The effect of Prede~ 2X and Flucort® on blood...
Transcript of The effect of Prede~ 2X and Flucort® on blood...

The effect of Prede~ 2X and Flucort® on blood metabolites, immune function and milk composition in
Holstein dairy cows
Dy
Madhu Rani Sindhwani
Department of Animal Science McGiII University
February,2007
A thesis submitted to the Faculty of Graduate Studies and Research in partial fultlUment of the requirements for the degree of
MASTER OF SCIENCE
~adhu Rani Sindhwani, 2007

1+1 Library and Archives Canada
Bibliothèque et Archives Canada
Published Heritage Branch
Direction du Patrimoine de l'édition
395 Wellington Street Ottawa ON K1A ON4 Canada
395, rue Wellington Ottawa ON K1A ON4 Canada
NOTICE: The author has granted a nonexclusive license allowing Library and Archives Canada to reproduce, publish, archive, preserve, conserve, communicate to the public by telecommunication or on the Internet, loan, distribute and sell th es es worldwide, for commercial or noncommercial purposes, in microform, paper, electronic and/or any other formats.
The author retains copyright ownership and moral rights in this thesis. Neither the thesis nor substantial extracts from it may be printed or otherwise reproduced without the author's permission.
ln compliance with the Canadian Privacy Act some supporting forms may have been removed from this thesis.
While these forms may be included in the document page count, their removal does not represent any loss of content from the thesis.
• •• Canada
AVIS:
Your file Votre référence ISBN: 978-0-494-32784-5 Our file Notre référence ISBN: 978-0-494-32784-5
L'auteur a accordé une licence non exclusive permettant à la Bibliothèque et Archives Canada de reproduire, publier, archiver, sauvegarder, conserver, transmettre au public par télécommunication ou par l'Internet, prêter, distribuer et vendre des thèses partout dans le monde, à des fins commerciales ou autres, sur support microforme, papier, électronique et/ou autres formats.
L'auteur conserve la propriété du droit d'auteur et des droits moraux qui protège cette thèse. Ni la thèse ni des extraits substantiels de celle-ci ne doivent être imprimés ou autrement reproduits sans son autorisation.
Conformément à la loi canadienne sur la protection de la vie privée, quelques formulaires secondaires ont été enlevés de cette thèse.
Bien que ces formulaires aient inclus dans la pagination, il n'y aura aucun contenu manquant.

Abstract
Glucocorticoids are commonly used to treat cows with clinical ketosis and fa~ liver disease. This study investigated the effects of 10 mg/mL of Flucort and Predefi' 2X on the day of calving on blood metabolites, immune function and milk composition on 30 transitional Holstein cows. Sample of blood and milk were analyzed for energy metabolites (glucose, NEF A, BHB and insulin), mineraI metabolites (Ca, P, Na, K, Cl and Mg), energy function parameters (antibody, lymphocyte), milk compositional parameters (protein, fat, lactose, SCC). There were no differences in glucose, Na, Cl, Mg, antibody, lymphocyte and milk fat, were observed among treatments. Flucort® treated cows had significantly lower NEFA on Dl, higher BHB on D21 and D28, lower insulin on D 14, higher Ca on Dl and lower P on Dl. Predefi' 2X treated cows had significantly higher BHB on D21, higher insulin on D7, lower Ca on Dl, higher sec on Dl and higher milk protein on DI. With respect to the significant data in this study, the use of glucocorticoids Flucort® and Prede~ 2X in a single intramuscular injection on dl for the treatment of ketosis is not warranted.
II

Résumé
Les glucocorticoIdes sont couramment utilisés pour traiter les vaches atteintes avec la cétose clinique ainsi qu'avec le syndrome du foie gras. Cette étude avait pour but de déterminer les effets de l'administration de 10 mg/ml de Flucort® et Prede~ 2X suite au vêlage sur les métabolites sanguins, le système immunitaire et la composition du lait de 30 vaches Holstein en période de transition. Les échantillons de sérum et de lait ont été analysés pour les métabolites énergétiques (glucose, AGL, acides B-hydroxybutyriques et l'insuline), les métabolites minéraux (Ca, P, Na, K, Cl et Mg), les paramètres fonctionnels énergétiques (anticorps, lymphocytes) et les paramètres de la composition du lait (protéines, gras, lactose, taux de cellules somatiques). Aucune différence dans le taux de glucose, Na, Cl, Mg, anticorps, lymphocytes et gras dans le lait n'a pu être détectée entre les traitements administrés. Les vaches traitées au Flucort® avaient une concentration d'AGL significativement plus basse au jour 1, un taux d'acides B-hydroxybutyriques plus élevé au jour 21 et 28, une concentration d'insuline plus basse aujour 14, un taux de Ca plus élevé au jour 1 et un taux de P plus bas au jour 1. Les vaches traitées avec Prede:f!' 2X avaient une concentration d'acide Bhydroxybutyrique significativement plus élevée au jour 21, une concentration d'insuline plus élevée au jour 7, un taux de Ca plus bas au jour 1, un taux de cellules somatiques plus élevé au jour 1 et un taux de protéines plus élevé dans le lait au jour 1. Basé sur les données statistiquement significatives obtenues lors de cette étude, l'utilisation des glucocorticoïdes Flucort® et Prede~ 2X en une seule injection intramusculaire ne peut garantir le traitement d'une cétose chez la vache.
III

Acknowledgements
1 would like to express my great gratitude to those who generously provided their supervision and assistance. They were:
Dr. Xin Zhao, my thesis supervisor, for his valuable guidance, patience, and advice during this study.
Dr. Ken Leslie, Dr. Arif Mustafa, Dr. Ng-Kwai-Hang, my committee members, for their advice, opinions and critical evaluations.
Dr. Roger Cue and Dr. Todd Duffield, for their help with the statistical analysis.
Nicole Perkins, Erin Vemooy, Cindy Todd, technicians at the AHL of Guelph University, Hélène Lalande, Jai-Wei Lee, Ming-Kuei Lee, and PATLQ for their help with my experiments.
Paul Meldrum, Phil Lavoie, Nancy Lavigne, Natasha, Abou, Judy, Ali, Lauren, Stephane, Eric, Brad, Alain, José, Martin, Milène, Isabella and Jason at the Macdonald Campus Farm, for their tremendous help with the animaIs.
Barbara Stewart, Sandra Nagy, Cinthya Horvath and Leslie Ann Laduke for their encouraging smiles.
AlI other prof essors, staff, and students at Macdonald Campus who have contributed to the completion of my M.Sc. thesis.
Annie, Josée, Pascale, Christian, Fadi, Malek, Charbel, Ming-Kai, Dana, Marsha and aU the "Girls", for their friendships and encouragement.
My girls at the barn, Viper (6898), Chives (881), Nala (1168), Ruby (6899), Feria (879), Saab (3734), High Society (1618), Happy Time (1613), Babel (893), Mistletoe (6537), Honey Suckle (1188), Muskoka (882), Saraby (1581), Meli Melo (894), Devil (8481), Lotessie (7484), Rocalle (2552), Heather (113), Boo (902), Hokus (1179), Macarena (3448), Mini Me (1626), Muffin (891), Fate (886), Pam (885), Justine (1155), JobeU (1160), Punch (858), Salsa (8401) and Dolly (1612) for being part of my Masters research and being so wonderful to work with.
My amazing parents, my brothers Rav and Maughan and their families for their love and encouragement.
Finally, my wonderful Fiancé, Jag, whom without his support, encouragement and love, this would not be possible.
IV

Table of Contents
Page
Abstract ................................................................................. .11
Résumé ................................................................................. .111
Acknowledgments .................................................................... .IV
Table of Contents ....................................................................... V
List of Tables ............................................................................ X
1. Introduction ........................................................................... 1
II. Literature Review ................................................................... 3
1. Transition Period ............................................................. 3
1.1. Hormone Changes during the Transition Period ............. 3
1.1.1. Estrogen ................................................. 4
1.1.2. Progesterone ........................................... 4
1.1.3. Insulin ................................................... 4
1.1.4. Growth Hormone ...................................... 5
1.1.5. Thyroxine and 3,5,3'-triiodothyronine ............ .5
1.1.6. Glucocorticoid .......................................... 6
1.2. Feed intake and energy balance during the transition
period ............................................................... 6
1.2.1. Nutrient Requirements ................................ 6
1.2.2. Dry matter intake (DMI) ............................. 6
1.2.3. Energy balance ........................................ 7
1.3. Glucose demand .................................................. 7
1.4. Gluconeogenesis in the cow ..................................... 8
1.4.1. Propionate ............................................... 9
1.4.2. Amino acids ............................................. 9
1.4.3. Lactate and glycerol.. ............................... 10
1.5. Metabolism ofnon-esterified fatty acids (NEFAs) ......... 1O
1.6. Fatty Liver ....................................................... 12
1.7. Effect ofBCS on fat mobilization ............................ 14
1.8. Further metabolism ofNEFAs ............................... .15
1.9. Ketosis ............................................................ 17
V

2. The immune system ofthe cow ............. , ............................. 18
2.1. Nonspecific Immune Response ............................... 19
2.2. Specifie Immune Response .................................... 19
2.2.1. B Lymphocytes: Antibody-Mediated
Immunity .................................................... 20
2.2.2. T Lymphocytes: Cell-Mediated Immunity ....... 20
2.3. Changes in the immune system during the peripartum
period .................................................................. 21
2.4. Major diseases during the transitional period ............... 24
2.4.1. Mastitis ................................................. 25
2.4.2. Udder Defense ........................................ 26
2.4.3. Compromised Reproduction ....................... .26
3. Adrenal Cortex ............................................................. 28
3.1. Corticosteroids ................................................... 28
3.1.1. Mineralocorticoids ................................... 29
3.1.2. Glucocorticoids ....................................... 29
3.1.2.1. CortisoL .................................... 29
4. Synthetic Glucocorticoids ................................................. 30
4.1. Flucort® ........................................................... 34
4.2. Predef!> 2X ........................................................ 35
III. Hypothesis and Objectives .................................................... .39
IV. Materials and Methods .......................................................... 40
1. Experimental design ....................................................... 40
2. Feeding ...................................................................... 41
2.1. Close-Up Dry Ration ........................................... 41
2.2. Fresh Cow Ration ............................................... 41
3. Blood Serum Collection .................................................. .42
4. Milk Sampling .............................................................. 42
S. Milk Sodium and Milk Potassium Analysis ........................... .42
6. Body Condition Scoring ................................................... 43
7. Serum Biochemical Analysis ............................................ .43
8. Antibody Production (ELISA) .......................................... .43
9. ConA-Induced Lymphocyte Proliferation .............................. .44
10. Serum Insulin Analysis .................................................. .45
VI

11. Statistical Analysis ...................................................... .45
V. Results and discussion ............................................................ 48
1. Flucort® ..................................................................... 49
1.1. Energy Status .................................................... 49
1.1.1. Glucose ................................................ 49
1.1.2. Insulin ................................................. 54
1.1.3. Non-esterified fatty acids ........................... 54
1.1.4. p-hydroxybutyrate .................................... 56
1.2. Mineral status .................................................... 59
1.2.1. Calcium ................................................ 59
1.2.2. Phosphorus ........................................... 61
1.2.3. Potassium .............................................. 61
1.2.4. Sodium ................................................. 62
1.2.5. Chloride ............................................... 63
1.2.6. Magnesium ........................................... 64
1.3. Immune Function ................................................ 64
1.3.1. Antibody Production ................................ 64
1.3.2. Lymphocyte proliferation ........................... 65
1.4. Milk Status ........................................................ 67
1.4.1. Milk Yield (kg) ....................................... 67
1.4.2. Protein .................................................. 68
1.4.2.1. Protein (%) ................................. 68
1.4.2.2. Protein Yield (kg) .............. " ......... 70
1.4.3. Fat ...................................................... 70
1.4.3.1. Fat (%) ..................................... 70
1.4.3.2. Fat Yield (kg) ............................. 71
1.4.4. Lactose ................................................ 71
1.4.4.1. Lactose (%) ................................ 71
1.4.4.2. Lactose Yield (kg) ........................ 71
1.4.5. Milk potassium (mgIL) .............................. 72
1.4.6. Milk sodium (mgIL) ................................. 72
1.4.7. Somatic cell count ................................... 73
2. Prede~ 2X .................................................................. 74
2.1. Energy Status .................................................... 74
VII

