- samine) of each O-linked tetrasaccharide chain, which is added to the protein at the

The LDL receptor 133earliest time point that can be studied (approximately lOmin after synthesis) (Tolleshaug et al. 1983; Cummings et al. 1983). These O-linked core sugars are added before the mannose residues of the N-linked chains are trimmed, i.e. while the receptor is still in the endoglycosidase H-sensitive stage. Thus, the O-linked core sugars must be added either in the endoplasmic reticulum, or in the transitional zone between the endoplasmic reticulum and the Golgi apparatus.
Within 30min after its synthesis, the receptor decreases in mobility on sodium dodecyl sulphate (SDS)-containing gels from a mobility corresponding to 120 000 M r to a mobility corresponding to 160000 Mr (Tolleshaug et al. 1983; Cummings et al. 1983). This change is coincident with the enzymic reactions that modify the highmannose N-linked oligosaccharide chains to the complex, sialic acid-containing, endoglycosidase H-resistant type. At the same time, the core A^-acetylgalactosamine of each O-linked chain is elongated by the addition of one galactose and two sialic acid residues. The net amount of carbohydrate that is added is not sufficient to account for a true increase of 40 000 in the M r, and so we believe that the bulk of the change is due to conformational alterations in the protein and changes in the amount of SDS binding that retard its mobility in SDS-containing gels.
About 45 min after its synthesis, the LDL receptor appears on the surface, where it clusters together with other receptors in indented regions of the plasma membrane that are coated on the cytoplasmic surface with a protein called clathrin. These are the so-called ‘coated pits’ that are responsible for the receptor-mediated endocytosis of a variety of receptor-bound molecules (Goldstein et al. 1979). While in the coated pit, the receptor binds LDL by attaching to the protein component of the lipoprotein. Within 3-5 min of their formation, the coated pits invaginate to form coated endocytic vesicles that pinch off from the plasma membrane and exist for a brief moment (less than 2 min) as free structures in the cytoplasm. Very quickly, the clathrin coat dissociates from the surface of the coated vesicle, which now appears as a smooth-surfaced structure. Multiple endocytic vesicles fuse with each other to create larger membrane-enclosed sacs of irregular contour called endosomes (Brown, Anderson & Goldstein, 1983). The pH of the endosomes is lower than that of the surrounding cytoplasm, owing to the presence of proton pumps in the membrane of the endosome that acidify its contents (Helenius, Mellman, Wall & Hubbard, 1983). Under the influence of the acid pH, the LDL dissociates from the receptor. The receptor can then return to the surface, apparently by clustering together with other receptors in a segment of the endosome membrane that pinches off to form a recycling vesicle that carries the receptors back to the surface (Brown et al. 1983). Once it reaches the surface the receptor binds another LDL particle and initiates another cycle of endocytosis and recycling. Each receptor makes one round-trip every 10 min in a continuous fashion.
The LDL that dissociates from the receptor remains within the lumen of the endosome and is eventually delivered to a lysosome when the membranes of the endosome and lysosome fuse. The LDL is now exposed to a variety of acid hydrolases. Its protein component is hydrolysed to amino acids and its cholesteryl esters are hydrolysed by an acid lipase, liberating cholesterol for use in the synthesis