2.1.1. Glucose ................................................ 74
2.1.2. Insulin ................................................. 75
2.1.3. Non-esterified fatty acids ........................... 76
2.1.4. p-hydroxybutyrate .................................... 77
2.2. Serum Mineral status ............................................ 79
2.2.1. Calcium ................................................ 79
2.2.2. Phosphorus ........................................... 79
2.2.3. Potassium .............................................. 80
2.2.4. Sodium ................................................. 81
2.2.5. Chloride ............................................... 81
2.2.6. Magnesium ........................................... 82
2.3. Immune Function ................................................ 82
2.3.1. Antibody Production ................................ 82
2.3.2. Lymphocyte proliferation ........................... 83
2.4. Milk Status ........................................................ 84
2.4.1. Milk Yield (kg) ....................................... 84
2.4.2. Protein .................................................. 84
2.4.2.1. Protein (%) ................................. 84
2.4.2.2. Protein Yield (kg) ......................... 85
2.4.3. Fat ...................................................... 86
2.4.3.1. Fat (%) ..................................... 86
2.4.3.2. Fat Yield (kg) .............................. 86
2.4.4. Lactose ................................................ 87
2.4.4.1. Lactose (%) ................................ 87
2.4.4.2. Lactose Yield (kg) ........................ 87
2.4.5. Milk potassium (mgIL) .............................. 88
2.4.6. Milk sodium (mgIL) ................................. 88
2.4.7. Somatic cell count ................................... 88
3. Flucort® versus Prede~ 2X ............................................... 89
3.1. Energy Status .................................................... 89
3.2. Serum Mineral Status .......................................... 89
3.3. Immune Function ................................................ 90
3.4. Milk Status ....................................................... 90
VI. CODclusioD .......................................................................... 92
VIII

VII. References ........................................................................ 94
Appendices ............................................................................ 111
Animal Use Protocol. ....................................................... 112
Amendment to Animal Use Protocol. .................................... 113
Certificate of Radiation Safety ............................................. 114
McGill University InternaI Radioisotope Permit.. ...................... 115
IX

List of Tables
Table 1. Comparison of Drug Duration and Action Potency* (Dowling, 2004) .......................................................................................................... 33
Table 2. Frequency ofparities (1 to >5) within treatment ....................... .40
Table 3. Frequency of calving seasons within treatment.. ....................... .40
Table 4. Diet Composition (% of DM) ofClose-Up Ration ..................... .41
Table 5. Diet Composition (% of DM) ofFresh Cow Ration ................... .41
Table 6. F value from mixed models for aU parameters in cows that were either treated day of calving with a Flucort®, Prede~ 2X or served as negative controls1
................................................................................. 48
Table 7. Overall least squares means from mixed models for serum biochemical parameters, immune function parameters and milk parameters in cows that were either treated day of calving with a Flucort®, Prede~ 2X or placebo control l
........................................................................ 50
Table 8. Least squares means from mixed models for serum energy parameters in cows on D-IO, D-5, DO, Dl, D7, D14, D21 and D28 relative to calving that were either treated on day of calving with a Flucort®, Predef!> 2X or placebo l
.......................................................................................... 52
Table 9. Frequency of cows with high NEFA levels at D-IO, D-5, DO, Dl, D7, D14, D21 and D28 by treatment group ............................................ .55
Table 10. Frequency of multiparous cows with high NEF A levels at D-IO, D-5, DO, Dl, D7, D14, D21 and D28 by treatment group ............................... .56
Table Il. Frequency ofprimiparous cows with high NEFA levels at D-I0, D-5, DO, Dl, D7, D14, D21 and D28 by treatment group ........................... 56
Table 12. Frequency of body condition score within treatment and within day ........................................................................................ 56
Table 13. Distribution of subclinical ketosis at D-IO, D-5, DO, Dl, D7, D14, D21 and D28 by treatment group .................................................... 57
Table 14. Distribution of subclinical ketosis in multiparous cows at D-IO, D-5, DO, Dl, D7, D14, 021 and D28 by treatment group ........................... .57
Table 15. Distribution ofsubclinical ketosis in primiparous cows at D-IO,D-5, DO, Dl, D7, D14, D21 and D28 by treatment group ..................................... 57
Table 16. Least squares means from mixed models for average dry matter intake in cows on D-I0, D-5, DO, Dl, week 1, week 2, week 3, and week 4
x

post-calving that were either treated day of calving with a Flucort®, Prede~ 2X or served as negative controls l
................................................... 58
Table 17. Least squares means from mixed models for serum minerai parameters in cows on D-IO, D-5, DO, Dl, D7, D14, D21 and D28 relative to calving that were either treated on day of calving with a Flucort®, Predef!1 2X or placebo! ............................................................................... 60
Table 18. Least squares means from mixed models for serum minerai parameters in cows on D-IO, D-5, DO, Dl, D7, D14, D21 and D28 relative to calving that were either treated on day of calving with a Flucort®, Predef!1 2X or placebo! ............................................................................... 63
Table 19. Least squares means from mixed models for antibody production values in cows DO, D7, D14, D21 and D28 relative to calving that were either treated day of calving with a Flucort®, Predef!1 2X or served as negative controls! .................................................................................. 65
Table 20. Least squares means from mixed models for lymphocyte proliferation values in cows DO and D7 relative to calving that were either treated day of calving with a Flucort®, Predef!1 2X or served as negative controIs· ........................ " ............... " ........ " .... " ......................................................................................... 66
Table 21. Least squares means from mixed models for average milk yield in cows on week 1, week 2, week 3, and week 4 post-calving that were either treated day of calving with a Flucort®, PredefM> 2X or served as negative controls1
.................................................................................. 68
Table 22. Least squares means from mixed models for milk component parameters in cows DI, D7, D14 and D21 relative to calving that were either treated day of calving with a Flucort®, Predef!1 2X or served as negative controls1
................................................................................... 69
Table 23. Frequency of mastitis, as denoted by sec > 283,000 cells/ml (Guidry, 1985; Reneau, 1986), percentages of cows by treatment group ....... 74
XI

1. Introduction
The transition period is a critical time for the dairy cow as the
periparturient period is characterized by tremendous metabolic adaptation of
the cow from the demands of late pregnancy to those of early lactation
(Grummer, 1993). Dairy cows increase their total nutrient requirements and
their demand for energy supply to satisfy the requirements of the uterus, fetal
development and the onset of lactation (Bell, 1995), therefore changes are
necessary in body tissue metabolism to meet the requirements of energy (Bell,
1995; Overton et al., 2001). During the final 3 weeks of gestation, a 20 to 40%
decline in dry matter intake (DMI) occurs which may initiate a negative
energy balance (Hayirli et al., 2002). If energy intake is insufficient to meet
energy requirements, one of the major metabolic adaptations involves the
mobilization of fatty acids from adipose tissue. Mobilization of adipose tissue
results in the release of non-esterified fatty acids (NEFAs) into the blood
stream. The plasma NEF A are used as a fuel source by muscle and taken up by
the liver. The liver takes up NEF A in proportion to their concentrations in
plasma, but typically does not have sufficient capacity to completely dispose
ofNEFA through export into the blood or catabolism for energy (Grum et al.,
1996). When nutrient intake is insufficient and large amounts of NEF A are
released into the blood, the liver begins to accumulate and store NEF A which
may be esterified to form triglycerides (TG) and the acetyl-CoA that is not
into the tricarboxylic acid (TCA) cycle is converted to ketone bodies, such as
p-hydroxybutyrate (BHB) (Goff and Horst, 1997) resulting in increased ketone
production which can cause ketosis and is detrimental to overall cow health
and performance (Grummer, 1993; Drackley et al., 2001). Elevated plasma
NEF A is associated with hepatic triglyceride accumulation, consequently it
can cause fatty liver which can ultimately lead to prolonged recovery from
other disorders, increased incidence of health problems, and compromised
Iiver function (Drackley, 1999; Grummer, 1993; Veenhuizen et al., 1991).
Failure of the transition cow to appropriately adjust her metabolism to support
increased nutrient requirements of early lactation may result in the occurrence
of metabolic disorders, poor reproductive performance, and decreased milk
production during the upcoming lactation (Bell, 1995; Grummer, 1995;
1

Drackley, 1999). In addition, the risk of displaced abomasum, retained
placenta and clinical mastitis is significantly increased (Duffield, et al., 2002).
It is hypothesized that preventive therapy could be administered to
promote reversai of the ketogenic processes in the liver and assist with the
needed increase in glucose supply. Administration of oral propylene glycol,
intravenous dextrose, exogenous insulin and various glucocorticoids have been
experimented for this purpose. However, to date, no consistant successful
therapeutic options have been reported. G1ucocorticoid therapy has been used
widely in dairy cattle for the treatment of ketosis as they enhance the
mobilization of glucose precursors (amino acids) and stimulate the rate of
gluconeogenesis. However, use of synthetic glucocorticoids in cows as a
preventive measure is limited since a great number of veterinarians share the
common perception that these drugs are contraindicated due to their potential
to induce immunosuppression.
Flumethasone sterile suspension (Flucort®, Wyeth Animal Health) is
approved for the treatment of ketosis in dairy cattle in Canada. Flucort®
contains a potent corticosteroid with reportedly 60 to 80 times greater
glucocorticoid activity than prednisolone (Flucort® package insert). On the
other hand, Prede:t«' 2X (9-alpha-fluoroprednisolone) is an isoflupredone
acetate sterile suspension made for injection by Pharmacia & Upjohn. It
contains potent corticosteroid and has greater glucocorticoid activity (10 times
more potent) than an equal quantity of prednisolone in its ability to elevate
blood glucose level. Prede:t«' 2X is also long-lasting (48 h gluconeogenic
activîty). It îs reported that blood glucose levels retum to normal or above
within 8 to 24 h after injection, following a reduction in blood and urine
ketone levels by increasing its gluconeogenic and glycogen disposition
activity.
In summary, an effective preventive therapy for bovine ketosis could
reduce and avoid metabolic disorders and production losses. Prede~ 2X and
Flucort® are two synthetic glucocorticoids that were investigated in the present
study. The effects of these veterinary drugs injected on the day of calving in
Holstein primiparous and multiparous cows were evaluated by monitoring
parameters of blood metabolites, immune function and milk composition
during the transition period.
2

II. Literature Review
1. Transition Period
The transition period is defined as the period 2 to 4 weeks prior to
calving (close-up) through 2 to 4 weeks after calving (fresh cow) and is a
critical period that detennines both productivity and profitability in a dairy
herd. During the transition period, the cow experiences a number of changes
such as the initiation of lactation, ration shifts, location and social group
changes and rapid changes in both hormonal and metabolic systems. AH of
these may increase the level of stress in the cow during this period and special
attention to nutrition and management practices may help minimize the level
of stress.
The transition period can also be detined as the transition of feeding
regimens. Changes in nutrient demand require cows to make metabolic
adaptations to meet the demands of late pregnancy to those of early lactation
(Bell, 1995; Overton et al., 2001), essentiaHy a cow must make a transition
from consuming a high fiber ration for dry cows to a lactation diet that is high
in energy and lower in fiber (Radostits et al., 1994).
1.1. Hormone Changes during Transition Period
Major changes in honnonal status in particular estrogen, progesterone
and insulin occur during the transition period and these changes contribute to
changes in blood metabolites. Changes in honnones around calving such as
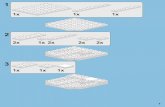
estradio~ progesterone and growth honnone are illustrated in Figure 1.
3

Glucocorllcoids 12
(fl9ImI serum) e 4
Growln 9 HoI"mone
6 (1IQ/mlsel'l.mt
Proloclln 200 (rI\1lm1 serum) 100
vt:========:::::::::::- -----1--------Progesterone
(rlQlml serum)
Eltrodlol-17 p (po/ml .erum)
·26 -22 -19 -15 -12 -9 Days 'rom Pa,IuriNon
Figure 1. Hormonal changes around calving (Bell, 1995)
1.1.1. Estrogen
Estrogen, primarily estrone of placental origin, increases in plasma
during late gestation but decreases immediately at calving (Chew et al., 1979).
The surge of estrogen that occurs on the day prior to calving may act as a
regulator of hepatic fatty acid metabolism in ruminants (Green et al.,1999). It
has been demonstrated that changes in blood estrogen or estrogen:
progesterone ratio may influence feed intake (Grummer et al.,1990).
1.1.2. Progesterone
Progesterone concentrations during the dry period are elevated for
maintenance of pregnancy but decline rapidly approximately 2 d before
calving (Chew et al., 1979). In lactating dairy cows, energy balance during the
first few weeks post-calving is positively related to concentrations of plasma
progesterone (P4) during the first post-calving estrous cycle (Villa-Godoy et
al., 1988; Spicer et al., 1990).
1.1.3. Insulin
Insulin is important for proper function of the reproductive processes
as it facilitates the partitioning of nutrients between the rapidly growing fetus
and the mammary tissue (Ebling et al., 1990). Insulin stimulates the synthe sis
of glycogen, increases uptake of glucose by muscle and adipose tissues,
4

promotes the uptake of branched-chain ammo acids by muscle, which
promotes muscle tissue synthesis and reduces protein catabolism (Jim
Quiqley, 2001). Insulin reduces ketogenesis and gluconeogenesis in the liver
but stimulates muscle tissue uptake of glucose, amino acids and ketone bodies.
In ruminants, acetate is the main precursor of lipogenesis and its uptake is
stimulated by insulin (Sterbauer, 2005). As a cow progresses from late
gestation to early lactation, plasma insulin decreases (Grummer 1995), with an
acute surge at calving (Kunz et al., 1985). During the transition period, the
adipose tissue becomes highly resistant to insulin (Bell, 1995) leading to
lowered responsiveness and sensitivity of extra hepatic tissues to insulin (Sano
et al., 1991). The decrease in sensitivity of adipose tissue to insulin may also
explain increased lipolysis and mobilization of NEF A from the adipose tissue
(Bell, 1995).
1.1.4. Growtb Hormone
Unlike insulin. endogenous plasma concentration of growth hormone
rises during late pregnancy, with a peak at calving (Bell, 1995). Therefore, as
growth hormone levels rise, insulin levels decrease. Under conditions of
negative energy balance, it has been shown that growth hormone affects fat
mobilization (Etherton and Bauman, 1998). Growth hormone has been shown
to decrease lipogenic enzyme activity and lipogenesis, thus opposing the
actions of insulin (Bell, 1995). Furthermore it can reduce glucose pool and
distribution space by enhancing glucose transport to the uterus and the udder
(Arieli et al., 2001).
1.1.5. Tbyroxine and 3, 5, 3'-trüodothyronine
Concentrations of some other hormones also change during the
transitional period, including thyroid hormones and glucocorticoids. The
thyroid gland principaUy synthesizes thyroxine (T4), considered a prohormone
which is transformed into the metabolically active 3,5,3'-triiodothyronine (T3)
by enzymatic 5'-deiodination in the liver (Chopra et al., 1978). Thyroid
hormones play a role in maintaining energy expenditure for high priority
functions (Bauman and Currie, 1980). Thyroxine (T4) concentrations gradually
increase during late gestation and then decrease approximately 50% at calving
(Kunz et al., 1985). Similar, but less pronounced, changes occur in 3,5,3'
triiodothyronine (Tj). There is an inverse relationship between milk yield and
5

the levels of these thyroid hormones during early lactation, hence decreased
levels ofT3 and T4 result in high milk levels.
1.1.6. Glucocorticoid
Cortisol and lesser amounts of corticosterone are the most important
glucocorticoid hormones secreted by the bovine adrenal gland. Cortisol is
generally considered a powerful immune suppressive agent and likely
exacerbates the immune suppression normally observed in the periparturient
period (Goff and Kimura, 2002). Cortisol exacerbates the immune suppression
rather than causes it, because most studies suggest that immune suppression
begins 1-2 weeks before calving (Kehrli et al., 1989 a, b), and the cortisol
surge occurs on the day of calving and the day after calving (Goff and Kimura,
2002). Edgerton and Hafs (1973) reported glucocorticoid concentrations
increase on the day of calving and return to near pre-calving concentrations
the following day. The actions of glucocorticoids on carbohydrate, protein,
and lipid metabolism result in sparing of glucose and a tendency to
hyperglycemia and increased glucose production. In addition, they decrease
lipogenesis and increase lipolysis in adipose tissue, which results in the release
of glyceroi and free fatty acids.
1.2. Feed intake and energy balance during the transition period
Like hormone changes, changes in dry matter intake during the
transition period also influences metabolism.
1.2.1. Nutrient Requirements
Dairy cows increase their total nutrient requirements and their demand
for energy supply by 23% for maintenance and pregnancy during the last
month of gestation to satisfy the increased requirements of the uterus and fetal
development (Bell, 1995). Cows carrying twins have higher fetai
requirements. Additionally, there is an increase in energy demand by the onset
of lactation at calving for milk synthesis.
1.2.2. Dry matter intake (DMI)
Due to metabolic and fill constraints, intake of feed and energy by
transition cows are limited (Bertics et al., 1992). The causes for decreased pre
calving DMI are not known but may be endocrine-related For example,
changes in blood estrogen or estrogen:progesterone ratio may influence feed
intake (Grummer et al., 1990). Dry matter intake decreases by 20-30%, 1 or 2
6

d before calving, and does not recover until 1 to 2 d after calving. Cows
carrying twins start to reduce their dry matter intake earlier. It has also been
observed that heifers have a lower dry matter intake than cows.
Previous studies have implicated leptin as one of the modulators of
feed intake (lngvartsen and Andersen, 2000; Meister, 2000). Leptin is
produced by adipocytes, and leptin concentrations rise in parallel with body
condition score (BCS) (Delavaud et al., 2000; Ehrhardt et al., 2000).
Kadokawa et al. (2000) and Block et al. (2001) reported that plasma leptin
concentrations decrease dramatically during the periparturient period, increase
slightly during the tirst 4 weeks post-calving, and remain relatively unchanged
after week 4 post-calving in dairy cows.
1.2.3. Energy balance
The inability to consume adequate amounts of feed causes cows to
enter a state of negative energy balance Le. energy requirements exceed
energy intake. Therefore transitional cows need to make necessary changes in
body tissue metabolism by mobilizing body reserves to meet nutrient and
energy requirements for maintenance, gestation and milk synthesis during
early lactation (Bell, 1995; Overton et al., 2001). Primiparous cows exhibit
negative energy balance in early lactation similar to that of multiparous cows
(Lin et al., 1984).
Bertics et al (1992) demonstrated the effects of reduced dry matter
intake with cows that were force-fed via ruminai fistulas to maintain feed
intake versus control cows before calving. Control cows having lower feed
intake pre-calving had higher liver triglyceride (35 vs. 3%, DM basis of liver
sample) and NEFA (1,392 vs. 667 pEqlL) and lower blood glucose (74 vs. 55
mg/dL) at d 1 post-calving than the force-fed cows. At d 14 post-calving, the
control cows had higher plasma BHB than the force-fed cows (15 vs. 9
mg/dL). A decrease in pre-calving intake seems unavoidable, but the
magnitude and duration of deerease ean vary (Berties et al., 1992; Vazquez
Anon et al., 1994; Grummer et al., 1995).
1.3. Glucose demand
Glucose is required in large amounts during the transition period as a
fuel (energy) for the uterus, mammary gland, peripheral tissues, central
nervous system, red blood cells, gastrointestinal tract, and is also required for
7

the synthe sis of lactose, which largely controls milk volume. During late
pregnancy, the gravid uterus consumes 46% of the maternaI glucose
production, whereas the glucose demands associated with lactation account for
85% of the glucose entry (Bell, 1995).
Two weeks before calving, the drive for glucose transport into the
uterus and the mammary gland is strong (Arieli et al., 2001). Plasma glucose
concentrations remain stable or increase slightly during the pre-calving
transition period, increase dramatically at calving, and then decrease
immediately post-calving (Kunz et al., 1985; Vazquez-Anon et al., 1994).
The estimates of whole-body glucose demand of gestating dairy cows
were approximately 1000 to 1100 gld during the last 21 days pre-calving and
the demand increased sharply after calving and was approximately 2.5 times
greater at d 21 post-calving compared with that during the three weeks
preceding calving (Overton, 2001). However, glucose demand during early
lactation is greater than that which can he supported from diet during that
time. Estimated dietary supply of glucose and precursors to support
gluconeogenesis is sufficient for much of the dry period in well-fed cows.
Before calving, dry matter and glucose uptake from the gut decreases, glucose
supply is almost equal to demanda After calving, glucose supply is insufficient
(about -500 gld) to support the demand hecause dry matter intake increases
more slowly than nutrient demand (Bell, 1995).
At the onset of lactation, the cow compensates for this situation in part
by decreasing glucose oxidation by tissues that do not absolutely require it
(Le., muscle). Furthermore, synthesis offat in adipose tissue is essentially shut
down and glucose is not required to make glycerol and provide energy in
support of fat synthesis in adipose tissue. The cow also increases
gluconeogenesis to meet this increased glucose demand (Overton, 2001).
1.4. Gluconeogenesis in the cow
Glucose, amino acids, and fatty acids can be used as energy sources for
maintenance of the cow, fetal growth and milk production. Furthermore, the
sources of each may vary widely through the course of the transition period.
Thus, these three nutrients will he considered.
The liver of the cow must more than double its glucose production in
the immediate postooealving period in order to meet the demand for glucose
8

(Overton and Waldron, 2004). Gluconeogenesis, the formation of glucose
ftom nonhexose precursors, occurs largely in the liver and to a smaller extent
in the renal cortex. The glucose produced passes into the blood to supply other
tissues.
The increase in plasma glucose at calving may result from increased
glucagon and glucocorticoid concentrations that promote depletion of hepatic
glycogen stores (Grummer, 1995). Although the demand for glucose by the
mammary tissue for lactose synthesis continues after calving, hepatic glycogen
stores begin to replete and are increased by d 14 post-calving (Vazquez-Anon,
1994). This probably reflects an increased gluconeogenic capacity to support
lactation.
The substrates for gluconeogenesis are propionate, amino acids, lactate
and glycerol.
1.4.1. Propionate
The contribution ofpropionate to gluconeogenesis is 32-73% (Seal and
Reynolds, 1993). Lomax and Baird (1983) reported that propionate produced
by ruminaI fermentation as the primary substrate for hepatic gluconeogenesis
in the dairy cow accounts for 50 to 60% of total glucose entry in fed animaIs.
Propionate is produced ftom the breakdown of grains by rumen fermentation
and remains the principal gluconeogenic substrate during the transition period
(Overton et al., 1998). Propionate is the principal substrate used to make
glucose by Iiver followed under normal conditions by amino acids, lactate, and
glycerol (Overton, 2001) and can contribute up to 60% of the substrate
necessary for gluconeogenesis in ruminants (DiCostanzo et al., 1999).
Furthermore, the capacity of the liver to make glucose from propionate
appears to he supply related, especially during the ftrst 21 days of lactation
(Overton et al., 1998).
1.4.2. Amino acids
The contribution of atnino acids to gluconeogenesis is 10-30% (Seal
and Reynolds, 1993). The amino acids used for gluconeogenesis in the Iiver
after calving come from skeletal muscle as weIl as dietary amino acids (Bell
1995). Data from Overton et al. (1998) and Simmons et al. (1994) support
increased degradation of skeletal muscle protein during the ftrst 21 days of
lactation. This may explain why feeding diets containing more CP than the
9