134 J. L. Goldstein and M. S. Brownof new membranes (Goldstein & Brown, 1977). After leaving the lysosome, the cholesterol liberated from LDL regulates the cell’s cholesterol metabolism, thereby assuring a steady level of cholesterol within the cell (Goldstein & Brown, 1977). To accomplish this regulation, the incoming cholesterol modulates several reactions (Goldstein & Brown, 1977), the most important of which involves the receptor itself. In this reaction, the build-up of cholesterol within the cell causes the cell to shut off the synthesis of new LDL receptors (Goldstein & Brown, 1977). Through this ‘feedback’ mechanism, cells adjust the production of receptors to supply sufficient cholesterol to meet their varying demands, but not enough to overload themselves with cholesterol. When cells are growing actively and producing new membranes, they synthesize a maximal number of LDL receptors (approximately 40 000 per cell) to supply the necessary amounts of cholesterol. On the other hand, when cells cease to grow, they have little demand for cholesterol. Consequently, the cholesterol begins to build up in the cell, and the production of LDL receptors is suppressed to levels as low as 10% of the maximum. In this way, intracellular accumulation of excessive cholesterol is prevented.
G E N E T I C SThe importance of the LDL receptor in normal physiology was first appreciated
when its absence was shown to produce a severe disease, in this case familial hypercholesterolaemia (FH). FH exists clinically in two forms: the less severe heterozygous form and the more severe homozygous form (Goldstein & Brown, 1983). Heterozygotes, who inherit one mutant gene, are quite common, accounting for one out of every 500 persons among most ethnic groups throughout the world. These heterozygotes have plasma LDL levels that are twofold above normal even before birth, and they begin to have heart attacks as early as age 35 years. Among people under age 60 who suffer heart attacks, 5 % have the heterozygous form of FH - 25-fold more than in the general population.
Ordinarily, each FH heterozygote passes a single copy of the mutant gene to half of his or her offspring, and these offspring then have heterozygous FH. If two FH heterozygotes marry, as occurs in one out of 250000 marriages, each offspring has a one in four chance of inheriting two doses of the mutant gene, one from each parent. Such an offspring is an FH homozygote. FH homozygotes number about one in a million persons in the population. They have LDL levels that are more than six times above normal. Heart attacks can occur as early as age 2 and are almost inevitable by age 20. It is notable that these children have no risk factors for atherosclerosis other than an elevated level of LDL. They have normal blood pressure, they do not smoke, and they do not have an elevated blood glucose level. The homozygous form of FH is a vivid experiment of nature that demonstrates unequivocally the causal relationship between elevated LDL levels and atherosclerosis.
Eleven years ago, we began to study FH with the hope of learning the mechanism for the elevation of LDL in these patients (Goldstein & Brown, 1973; Brown & Goldstein, 1974). We soon discovered that cultured skin fibroblasts and circulating

The LDL receptor 135blood cells from FH homozygotes produce few or no functional L D L receptors. They are therefore unable to bind, internalize and degrade L D L with normal efficiency. The receptor deficiency arises because FH homozygotes inherit two defective copies of the gene for the LD L receptor, one from each parent. They have no copy of the normal gene and therefore can produce no normal L D L receptors.
COATEDPIT
Classof
MutationSynthesis
Transport from ER
to Golgi
Bindingof
LDL
Clusteringin
Coated Pits
® X© -►X© -►X
r * .
Fig. 2. Four classes of mutations in the structural gene for the LD L receptor have been identified in patients with familial hypercholesterolaemia. Each mutation affects a different region in the gene and thus interferes with a different step in the normal process by which the receptor is synthesized, processed in the Golgi complex, and transported to coated pits where it is available to bind LDL. Each class of mutation can be further subdivided into different mutant alleles that have been reviewed by Goldstein & Brown (1984). (Reprinted from Journal of Lipid Research, with permission.) ER, endoplasmic reticulum.

136 J. L. Goldstein and M. S. BrownCells from their parents (and from other FH heterozygotes) have one normal gene and one mutant gene for the receptor. They synthesize half the normal number of LDL receptors and are therefore able to bind, internalize and degrade LDL at half the normal rate. All individuals with FH studied so far have mutations in the gene encoding the LDL receptor, but the mutations are not all the same. The mutations fall into four different classes, depending on the site in the gene at which the mutation has occurred (Tolleshaug et al. 1983; Goldstein & Brown, 1973).
Fig. 2 presents a summary of the four classes of LDL receptor mutations that have been identified in patients with FH. One class of mutant genes produces no detectable receptors (so-called ‘null’ alleles). The second class produces receptors that are synthesized in the rough endoplasmic reticulum, but cannot be transported to the cell surface and therefore cannot perform their normal function. The third class of mutations produces receptors that move to the cell surface normally, but are unable to bind LDL owing to an abnormality in the binding site. The fourth class of mutations produces receptors that are transported to the surface and bind LDL, but are unable to enter coated pits and therefore cannot carry LDL into cells (so-called ‘internalization defective’ alleles) (Tolleshaug et al. 1983; Cummings et al. 1983; Brown et al. 1983; Goldstein & Brown, 1983).
Studies of the molecular basis of the mutations in the LDL receptor are beginning to reveal those parts of the protein that signal transport to the plasma membrane and those that signal the incorporation into coated pits. Thus, these mutations are providing new insights into fundamental cell biology (Goldstein & Brown, 1984; Lehrman et al. 1985).
The authors’ research is supported by grants from the National Institutes of Health (HL 20948) and the Moss Heart Fund.
R E F E R E N C E SB r o w n , M. S ., A n d e r s o n , R. G . W. & G o l d s t e in , J. L. (1983). Recycling receptors: The
round-trip itinerary of migrant membrane proteins. Cell 32, 663—667.Br o w n , M. S. & G o l d s t e in , J. L. (1974). Familial hypercholesterolemia: Defective binding of
lipoproteins to cultured fibroblasts associated with impaired regulation of 3-hydroxy-3- methylglutaryl coenzyme A reductase activity. Proc. natn. Acad. Sci. U.SA. 71, 788-792.
Br o w n , M. S. & G o l d s t e in , J. L. (1983). Lipoprotein receptors in the liver: Control signals for plasma cholesterol traffic, jf. clin. Invest. 72, 743-747.
Br o w n , M. S., K o v a n e n , P. T. & G o l d s t e in , J. L. (1979). Receptor-mediated uptake of lipoprotein-cholesterol and its utilization for steroid synthesis in the adrenal cortex. Recent Prog. Hormone Res. 35, 215-257.
C u m m in g s , R . D . , K o r n f e l d , S ., S c h n e id e r , W . J . , H o b g o o d , K . K . , T o l l e s h a u g , H . , B r o w n , M. S. & G o l d s t e in , J . L. (1983). B io sy n thesis o f th e N- a n d O -lin k e d o lig o sac ch arid es o f th e low d e n sity lip o p ro te in re c e p to r . J '. biol. Chem. 258, 15 261-15 273.
G o l d s t e in , J. L., A n d e r s o n , R. G . W. & Br o w n , M. S. (1979). Coated pits, coated vesicles and receptor-mediated endocytosis. Nature, Land. 279, 679-685.
G o l d s t e in , J. L. & B r o w n , M. S. (1973). Familial hypercholesterolemia: Identification of a defect in the regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity associated with overproduction of cholesterol. Proc. natn. Acad. Sci. U.SA. 70, 2804-2808.
G o l d s t e in , J. L. & B r o w n , M. S. (1977). The low-density lipoprotein pathway and its relation to atherosclerosis. A. Rev. Biochem. 46, 897-930.