NRC requirements (2001) (18% CP) in early lactation cows has proven to be
effective. The rate of gluconeogenesis from amino acids peaks near calving
(Bell et al., 2000). AIl amino acids except leucine and lysine can make a net
contribution to gluconeogenesis (Bergman and Heitmann, 1978). Alanine and
glutamine have been reported to be the most gluconeogenic of aIl amino acids
(Bergman and Heitmann, 1978). Similar to propionate, utilization of amino
acids for gluconeogenesis may he supply-dependent.
1.4.3. Lactate and glycerol
Lactate and glycerol contribute a small amount to gluconeogenesis
(Seal and Reynolds, 1993). The maximal contribution of lactate to
gluconeogenesis is 15%. Glycerol, released from adipose tissue as a
consequence of lipolysis, may contribute as much as 15 to 20% of the glucose
demand around calving (Bell, 1995) or during feed deprivation (Baird et al.,
1980).
Amino acids, lactate and glycerol contribute a greater percentage of
total glucose synthesis when DMI or propionate availability declines (Danfaer
et al., 1995; Donkin and Armentano, 1993; Reynolds et al., 1988; Lomax and
Baird, 1983). Propionate and amino acids are considered supply related during
the first 21 days of lactation. Therefore, when glucose synthesis is inadequate
due to insufficient amounts of propionic acid, altemate sources of energy must
be found and body fat stores hegin to he broken down.
1.5. Metabolism ofnon..esterified fatty acids (NEFAs)
There is a normal breakdown of body fat around calving time because
ofthe hormonal changes associated with calving. Concentrations of circulating
lipolytic hormones increase near the time of calving (Bremmer et al., 1998) and
contribute to fatty acid mobilization from adipose tissue. Stress situations also
increase the mobilization ofbody fat stores. In addition, triacylglycerols stored
in adipose tissue are mobilized to be used as an altemate energy source due to
the reduction in energy intake. Low levels of glucose in the blood cause
hormones (epinephrine and glucagon) to activate the enzyme adenylyl cyclase
in the adipocyte plasma membrane which produces an intracellular second
messenger, cyclic AMP (cAMP). A cAMP-dependent protein kinase
phosphorylates and thereby activates hormone-sensitive triacylglycerol lipase,
which catalyzes hydrolysis of the ester linkages of triacylglycerols. The fatty
10

acids are mobilized and released into the bloodstream in the form of NEF As
where they bind to blood prote in serum albumin. These bound NEF As are
carried to body tissues such as skeletal muscle, heart, and renal cortex where
they will dissociate from albumin and transported into ceUs to he oxidized for
energy production (Grummer, 1993).
Fatty acids are utilized as fuels for skeletal muscle, liver, and other
organs of the cow. Approximately 50% of fatty acids found in milk fat come
from either the diet or from lipoprotein TG in blood. Fatty acids used by the
mammary gland during synthesis of milk fat can also be provided by NEF A in
the blood, which are released during mobilization of adipose tissue (Overton,
2001). Bell (1995) suggested that 40% offatty acids in milk fat during the first
week of lactation may come from blood NEF A.
During the last week before calving, the concentration of NEF A
increase slowly as the cow approaches calving, and usually range from 200 to
300 f.1M. Values increase sharply from 2 to 3 days hefore calving and
generally peak at 800 to 1200 f.1M on the day of calving (Grummer, 1995). The
rapid rise in NEF A at calving is presumably due to the stress of calving
(Grummer, 1995). It is not known how much of the initial increase in plasma
NEF A can he accounted for by changing endocrine status versus energy
restriction (Grummer, 1995). Bertics et al (1992) demonstrated that force
feeding cows during the transition period reduced the magnitude of NEF A
increase but did not completely eliminate it. An increase in plasma NEF A was
observed d 1 pre-calving in cows that did not experience dry matter intake
depression (Vazquez-Anon et aL, 1994). These observations indicate at least
part of the pre-calving increase in plasma NEFA is hormonally induced.
After calving, NEF A concentrations decrease rapidly, but
concentrations remain higher than they were before calving (Vasquez-Anon et
al.,1994). By 3 weeks after calving, values should again be below 300 f.1M.
Heifers experience a higher tevet ofNEFA which may be associated with their
greater nutrient demands for growth. Values greater than 700 f.1M heyond d 7
after calving, indicate severe negative energy balance or health problems
(Drackely, 1999).
11

1.6. Fatty Liver
When nutrient intake is insufficient and large amounts of NEF A are
released into the blood, the liver begins to accumulate and store NEF A in
proportion to their concentrations in plasma. In liver, NEF A will be esteritied
to form TG, which can either he exported as part of very low-density
lipoprotein (VLDL), or stored. In ruminants, export to blood or disposai of
NEF A occurs at a very slow rate (low capacity) relative to many other species
and mechanisms regulating this export are unknown (Grummer, 1993). Under
conditions of increased hepatic NEF A uptake and esteritication, triglyceride
accumulation occurs, causing fatty liver disease. Fatty liver is a major
metabolic disease that affects up to 50% of dairy cows in early lactation. High
concentrations of liver TG promote the synthesis of additional triglyceride and
decrease the oxidation of NEF A (Grum et al., 1996), thereby further increasing
accumulation of triglyceride in the liver. Fatty liver occurs when the rate of
hepatic triglyceride synthesis exceeds the rate of triglyceride disappearance
through either hydrolysis or secretion via very low density lipoproteins
(VLDL), (i.e. export oftriglyceride as VLDL from the liver cannot keep pace
with increased NEF A uptake and triglyceride synthesis by the liver)
(Grummer, 1993).
Apolipoprotein BI00 (Apo BI00) is the major prote in of VLDL, and
its concentration in the liver is inversely related to hepatic triglyceride
concentration. Gruffat et al. (1997) examined stage of lactation-dependent
regulation of Apo B 100 in high producing dairy cows during the tirst 12
weeks of lactation. Cows were fattened during gestation and were underfed
just after calving to increase fat mobilization and induce hepatic lipidosis.
Concentration of Apo BI 00 in liver was approximately 25% lower during the
tirst 4 weeks of lactation than during late pregnancy. Hepatic Apo BI 00
concentrations retumed to pre-calving levels by 12 weeks of lactation.
Accumulation of lipid in the liver commonly occurs prior to or at
calving (Bertics et al.,1992) although the greatest increase in liver triglyceride
typically occurs at calving. In a study by Vasquez-Anon et al. (1994), lipid did
not accumulate in the liver until after the concentration of NEF A in plasma
increased at calving. By d 1 after calving, the largest increase in hepatic
triglyceride has occurred and concentration in the liver remained constant or
12

increased slightly during the post-calving transition period (Bertics et al.,
1992; Vasquez-Anon et al., 1 994). Heifers seem to be less susceptible to fatty
liver at d 1 post-calving, however reasons are unknown (Grummer et al.,
1995).
The extent to which feed intake is depressed before and after calving or
during disease moderates the degree of infiltration of TG. Fatty liver can
develop within 24 h of an animal going off feed. Because of the slow rate of
triglyceride export as lipoprotein, once fatty liver has developed, it will persist
for an extended period of time. Depletion of TG from the liver usually begins
when the cow reaches a state of positive energy balance about 5 to 10 weeks
after calving and may take several weeks to be completed.
Fatty liver is a consequence of negative energy balance, not positive
energy balance. Energy consumption above requirements for maintenance and
production purposes will not directly result in deposition of triglyceride in
hepatic tissue. Triglyceride deposition will occur only if the cow becomes
overconditioned and, consequently, reduces feed intake. Fatty liver is likely to
develop concurrently with other diseases, typically disorders that are seen at or
shortly after calving, including metritis, mastitis, displaced abomasum,
acidosis, and hypocalcemia. Cows that are slow to increase in milk production
and feed intake after calving are likely to have fatty liver. However, fatty liver
is the result of poor feed intake, which is a condition that leads to low blood
glucose, which also contributes to fatty liver because insulin suppresses fat
mobilization from adipose tissue. Fatty liver is often associated with obese
cows and downer cows.
Fatty liver syndrome (> 20% fat) impairs the function of the liver,
increases disease incidence, pro longs recovery from other disorders, reduces
fertility, and sometimes leads to death (Drackley, 1999; Grummer, 1993;
Veenhuizen et al., 1991; Overton and Waldron, 2004). There are no known
clinical signs that are unique to cows with fatty liver. Fatty liver has been
associated with low milk production, increased clinical mastitis, and poor
reproductive performance. However, cause and effect have not been
established, and the metabolic consequence oftriglyceride accumulation in the
liver has not been determined. Field observations suggest that response to
13

treatment of concurrent disorders is poor if triglyceride infiltration of the liver
is extensive.
Liver biopsy is the only reliable method to detennine severity of fatty
liver in dairy cattle. Measurement of total lipid or triglyceride content by
analytical methods after extraction from tissue by organic solvents is
necessary for quantitative assessment of fatty liver; however, these assays are
not routinely conducted in commerciallaboratories.
Blood metabolites, urine metabolites or blood enzyme activity have
been proposed as diagnostic tools for the detennination of fatty liver. When
conditions are conducive to the development of fatty liver, blood glucose
concentrations are low and blood NEF A and BHB concentrations are high.
Blood cholesterol concentration is usually low when fatty liver occurs, and
this may reflect impairment in the ability of the liver to secrete lipoproteins.
However, blood metabolites or enzymes are poor indices of fatty liver because
baseline (nonnal) concentrations vary tremendously among animais.
Microscopie evaluation can he used to estimate the volume of the
tissue occupied by fat. Mild, moderate, and severe fatty Iiver are often defined
as <20%, 20-40%, and >40% fat (percentage of cell volume), respectively.
However, these values have litt le meaning relative to impact of physiological
functions or clinical signs of fatty liver.
1.7. EtTect ofBeS on fat mobilization
The current National Research Council (NRC, 2001) recommendation
is that cows should not gain B W during the dry period, except for B W
associated with growth of the fetus and fetai membranes. Furthermore, cows
should end lactation with the same body condition score (BCS) as desired at
the start of the next lactation (3.5 to 3.75 on a five-point scale, 1 =- thin to 5 = fat). This will allow the cow to calve with an adequate but not excessive body
fat reserve.
Early studies (Garnsworthy, 1988) using lactating dairy cows showed
that the delay between maximum milk yield and maximum DMl might be
related to body condition at calving. Cows calving with high body condition
slowly increased DMl and then reached maximum DMllater than did those
cows calving with poor body condition. Over-conditioned cows are more
likely to have poor appetites post-calving (Holter et al., 1990). A fat cow is
14

more prone to mobilize fat and to have a depressed appetite causing a greater
loss of body condition in early lactation (de Ondarza, 1998). This high lipid
mobilization after calving could lengthen the delay to reach maximum DMI
(Bareille and Faverdin, 1996). Concentrations of NEF A and BHB in plasma
and TG in liver were elevated in primiparous cows calving at heavier BW and
higher BCS indicating a positive correlation between high BCS and BW with
increased levels of BHB and NEFA (Grummer et al., 1995). Excessive
deposition of adipose tissue pre-calving is highly correlated to post-calving
metabolic disorders, such as ketosis and fatty liver syndrome (Baird, 1982).
On the other hand, cows that begin lactation with a BCS <3.25 may not
be capable of mobilizing enough energy to support maximal milk production
(Otto et a1.,1991). Thin cows have greater mobilization of body fat due to
decreased insulin levels and a greater conversion of the resulting NEF As to
TG and ketones. Under-conditioned cows have insufficient energy reserves.
Rapid and/or excessive body weight losses can increase the incidence of
metabolic disorders.
Failure of the transition cow to appropriately adjust her metabolism to
support increased nutrient requirements of early lactation may result in the
occurrence of metabolic disorders, poor reproductive performance, and
decreased milk production during the upcoming lactation (Bell, 1995;
Grummer, 1995; Drackley, 1999).
1.8. Furtber metabolism orNEFAs
The liver is a major site of long-chain fatty acid metabolism, especially
during feed deprivation or early lactation when mobilization of adipose tissue
TG Ieads to increased NEF A concentrations in blood. The mechanism for the
disposaI of NEF A during excessive lipid mobilization is the increased hepatic
uptake ofNEFA (Grum et al., 1996). In the liver, NEFAs are transported into
mitochondria where the enzymes for acid oxidation are located. The free fatty
acids that enter the cytosol from the blood cannot pass directly through the
mitochondrial membranes and therefore must undergo a series of enzymatic
reactions to he activated, and the activated fatty acid can then enter the
mitochondria via an acyl-camitine/camitine transporter. Mitochondrial J3-oxidation takes place in three stages. The first stage is composed of four steps;
first, dehydrogenation; second, the addition of water to the resulting double
15

bond; third, oxidation of the f3-hydroxyacyl-CoA to a ketone; fourth, thiolytic
cleavage by coenzyme A. During stage one, fatty acids undergo oxidative
removal of successive two-carbon units in the form of acteyl-CoA starting
from the carboxyl end of the fatty acyl chain. In the second stage, the acetyl
CoA produced from the oxidation of fatty acids can be oxidized to C02 and
H20 by the citric acid cycle, which also takes place in the mitochondrial
matrix. The frrst two stages of fatty acid oxidation produce the reduced
electron carriers NADH and F ADH2' which in the third stage donate electrons
to the mitochondrial respiratory chain, through which electrons pass to oxygen
with the concomitant phosphorylation of ADP to ATP. Therefore, the energy
released by fatty acid oxidation is used for A TP synthesis. The number of
reaction required for f3-oxidation will vary depending on the nature of the fatty
acid (ie. saturated, degree ofunsaturation, odd-number fatty acids).
Although the major site of fatty acid oxidation is the mitochondrial
matrix, peroxisomes contain enzymes capable of oxidizing fatty acids to
acetyI-CoA by a similar pathway. Peroxisomes are membrane-enclosed
cellular compartments in which fatty acid oxidation produces H202, which is
then enzymatically destroyed. The difference between peroxisomal and
mitochondrial oxidation is in the frrst step. In peroxisomes, the flavoprotein
dehydrogenase that introduces the double bond passes electrons directly to O2
producing H20 2, which is immediately cleaved to H20 and O2. The resulting
energy produced from peroxisomal oxidation is dissipated as heat and not
conserved as ATP such as in mitochondrial oxidation. Liver peroxisomes do
not contain the enzymes of the citric acid cycle and cannot catalyze the
oxidation of acetyl-CoA to CO2• The fatty acid produced from peroxisomal
oxidation can enter the mitochondria to be oxidized.
Acetyl-CoA formed in the liver during oxidation of fatty acids can
enter the citric acid cycle or can be converted to ketone bodies (acetone,
acetoacetate and J3-hydroxybutyrate) for export to other tissues. Acetone is
produced in smaller quantities than other ketone bodies. Acetoacetate and 13-hydroxybutyrate are transported to the blood and extrahepatic tissues, where
they are oxidized in the citric acid cycle to provide most of the energy required
by skeletal muscle, heart muscle, and renai cortex. The brain, which usually
16

uses glucose for energy, can adapt to the use of acetoacetate or ~
hydroxybutyrate when glucose is not available or under starvation conditions.
The production and export of ketone bodies from the liver to extrahepatic
tissues allows continued oxidation of fatty acids from the liver when acetyl
CoA is not being oxidized in the citric acid cycle. During starvation,
gluconeogenesis depletes citric acid cycle intermediates, diverging acetyl-CoA
to ketone body production. Fatty acids therefore enter the mitochondria to be
degraded to acteyl-CoA, which cannot pass through the citric acid cycle
because cycle intermediates (such as oxaloacetate) have been drawn off for
use as substrates in gluconeogenesis. The rate of fatty acid mobilization from
adipose tissue exceeds that of their oxidation. The accumulation of acetyl
CoA, which is not incorporated into the citric acid cycle builds up in the liver
and accelerates the formation of ketone bodies beyond the capacity of
extrahepatic tissues to oxidize them. The increased blood levels of
acetoacetate and ~-hydroxybutyrate, lowers the blood pH causing acidosis.
Extreme acidosis can lead to coma and in sorne cases death. Severe starvation
leads to high concentrations of ketone bodies in the blood and urine causing
ketosis.
1.9. Ketosis
Ketone bodies production is favored when blood glucose
concentrations are low and is detrimental to overall cow health and
performance (Grummer, 1993; Drackely et al., 2001). The characteristics of
ketosis include reduced milk yield, loss of body weight, loss of appetite, and
occasionally, signs of nervousness. Sometimes these signs are clearly
recognized (clinical) but, often, they are not easily seen (subclinical).
Although symptoms of subclinical ketosis are not detectable, it is potentially
serious because it usually remains undetected, untreated, and could progress to
a clinical condition (Baird, 1982). Subclinical ketosis has also been associated
with a 10ss of milk production of 1.0 to 1.4 kg/d (Doohoo & Martin, 1984).
This loss in production is economically significant to the dairy producer, since
the reported prevalence for subclinical ketosis ranges from 8.9 to 34%
(Kauppinen, 1984).
Subclinical ketosis is a disorder that is associated with increased levels
of circulating ketone bodies and can he identified by the concentrations of
17

ketone bodies in the serum, milk and urine (Grum et al., 1996). Reported
threshold concentrations of serum BHB that are used to define subclinical
ketosis range from 1000 to 1400 mmol/L (Nielen et al., 1994). The primary
risk period for subclinical ketosis is the first 2 months of lactation, but the
peak prevalence occurs during the Ist month (Andersson & Emanuelson,
1985; Dohoo & Martin, 1984).
In genera~ fatter cows (Bes > 3.75) will experience more ketosis (de
Ondarza, 1998). Duffield et al. (1998) reported that higher BHB levels were
observed in fat cows and that the risk of clinical ketosis was three times
greater for fat cows than for thin or fair-conditioned cows. Several other
factors influence the prevalence of hyperketonemia or ketosis, including age
(Andersson, 1988; Dohoo & Martin, 1984), season (Tveit et al., 1992), and
breed (Andersson, 1988). However, the influence of heritability is thought to
be relatively low (Tveit et al., 1992).
Ketosis has been found to occur after a cow develops a fatty liver.
Liver triglyceride:glycogen ratio at calving may be an indicator of a cow's
susceptibility to ketosis (Veenhuizen et al.,1991). The etiology of fatty liver
and ketosis are similar, and in both cases liver function is impaired (DeBoer et
al., 1985; Drackley et al., 1992). In addition, fatty liver and ketosis are
common metabolic disorders in transition cows and increase predisposition to
other post-calving health problems.
2. The immune defense system of the cow
Immunity refers to the body's ability to resist or eliminate potentially
harmful materials or abnormal cells. The immune defense system of the
ruminant plays a key role in recognizing and either destroying or neutralizing
materials within the body that are foreign to the "normal self'. The immune
defense system provides protection against foreign and abnormal cells and
removes cellular debris. Pathogenic bacteria and viruses are the major targets
of the immune defense system. Leukocytes (white blood celIs) and their
derivatives (neutrophils, eosinophils, basophils, B lymphocytes, T
lymphocytes and monocytes) are the effector cells of the immune defense
system. Immune responses may he either nonspecific or specifie.
18

2.1. Nonspecific Immune Response
Nonspecific defenses include inflammation, interferon, neutrophils,
natural killer ceUs, macrophages, lysozyme and the complement system.
Inflammation refers to an innate, nonspecific series of highly interrelated
events that are set into motion in response to foreign invasion, tissue damage
or both. The ultimate goal of inflammation is to bring to the invaded or injured
area phagocytes and plasma proteins then can (1) isolate, destroy or inactivate
the invaders; (2) remove debris; and (3) prepare for subsequent healing and
repair.
Numerous drugs can suppress the inflammatory response, the Most
effective are the salicylates and glucocorticoids (drugs similar to the steroid
hormone cortisol, which is secreted by the adrenal cortex). Glucocorticoids,
which are potent anti-inflammatory drugs, suppress almost every aspect of the
inflammatory response. In addition, they destroy lymphocytes within
lymphoid tissue and reduce antibody production. By suppressing inflammatory
and other immune responses that localize and eliminate bacteria, such therapy
also reduces the body's ability to resist infection.
2.2. Specifie Immune Response
Specific immune defenses include lymphocytes, macrophages and
immunoglobulins. Specifie immunity can he divided into two types of
responses, antibody-mediated immunity accomplished by B lymphocyte
derivatives and cell-mediated immunity accomplished by T lymphocytes.
Lymphocytes, categorized under Band T lymphocytes, are responsible for
specifie recognition of the antigen but have different functions.
Both Band T ceUs must he able to specificaUy recognize unwanted
ceUs and other materials to he destroyed or neutralized as heing distinct from
the body's own normal cells. The presence ofantigens enables lymphocytes to
make this distinction. An antigen is a large, complex Molecule that triggers an
immune response against itself when it gains entry into the body. In general,
the more complex a Molecule results in greater antigenicity. Foreign proteins
are the MOst common antigens because oftheir size and structural complexity.
Bach B and T cell bas receptors on its surface for binding with one particular
type of the Many possible antigens. For B ceUs, binding with antigen induces
the cell to differentiate into a plasma ceU, which produces antibodies that are
19

able to combine with the specifie type of antigen that stimulated the
antibodies' production. Each individual must be able to produce thousands of
different antibody molecules in order to identify the large array of potential
antigens and pathogens. The functions of antibodies include their ability to
bind to antigen and to increase phagocytosis or killing by polymorphonuclear
ceUs and macrophages, complement activation, direct inactivation of virus or
toxin, and enhancement of antigen clearance.
2.2.1. B Lymphocytes: Antibody-Mediated Immunity
The B lymphocytes express surface Ig and are the precursors of plasma
ce Us that synthesize antibodies that indirectly lead to the destruction of foreign
material. B lymphocytes are responsible for antibody-mediated immunity
involving antibodies that amplify the inflammatory response to promote
destruction of the antigen that stimulated their production. Bach antigen
stimulates a different clone of B lymphocytes to produce antibodies. The
production of antibodies as a result of exposure to an antigen is referred to as
active immunity against the antigen. Another way to acquire antibodies is
passive immunity, which is achieved by direct transfer of antibodies actively
formed by another animal.
2.2.2. T Lymphocytes: Cell-Mediated Immunity
T lymphocytes are responsible for ceU~mediated immunity involving
direct destruction of virus-invaded ceUs and mutant ceUs through
nonphagocytic means. The T lymphocytes are divided into three discrete
subpopulations based on the expression of cell surface receptors.
Cytotoxic T ceUs (killer T ceUs or CD 8 ceUs) destroy host ceUs
bearing foreign antigen. Helper T cells (CD4 ceUs) enhance the development
of antigen-stimulated B ceUs into antibody-secreting ceUs, enhance the activity
of the appropriate cytotoxic and suppressor T cells, and activate macrophages.
Suppressor T ceUs suppress both B cell antibody production and cytotoxic and
helper T cell activity. These functions are mediated by the production of a
wide range of cytokines and enzymes.
Measures of lymphocyte activity, such as antibody and cytokine
production, cytotoxicity, and proliferation, have been used to indicate the
functional status of the immune system (MaUard et al., 1998). In the current
study, lymphocyte proliferation and antibody production in response to
20

chicken ovalbumin were examined during treatment with Flucort® and Prede~
2X administered at calving.
2.3. Changes in the immune system during the transition period
Around the time of calving, host defense is impaired and the dairy cow
is immunosuppressed (Detilleux et al., 1995). These are associated with
changes in hormone profiles and metabolic and physical stresses ofpregnancy,
calving and lactation and largely mediated through the neuroendocrine
immune axis (perkins et a1.,200 1).
In dairy cows, the weeks before and after calving are periods with high
incidence of infections. Diseases may occur when the immune system is
unable to respond efficiently to invading pathogens. An efficient immune
response relies on the interaction and balance between different ceH types and
their products. In the periparturient period, large changes occur in hormonal
levels and metabolism, adapting the animal to a high metabolism and high
milk production (Holtenius et al., 1996; Bell and Bauman, 1997; Kehrli et al.,
1999). It has been suggested that negative energy balance in combination with
other factors involved in calving, such as increases in cortisol, may contribute
to periparturient immunosuppression and disease susceptibility (perkins et al.
(200 l, Preisler et al. 1999 and Goff and Kimura, 2002)
As calving approaches, the total number of white blood cells increases,
mainly as a consequence of higher numbers of neutrophils (Saad et al., 1989;
Gilbert et al., 1993). However, the functional capacities ofthe neutrophils are
impaired during this periode Neutrophils are the tirst line of host
immunological defense against bacterial infections. One of the key points in
the control and eradication of an infection is rapid migration and recruitment
of neutrophils to the site of infection (Heyneman et al., 1990; Burton and
Erskine, 2003). The tirst step in neutrophil migration depends on the
coordinated function of selectins and ~2-integrins (adhesion molecules on
leukocytes and endothelial ceIIs) (Kehrli et al., 1999). As a consequence,
migratinglmarginating cells stop their rolling and adhere tightly to the
endothelium, initiating diapedesis. However, down-regulation and shedding of
CD62L molecules from neutrophils have been reported around calving,
consequently less numbers of cells are able to migrate into peripheral tissue
(Lee and Kehrli, 1998; Paape et al., 2002). In addition, the phagocytic and
21