The LDL receptor 137G o l d s t e in , J. L. & Br o w n , M. S. (1983). Familial hypercholesterolemia. In The Metabolic Basis
of Inherited Disease (ed. J. B. Stanbury, J. B. Wyngaarden, D. S. Fredrickson, J. L. Goldstein & M. S. Brown) chap. 33, 5th edn, pp. 672-712. New York: McGraw-Hill Book Co.
G o l d s t e in , J. L. & Br o w n , M. S. (1984). Progress in understanding the LDL receptor and HMG CoA reductase, two membrane proteins that regulate the plasma cholesterol. X Lipid Res. 25, 1450-1461.
H e l e n iu s , A., M e l l m a n , I., W a l l , D. & H u b b a r d , A. (1983). Endosomes. TIBS 8, 245-250.L e h r m a n , M. A., S c h n e id e r , W. J., S u d h o f , T ., B r o w n , M. S ., G o l d s t e in , J. L. & R u s s e l l ,
D. W. (1985). Mutation in LDL receptor: Alu-Alu recombination deletes exons encoding transmembrane and cytoplasmic domains. Science 227, 140-146.
M a h l e y , R. W. & I n n er a r ity , T. L. (1983). Lipoprotein receptors and cholesterol homeostasis. Biochim. biophys. Acta 737, 197-222.
R u s s e l l , D. W., S c h n e id e r , W. J., Y a m a m o to , T ., L u s k e y , K. L . , Br o w n , M. S . & G o l d s t e in , J. L . (1984). Domain map of the LDL receptor: Sequence homology with the epidermal growth factor precursor. Cell 37, 577—585.
S c h n e id e r , W. J . , B e is ie g e l , U., G o l d s t e in , J . L. & B r o w n , M . S . (1 9 8 2 ). Purification of the low density lipoprotein receptor, an acidic glycoprotein of 164,000 molecular weight. J . biol. Chem. 257, 2 6 6 4 -2 6 7 3 .
S c h n e id e r , W. J., S l a u g h t e r , C . J., G o l d s t e in , J. L., A n d e r s o n , R. G . W., C a p r a , D. J. & B r o w n , M. S . (1983). Use of anti-peptide antibodies to demonstrate external orientation of NHi-terminus of the LDL receptor in the plasma membrane of fibroblasts. J . Cell Biol. 97, 1635-1640.
T o l l e s h a u g , H . , H o b g o o d , K. K., Br o w n , M. S . & G o l d s t e in , J. L. (1983). The LDL receptor locus in familial hypercholesterolemia: Multiple mutations disrupting the transport and processing of a membrane receptor. Cell 32, 941-951.
Y a m a m o to , T ., D a v is , C . G . , Br o w n , M. S ., S c h n e id e r , W. J., C a sey , M. L., G o l d s t e in , J. L. & R u s s e l l , D. W. (1984). The human LDL receptor: A cysteine-rich protein with multiple Alu sequences in its mRNA. Cell 39, 27—38.