killing ability of neutrophils are also impaired around calving (Saad et al.,
1989; Hoeben et al., 2000; Mehrzad et al., 2001).
As calving approaches, the proportion of blood lymphocytes and their
functional activities, such as cloning expansion and antibody production
decrease (Ishikawa et al., 1994; Detilleux et al., 1995; Kimura et aL, 1999).
The decrease in lymphocyte numbers is due to a net depression in CD4+, CD8+
and 10+ T lymphocytes (Van Kampen and Mallard, 1997; Kimura et al., 1999).
ln addition, functions of certain subpopulations change. It has been observed
that blood CD4+ T-cells preferentially produce IL-4 and IL-I0 around calving,
while they shift to IFN-y and IL-2 production during mid to late lactation
(Shafer-Weaver et al., 1999). Moreover, CD8+ lymphocytes of the suppressor
type predomina te at this time, which may also contribute to higher levels IL-4
and IL-lO, setting a humoral immune response (Shafer-Weaver and Sordillo,
1997). The changes in leukocytes and cytokine production observed around
calving result in a suppressed activity of the cellular immune response; which
is necessary to deal with intracellular bacteria and viruses, thereby making the
animal more susceptible to infections.
Numerous investigations (Ishikawa, 1987; Kehrli et al., 1989b; Saad et
al., 1989) have reported diminished lymphocyte responsiveness around
calving. Studies by Kehrli et aL (1989b) utilizing Holstein heifers
demonstrated that peripheral blood lymphocyte response to pokeweed mitogen
declined steadily from 2 weeks pre-calving until the week of calving and then
began to increase again at week 2 post-calving. This diminishing response was
substantiated by Saad et al. (1989), who reported a steady decline in
lymphocyte response of Swedish Red and White cows to concanavalin A and
pokeweed mitogen from 3 weeks pre-calving through to calving and recovery
at about week 2 or 3 post-calving. Those groups (Kehrli et al., 1989b; Saad et
al., 1989) speculated on the ability of reproductive hormones and
glucocorticoids to modulate this response in vivo. Those observations provide
sorne support for the notion that altered lymphocyte responsiveness around
calving is linked to increased mastitis susceptibility and that transition period
hormone changes may be influential.
Mitogens are often used to assess the proliferation ability of
lymphocytes and mitogens such as concanavalin A (ConA), pokeweed
22

mitogen (PWM), and lipopolysaccharide (LPS) are often used in such studies.
Concanavalin A is known to stimulate T -celI proliferation, whereas pokeweed
mitogen and bacterial lipopolysaccharide selectively stimulate B-cell
proliferation (Li et al., 2000).
Decline of certain subsets of blood lymphocytes in both healthy and
diseased cows may explain the increased disease incidence at calving.
Changes in lymphocyte subsets, particularly the ratios of CD4 to CDS have
been associated with immunosuppressive diseases in various species. It has
been shown that the proportion of certain T -cell subsets decreased
dramatically around calving, but B cells did not. In addition, the proportions of
T lymphocytes pre-calving and at calving were also significantly lower than
those of non-pregnant, non-Iactating cows of the same breed (Glass et
al., 1990). The percentage of T ceUs was lowest in milk (16%) during the
transition period, but increased to 62% in late lactation, whereas the
percentage of B ceUs and macrophages were reported at 25 and 69%,
respectively, during the same period, but declined in late lactation to 7 and
21 %, respectively (Glass et al., 1990).
As calving approaches, there are changes in the different T -cells
subsets, however, the percentage of B-Iymphocytes seems to remain fairly
constant (Shafer-Weaver et al., 1996). In contrast, Van Kimpen and Mallard
(1997) reported a higher proportion of B-cells in blood before and at calving
than after calving. In regards to functional activity of B-cells, a diminished
antibody production during the time of calving has been observed (Nagahata et
al., 1992; Detilleux et al., 1995).
Lacetera et al. (2004) demonstrated that high concentrations of fatty
acids impaired cow lymphocyte function in vitro. Results suggest that intense
lipomobilization with increased plasma concentrations of NEF As might be
factors that explain the higher incidence of infections observed in cows
suffering from energy deficit. Further, the same authors reported that the
increase of plasma NEF A is likely to exert negative effects on lymphocyte
functions in cows and that a deficiency of IFN production occurred in cows
with fat mobilization syndrome (Lacetera et al., 2004). Wentink et al. (1997)
documented that hepatic lipidosis due to intense lipomobilization is associated
with impaired immunodepression. High plasma NEF A is hypothesized as
23

directly responsible for impairment of physiological functions of live stock
suffering from energy deficit.
During the transition period, sorne animaIs experience a low antibody
response (Franco et al., 1990). Franco et al. (1990) reported that antibody
responses are suppressed due to stress released glucocorticoids. Although it
has been reported that low serum antibodies in cattle at calving may be due to
sequestration of immunoglobulin into the mammary gland (Detilleux et al.,
1995), the study done by Mallard et al (1997) suggest that lower specifie
antibody response in serum does not necessarily relate to immunoglobulin
transport. Mallard et al. (1997) demonstrated that animais with high serum
antibody response also supply higher concentrations of specifie antibody to the
mammary gland and that not aIl cows experience depression of antibody
response during the transition period.
2.4. Major diseases during the transitional period
Elevation of NEF A, BHB, and liver TG may predispose cows to high
incidence of metabolic disorders (Baird, 1982). Negative energy balance and
the degree of fatty acid mobilization before calving, as indicated by plasma
NEF A concentrations, has been positively related to the incidence of dystocia,
retained placentas, ketosis, fatty liver, displaced abomasums, and mastitis in
the transition period (Grummer, 1993). The risk of displaced abomasums,
retained placenta and clinical mastitis is significantly increased when marked
energy deficits occur prior to calving (Duffield et a1.,2002). Furthermore,
over-conditioned cows had a higher incidence of health problems within 75 d
post-calving. Profits are affected by metabolic disorders as expenses for most
metabolic disorders have been reported to vary from $200 to $400 per incident
(Harris, 2000).
Many of the resulting disorders are associated and are consequential of
each other. Studies of periparturient diseases suggest that clinical ketosis
precedes displaced abomasum in addition to increasing its risk (Curtis et al.,
1985; Grohn et al., 1989). In addition, subclinical ketosis has been identified
as a risk factor for metritis (Doohoo & Martin, 1984, Grohn et al., 1989) and
mastitis (Doohoo & Martin, 1984) and bas been associated with cystic ovaries
(Andersson & Emanuelson, 1985; Doohoo & Martin, 1984).
24

2.4.1. Mastitis
The dairy cow is at increased risk of infectious disease during the
transition period (Smith et al., 1985). Selection of dairy cows with superior
milk production traits has resulted in a steady increase in the incidence of
clinical mastitis (Harmon, 1994). As parity increases, so too does production
yield and the occurrence ofdisease (Dunklee et al., 1994).
Given the positive genetic correlation between selection for increased
milk production and the increased rate of clinical mastitis (Owen et al., 2000),
one might hypothesize that superior production is associated with unfavorable
changes in host defense mechanisms that could result in an increased
occurrence of mastitis.
More than 95% of somatic celIs (SC) (cells in bovine milk) are
leucocytes, including neutrophils, macrophages and lymphocytes. The somatic
cell count (SCC) is the number of SC/mL of milk and is a useful
approximation for the concentration of leucocytes in milk. Somatic celI counts
in milk are used as indicators of mammary health on the basis that they reflect
an immune response and thus the presence of infection. Although a raised
SCC is an accepted indicator of an existing bacterial infection, a very low SCC
has been associated with an increased subsequent susceptibility to clinical
mastitis. This suggests that SC may provide protection from bacterial
colonisation as weIl as being a marker of infection.
Mastitis is a common problem in modem dairy cows and a major cause
of lost income in the dairy industry. Mastitis is a disease that leads to reduced
milk yield and an increased number of clinical treatments and early cow
culling (Beaudeau et al., 1993; Lescourret and Coulon, 1994). This
inflammation of the mammary gland, usually a response to invasive agents,
can be characterized by an increase in SCC. A logarithmic transformation
calIed somatic celI score has been used as an indicator of udder health for
management and selection purposes (Rodriguez-Zas et al., 2000). Somatic cell
count values higher than 283, 000 cells/mL indicate the presence of mastitis
(Guidry, 1985; Reneau, 1986). Therefore, values lower than 283,000 cells/mL
do not reflect the health of the udder, but rather are associated with milk yield
(Hortet et al., 1999). The established association between milk production and
SCC in dairy cattle is increasingly used to estimate lost production due to
25

mastitis because important management decisions regarding cost-effective
prevention and control of mastitis are based on this relationship (Bartlett et al.,
1990). Jones (1986) suggested that sec of 600, 000 to 1, 000, 000 cells/mL
were associated with an 8 to 12% reduction in herd milk production.
According to Harmon (1994), mastitis or elevated sec is associated with a
decrease in lactose, a-Iactalbumin, and fat in milk because of reduced
synthetic activity in the mammary tissue.
2.4.2. Udder Defense
Wagner (2003) demonstrated that cows in the negative energy balance
period show an impairment of udder defense mechanisms. Lacetera et al.
(2001) reported immunodepression in ketotic ewes and negative relationships
between immune functions and plasma NEF A or BHB. Hyperketonemia is
hypothesized as one of the Most important factors leading to reduced udder
defenses. There are possible explanations for these effects via each of the
mechanisms of defense. Firstly, the capacity for phagocytosis by
polymorphonuclear cells and macrophages May be reduced in a state of
negative energy balance, furthennore bacterial killing capacity is impaired in
the presence ofketone bodies (Leslie et al., 2000). Secondly, lower amounts of
cytokines produced by lymphocytes in ketotic cows May cause udder
leukocytes to induce ceIl recruitment in intra·mammary infection (Leslie et al.,
2000). Finally, the capacity for blood leukocytes to migrate into the infected
gland is reduced (Leslie et al., 2000).
It is hypothesized that high plasma NEF A are directly responsible for
impairment of physiological functions of livestock suffering from energy
deficit (Drackley et al., 2001; Gillund et al., 2001). ResuIts from Lacetera et al.
(2004) suggest that intense lipomobilization May he potentially responsible for
immunodepression and also that plasma NEF A might represent biochemical
indicators of the immune reactivity of cows.
2.4.3. Compromised Reproduction
Lipid metaboHsm is related to the reproductive status of high yielding
dairy cows, for example during early lactation the rate of adipose tissue
lipolysis is high, but the rate of lipogenesis is almost negligible (Bareille and
Faverdin, 1996). In addition, subclinical ketosis has been associated with
26

decreased milk yield, increased risk of clinical ketosis, metritis, cystic ovarian
disease, and impaired reproductive performance (Doohoo and Martin, 1984)
Decreased reproductive performance can he explained partly by
delayed uterine involution (Higgins and Anderson, 1983). The delayed
involution can be explained by an increased incidence, length, and severity of
endometritis (Heinonen et al., 1987; Sheldon et al., 2002), which can be
caused by delayed and decreased immune response in the uterus (Zerbe et al.,
2000). Delayed initiation of ovarian activity is caused by a severe negative
energy balance (Herdt, 1991). Additionally, decreased concentrations of
insulin and elevated concentrations of NEF A can impair normal ovarian
function (Comin et al., 2002; Jorritsma et al., 2003).
The etiology of retained placenta is not completely understood,
however, impaired immune function may play an important role (Goff and
Horst, 1997). For example, after calving, reduced neutrophil chemoattraction
for fetal tissue has been observed in cows with retained placenta (Cai et al.,
1994). In addition, Gunnink (1984) observed that impaired leukocyte ability to
attack cotyledon material existed prior to calving in cows that subsequently
developed retained placenta.
Lucy et al. (1991) found that the number of small follicles «5 mm)
decreased while the number of large follicles (> 1 0 mm) increased between d 0
and d 25 post-calving, and energy balance was related to changes in follicular
populations. As the degree of negative energy balance increases during early
lactation, the interval from calving to first ovulation has been shown to be
lengthened (Butler & Smith, 1989). Butler and Smith (1989) suggested that
cows with a longer interval from calving to first ovulation experience a
decrease in pregnancy rate at frrst service because the conception rate is
related to the number of ovulatory cycles that occur before insemination
(Stevenson & Call, 1983). In addition, cows that express estrus before the frrst
post-calving ovulation have greater energy balance than cows that do not
express estrus (Spicer et al., 1990). Negative energy balance is, therefore, a
likely cause for poor reproductive efficiency in lactating dairy cows (Opsomer
et al., 1996).
Because plasma cholesterol (Carroll et al., 1990; Spicer et al., 1993),
insulin (Koprowski and Tucker; 1973) and IGF-I (Spicer et al., 1990, 1993)
27

increase, whereas plasma NEF A decrease (Staples et al., 1990) with increasing
week of lactation, those hormones and metabolites are primary candidates for
transmitting the metabolic status of a cow to its reproductive axis.
Concentrations of cholesterol and IOF-I in blood of cattle are modified by
variations in fat, prote in, and (or) energy intake, and increase as energy
balance increases (Grummer and Davis, 1984; Spicer et al., 1990). Moreover,
insulin and IOF-I stimulate mitogenesis and steroidogenesis of bovine ovarian
ce Us in vitro (Spicer and Echternkamp, 1995), and thus, negative energy
balance may affect ovarian activity by decreasing luteal progesterone (P4)
production (Orummer and Carroll, 1988; Spicer et al., 1993). Recent studies
also implicate leptin as a possible metabolic mediator of reproduction by
inhibiting steroidogenesis of bovine granulosa and theca ceUs (Spicer and
Francisco, 1997, 1998).
3. Adrenal Cortex
The adrenal cortex produces corticosteroids or a number of different
adrenocortical hormones, aIl of which are steroids derived from cholesterol.
Adrenal corticosteroids can he divided into three categories; (l)
mineralocorticoids; (2) glucocorticoids; and (3) sex hormones.
3.1. Corticosteroids
The adrenal glands are stimulated to produce corticosteroids by
adrenocorticotropic hormone (ACTH) released by the anterior pituitary gland
in the brain. Release of ACTH from the pituitary gland is influenced by factors
including exercise, stress, surgery, cold exposure, and hypoglycemia. The
major control of ACTH release is feedback inhibition by high blood levels of
corticosteroids. This occurs with the natural release of corticosteroids from the
adrenal glands, or with the administration of a corticosteroid drug formulation.
After release from the adrenal glands, corticosteroids circulate in the
bloodstream until they reach cellular targets. At the level of individual cells,
eortieosteroids enter the eell and bind to specifie protein receptors. The
corticosteroid-protein complex then enters the cell nucleus and alters the cell's
production of proteins. Changes in protein production result in altered cellular
functions, which can have a wide variety of effects in the body, depending on
the type of cell involved.
28

3.1.1. Mineralocorticoids
Mineralocorticoids (primarily aldosterone) have a major effect on
electrolyte (sodium and potassium) balance and blood pressure homeostasis.
Specifically aldosterone stimulates sodium reabsorption and potassium
secretion in the kidneys.
3.1.2. Glucocorticoids
Glucocorticoids have an important role in adaptation to stress, however
the natural function of glucocorticoids is to protect the supply ofblood glucose
critical for normal brain function. They increase blood glucose concentration
by counteracting the effect of insulin and by mobilizing fatty acids and amino
acids from body stores for additional glucose production by the liver.
Therefore, glucocorticoids have a breakdown (catabolic) effect on body
muscle and fat stores, but can cause excessive fat to accumulate in the liver.
The primary glucocorticoid, cortisol, plays an important role in carbohydrate,
prote in, and fat metabolism; exhibits significant permissive actions for other
hormonal activities; and helps resist stress.
3.1.2.1. Cortisol
The overall effect of cortisol is to metabolically increase the
concentration of blood glucose at the expense of prote in and fat stores.
Cortisol also plays a major role in glucose metabolism (carbohydrate, fat, and
protein) by stimulating hepatic gluconeogenesis, the conversion of non
carbohydrate (amino acids) into carbohydrate sources within the liver during
periods of starvation, in other words it mobilizes the body's nutrient stores so
that metabolic fuel is readily available to keep pace with the body's energy
needs when feed intake is low and to maintain normal blood glucose levels.
Cortisol inhibits glucose uptake and use by many tissues, but not the brain,
therefore sparing glucose for use by the brain. Cortisol stimulates prote in
degradation in Many tissues, especially muscle. Muscle proteins are broken
down into amino acids, increasing the blood amino acid concentration, which
are available for use in gluconeogenesis. Cortisol facilitates lipolysis, releasing
fatty acids into the blood which are available as an altemate metabolic fuel for
tissues that can use this energy source, therefore sparing glucose for the brain.
Cortisol plays a key role in adaptation to stress. The release of
glucocorticoids in response to stress May in part serve to control the
29

magnitude of an immune response (Blalock, 1994; Derijk and Sternberg,
1994). For example, cytokines released during inflammation or infection can
stimulate the eventual release of glucocorticoids (Vassilopoulou-Sellin. 1994;
Spangelo and Gorospe, 1995). The glucocorticoid cortisol delivers its
hormonal message to ceUs via cytoplasmic glucocorticoid receptors (Preisler
et al., 2000), which may act in a feedback loop to control the magnitude of the
immune response (Vassilopoulou-Sellin. 1994; Spangelo and Gorospe, 1995).
o 4 (1
4. Syntbetic Glucocorticoids
21jH20H
2OC=O 12 18CH3 _. -OH
17
Synthetic glucocorticoids are potent anti-inflammatory drugs that
suppress immune response, delay wound healing, and depress numbers of
circulating lymphocytes (Griffin, 1989). Synthetic glucocorticoids have been
developed to maximize anti-inflammatory and immunosuppressive effects
while minimizing the metabolic effect, which is useful for treating undesirable
immune responses such as allergic reactions and inflammation. Anti
inflammatory action has been attributed only to pharmacological levels of
glucocorticoids (administration of cortisol-like drugs that are higher in blood
concentrations than the nonnal physiological range). When phannacological
glucocorticoids are administered to yield higher than physiological
concentrations, the metabolic effects increase in magnitude in addition to
immunosuppressive and anti-inflammatory effects.
Glucocorticoid therapy has been used widely in dairy cattle for the
treatment of ketosis, however there is Httle current research documenting the
efficacy of these treatments, especially in the early stages of the condition.
Glucocorticoid concentrations are usually elevated in the serum of ketotic
cows. AlI of their actions except adipose lipolysis and increased ketogenesis
are favorable in terms ofketosis prevention (Morin, 2004).
30

Administration of large amounts of glucocorticoids inhibits almost
every step of the inflammatory response by acting in a negative-feedback
fashion to suppress the hypothalamus-pituitary axis that drives normal
glucocorticoid secretion and maintains the integrity of the adrenal cortex.
Glucocorticoids stimulate the cellular production of lipocortin, which inhibits
phospholipase A2, the enzyme responsible for cleaving arachidonic acid from
cell walls therefore inhibiting arachidonic acid release (Morin, 2004). The
corticosteroids inhibit both the lipoxygenase and cyclooxygenase pathways of
inflammation, blocking formation of leukotrienes as well as prostaglandins,
prostacyclin, thromboxan and in addition to blocking the inflammatory
Mediators, the corticosteroids suppress white blood cell functions and antibody
production, specifically destroying lymphocytes within lymphoid tissue which
are responsible for antibody production and destruction of foreign cells. This
is reflected in alterations in the numbers of white blood cells circulating in the
bloodstream and the white blood cell response to injured and infected tissues.
In acute inflammation, the corticosteroids maintain the integrity of the blood
vessels and reduce edema formation, and limit the movement of white blood
ceUs into injured tissues.
The use of glucocorticoids which are effective in the management of
anti-inflammatory disorders such as bovine mastitis is hampered by their
adverse effects on hormonal, metabolic and skeletal systems (Sigeal, 1985).
To overcome these drawbacks, research has been performed on the structural
modifications of glucocorticoids in an attempt to increase their potencies while
reducing their serious adverse systemic effects.
A hydroxyl group at carbon Il is essential for glucocorticoid and anti
inflammatory activity. The 4, 5 double bond and a 3 ketone on ring A is
essential for both mineralocorticoid and glucocorticoid activity. Synthetic
analogues of cortisone and hydrocortisone contain a double bond between
carbon 1 and 2 of the corticosteroid nucleus and have a greatly decreased
effect on electrolyte metabolism and increased glucocorticoid activity. In
addition, unsaturation of hydrocortisone at the number one carbon
(prednisolone) bas been shown to enhance its glucocorticoid properties four to
five times in lactating normal and ketotic dairy cattle. Methylation at carbon 6
or 16 and hydroxylation at carbon 16, have led to further decrease in
31

electrolyte imbalance noted with the naturally occurring glucocorticoids
(package insert). Fluorination at carbon 6 and/or 9 has led to an increase in
anti-inflammatory activity. It has also been shown that when hydrocortisone
bears fluorine in the 9-alpha position (9-alpha-fluorohydrocortisne), it
becomes more potent than the parent compound. Fluorination and methylation
prolongs the half life of glucocorticoids (package insert). Fusion of the
heterocyclic rings onto the steroid nuclei and fluorination at the 90. position,
have been very effective in improving the pharmacological activity of
glucocorticoids.
There are many corticosteroid preparations available for veterinary use.
• Methylprednisolone sodium succinate (Solu-Medrol'Iil)
• Prednisolone sodium succinate (Solu-Delta-Corte~)
• Dexamethasone sodium phosphate (Azium SP~
• Dexamethasone (Azium~
• Flumethasone (Flucort®)
• Methylprednisolone acetate (Depo-Medrol~
• Triamcinolone acetonide (Vetalog®)
• Isoflupredone acetate (Predef!' 2X)
• Betamethasone dipropionate (Betasone ~
Differences in chemical structure of these drugs determine the potency
of anti-inflammatory activity, duration of effect, and duration of suppression
ACTH release from the brain, shown in Table 1. The corticosteroids can be
classified by comparing them to hydrocortisone, which is identical to cortisol,
the natural corticoid hormone.
32

Table 1. Comparison of Drug Duration and Action Potency*
(Dowling, 2004)
Drug Duration Potency
Hydrocortisone 8-12 hrs 1
Prednisone 12-36 hrs 4
Methylprednisone 12-36 hrs 5
Triamcinolone 12-36 hrs 5
Isoflupredone 12-36 hrs 50
Dexamethasone 32-48 hrs 30
Betamethasone 32-48 hrs 30
Flumethasone >48 hrs 120
* Potency is determined by comparison to a cortisol value of 1.0.
Corticosteroid therapy is directed at modifYing the body's response to
inflammation and not at treating the underlying disease process (Dowling,
2004). A veterinarian will use the smallest dose that achieves the desired effect
in order to limit adverse side effects. Generally, anti-inflammatory doses are
10 times the physiological levels, doses to suppress the immune system are
twice the anti-inflammatory dose, and doses to treat shock are 5 to 10 times
the immunosuppressive dose (Dowling, 2004). Product formulations have
differences in onset and duration of action.
Flucort® and Prede~ 2X are two corticoid steroids used in this study
and their characteristics will he further described.
Solutions of free steroid alcohols such as Flumethasone (Flucort®;
Syntex Animal Health, Inc) are administered intravenously or intramuscularly
and their use is usually limited to acute, but not immediately life·threatening
conditions such as chronic obstructive pulmonary disease (heaves) attacks,
snake bites, vaccine reactions, and insect bite hypersensitivity (Dowling,
2004).
33

4.1. Flucort®
F
Flucort® solution is made by Fort Dodge. It is a chemical modification
of prednisolone, which possesses greater anti-inflammatory and gluconeogenic
properties than the parent compound when compared on an equivalent basis
(package insert).
Chemically it is 6a-9a-difluoro-l6a methylprednisolone. The active
ingredient of Flucort® solution is flumethasone which occurs as a white to
creamy white, odorless, crystalline powder. The appearance of Flucort®
solution is a clear colorless to slightly yellowish mobile liquid. Each mL of the
injectable preparation contains
• 0.5 mg flumethasone
• 420 mg polyethylene glycol 400
• 9 mg benzyl alcohol (as a preservative)
• 8 mg sodium chloride
• 0.1 mg citric acid
• Water for injection USP q.s.
• When necessary, pH is adjusted with hydrochloric acid and/or
sodium hydroxide
The eosinophil depression test in normal dogs and blood glucose
elevation and eosinophil depression in normal cattle have been used as
parameters of drug activity to compare prednisone and dexamethasone.
According to the company package insert, the assays indicate that
34

flumetbasone possesses greater anti-inflammatory and gluconeogenic activity
than these compounds, on an equivalent basis (Flucort® package insert).
Acetate and acetonide esters of flumethasone are given
intramuscularly, subcutaneously or intra-articular (into the joint) for a
prolonged effect. Absorption of drug into the systemic bloodstream oceurs
slowly, over days to weeks. Examples of long-acting formulations include
Isoflupredone acetate (PredefP 2X; Pharmacia and Upjohn) and
Betamethasone dipropionate (Betasone®; Schering-Plough Animal Health)
(Dowling, 2004).
4.2. PredefP 2X
I~H c=o
CHs, .'.OH
o
Since the successful treatment of bovine ketosis with cortisone and
hydrocortisone in 1950 (Hatziolos and Shaw, ] 950), a number of synthetic
glucocorticoids have been recommended for the use (Butler and Elliot, 1970),
and research continues to develop more potent steroids to do more specifie
jobs. The biological activity of (9a.-fluoroprednisolone acetate was first
reported in 1955 by Stafford et al. Their work in the rat demonstrated that this
compound was about 50 times as potent as hydrocortisone as a gluconeogenic
agent (measured by liver glycogen deposition assay), and almost 20 times as
potent as desoxycorticosterone acetate in causing sodium retention in the
adrenalectomized rat. Data such as these prompted experiments leading to the
possible use ofthis compound in the treatment of bovine ketosis. Goetsch et al
(1959) reported 9a-fluoroprednisolone to be about four times as potent as
prednisolone or 9a-fluorohydrocortisone in its ability to elevate blood glucose
of normal dairy cows.
Prede~ 2X is an isoflupredone acetate sterile aqueous suspension
made for intramuscular or intrasynovial injection by Pharmacia & Upjohn to
35

treat bovine ketosis and other conditions that require a potent gluconeogenic
and anti-inflammatory agent. It is noted for its gluconeogenic and glycogen
deposition activity and is an effective and valuable treatment for the endocrine
and metabolic imbalance of bovine ketosis. In secondary bovine ketosis,
where the condition is complicated by pneumonia, mastitis, endometritis, and
traumatic gastritis, isoflupredone acetate exerts an inhibitory influence on the
mechanisms and the tissue changes associated with inflammation. Vascular
penneability is decreased, exudation diminished, and migration of the
inflammatory ceUs markedly inhibited. In addition, systemic manifestations
such as fever and signs of toxemia may also be suppressed. White certain
aspects of this alteration of the inflammatory reaction may be beneficial, the
suppression of inflammation may mask the signs of infection and tend to
facilitate spread of microorganisms. Predet«' 2X therapy should he used in
conjunction with appropriate antibacterial therapy. Without concurrent use of
an antibiotic, the use of the adrenal honnones in animaIs with infections can
be hazardous. No sodium retention or potassium depletion has been observed
at the doses recommended in animaIs receiving 9-fluoroprednisolone acetate
(package insert).
Each mL of Predet«' 2X contains 2 mg of isoflupredone acetate. Non-
medicinal ingredients include (Package insert):
• 4.5 mg sodium citrate hydrous
• 120 mg polyethylene glycol 3350
• 1 mg polyvinylpyrrolidone (povidone)
• 0.201 mg Myristyl-gamma-picolinium chloride (as preservative)
• Water for injection USP q.s.
• When necessary, pH is adjusted with hydrochloric acid and/or
sodium hydroxide
According to the package insert, Predef> 2X results in a more potent
corticoid whose activity is greater than the additive effects of unsaturation and
fluorination. Predet«' 2X has greater glucocorticoid activity than an equal
quantity of prednisolone. Isoflupredone acetate has less than half the anti
inflammatory potency of dexamethasone (Langston, 1993). The glucocorticoid
36

activity of isoflupredone is approximately ] 0 times that of prednisolone, 50
times that of hydrocortisone, and 67 times that of cortisone as measured by
liver glycogen deposition in rats. Isoflupredone acetate is a less potent
glucocorticoid than dexamethasone, does not cause abortion, and presumably
has less risk of immunosuppression (Morin, 2004). Isoflupredone acetate has
less than half the anti-inflammatory potency of dexamethasone (Langston,
1993). However, isoflupredone acetate has more mineralocorticoid activity,
which can lead to hypokalemia and recumhency when repeated doses are used
in sick cows (Sielman et al.,1997; Sielman et al.,1997). Other sources state
that PredefP 2X is a safe substance at one 10mL dose (Radostits, et al. 2000).
And its gluconeogenic activity is based on its hyperglycemic effect in both
normal and ketotic cattle. A possible explanation for the increased potency of
PredefB> 2X over prednisolone is the difference in the rates of reduction of !:14_3
and C20 ketone groups of prednisolone and PredefP 2X by a liver enzyme
system. It has been shown that the ketone groups of prednisolone were
reduced to biologically inactive products more than twice as rapidly as those
of Predef> 2X. Consequently, Prede~ 2X remains present in a biologically
active form more than twice as long as prednisolone. This factor would
increase the length of time the active steroid remained in the circulatory
system and thus contributed to a longer elevated blood glucose level. Prede~
2X is long-lasting (48 h gluconeogenic activity). Isoflupredone is 10 times
more glucogenic than prednisolone (package insert), thus 10 mg of
isoflupredone acetate therapeutically equals 100 mg of prednisolone (package
insert). The usual intramuscular dose for cattle is 10 to 20 mg according to the
size of the animal and severity of the condition (package insert). This dose
may be repeated in 12 to 24 h if indicated (package insert). It is reported that
blood glucose levels return to normal or above within 8 to 24 h after injection,
following a reduction in blood and urine ketone levels by increasing its
gluconeogenic and glycogen disposition activity. It does not cause pregnancy
termination or induce calving. There is a decrease in the circulating amount
eosinophils. Usually the general attitude of the cow is much improved,
appetite retums, and milk production rises to previous levels within 3 to 5 d. In
secondary bovine ketosis, where the condition is complicated by pneumonia,
mastitis, endometritis, traumatic gastritis, etc, PredefB> 2X should he used
37

concurrently with proper local and parenteral antibacterial therapy, infusion
solutions, and other accepted treatments for the primary conditions (Package
insert). Milk from treated animais must not be used for food within 72 h after
the last treatment with the drug, in addition animaIs intended for human
consumption should not be slaughtered within 7 d of last treatment (package
insert).
In ruminants, the comparison and effects of Flucort® and Predefll 2X
on blood metabolites, immune function and milk composition has not been
investigated. The main objective of this study was therefore to determine
whether an intramuscular injection 10mg/mL of Flucort® or Prede~ 2X on the
day of calving improved the energy status of cows without depressing immune
function.
38

III. Hypothesis & Objectives
Hypothesis
1. We hypothesize that cows will respond to glucocorticoid treatments on
the day of calving by increasing gluconeogenic activities and reducing
production of ketone bodies, without compromising the immune
function.
Objectives
The objective of this research project was to evaluate specifie intervention
strategies for negative energy balance and observe its effects on immune
function. Specifically:
1. Determine the effects of glucocorticoid (Predet«' 2X and Flucort~
treatment on energy balance
2. Determine the effects of glucocorticoid (Predet«' 2X and Flucort®)
treatment on immune function.
3. Determine the effects of glucocorticoid (Predet«' 2X and Flucort®)
treatment on milk composition.
39

IV. Materials and Methods
1. Experimental design
Thirty Holstein cows (pre-calving first lactation heifers and
multiparous cows) were randomly selected and enrolled in the trial 10 d prior
to the expected calving date. Two different glucocorticoids were compared
with a placebo control group in a randomized double-blind design. Using
random number tables, animaIs were assigned to one of three treatment groups
to receive a 10 mg/mL intramuscular injection in the right hind leg of
Flucort®, Prede~ 2X or 10.5 mL of placebo on the day of calving. The
placebo solution contained 10 mL sterile water and 0.5 mL penicillin.
Approximately 33% cows were in flfSt lactation, 20% in second lactation, 17%
in third lactation, 17% in fourth lactation, and the remainder were fifth parity
or greater cows as shown in Table 2.
Table 2. Frequency of parities (1 to >5) within treatment
Parity Control Flueort'Ïl Prede~2X Total Total %
1 3 3 4 10 33.3
2 2 2 2 6 20.0
3 2 2 1 5 16.7
4 2 1 2 5 16.7
>5 1 2 1 4 13.3
For simplicity, parities 3 and greater were combined. Therefore
approximately 47% of the animaIs were in parity group ~3.
Twenty percent of the cows calved during the summer, over 70%
calved in the falI and approximately 7% calved in the winter as shown in
Table 3.
Table 3. Frequency of calving seasons within treatment
Prede~
Season of Calving Control Flutort@ 2X Total Total %
Summer 2 3 1 6 20.0
FaU 7 7 8 22 73.3
Winter 1 0 1 2 6.7
40

2. Feeding
AU cows were based in a tie-staU and fed total mixed rations. Daily
feed intakes were recorded from two weeks pre-calving until one month post
calving. The herd utilized two dry cow feeding groups (far-off and close-up).
Cows were fed twice per day with the first feeding occurring after the morning
milking and the second feeding before the afternoon milking. Feed intake was
recorded daily from day lOto day 28.
2.1. Close-Up Dry Ration
Specifie diet formulations were changed frequently, however the
predominant forages used were dry hay, haylage and corn silage; the main
components of the concentrate mix were high moisture corn, cracked corn,
raw soybean, soybean meal and commercial supplements (Anion Tech).
Table 4. Diet Composition (% of DM) of Close-Up Ration
Chemical Composition %
Crude Protein (CP) 15.5
Acid-Detergent Fiber (ADF) 25.8
Neutral-Detergent Fiber (NDF) 41.2
Non-Fiber Carbohydrate (NFC) 32
NEL, McaVkg of DM 1.51
2.2. Fresh Cow Ration
Specifie diet formulations were changed frequently, however the
predominant forages used were dry hay, haylage, corn silage and alfalfa hay;
the main components of the concentrate mix were high moisture corn, cracked
corn, soybean meal and raw soybean, and commercial supplements (Ener-g-II,
Melass Sec, RTM Amino).
Table 5. Diet Composition (% of DM) of Fresh Cow Ration
Chemical Composition %
Crude Protein (CP) 16.2-18
Acid-Detergent Fiber (ADF) 18-21.7
Neutral-Detergent Fiber (NDF) 28-32.5
Non-Fiber Carbohydrate (NFC) 41-41.2
NEL, Meal/kg of DM 1.6-1.71
41

3. Blood Serum Collection
Blood was collected from the coccygeal vein into 10 mL vacuum tubes
without anticoagulant (Monoject® red stopper blood collection tubes;
Sherwood Medical, St. Louis, MO) d-l0, d-5, dO, d 1, d 7, d 14, d 21, and d
28 relative to calving. Pre-calving sampling dates were assigned
retrospectively and labeled according to closest d-l0 or d-5 in the original
sampling schedule. Blood samples were taken at approximately the same time
of day. Blood samples were stored in an insulated cooler before samples were
returned to the laboratory where blood samples were allowed to clot and went
through clot retraction by centrifugation at 3,000 x g for 10 min. The serum
was separated from the clotted sample by aspiration and stored in a -20°C
freezer until further serum analysis.
4. Milk Sampling
Composite aseptic milk samples were taken weekly from dito d 21
post-calving and submitted directly to PATLQ (Programme d'Analyze
Traitement Laitiers du Quebec; Ste-Anne-de-Bellevue, Quebec) for analysis of
SCC, fat (%), protein (%) and lactose (%). In addition, daily milk yields were
reeorded from dIto d 28 post-calving. Fat, prote in and lactose yield were
calculated based on milk yield and the respective percentages.
5. Milk Sodium and Potassium Analysis
An extra composite aseptic milk sample was taken on d 1 post-calving
and an aliquot of 5 mL was placed in a 100 mL volumetrie flask, 50 mL of
24% (w/v) TCA was added to the sample and then diluted with deionized
water to a volume of 100 mL. Milk proteins including casein were precipitated
using trichloroacetic acid (TCA). Samples were shaken at 5-min intervals for
30 min and then filtered. A 5 mL aliquot of the filtrate was transferred to a 50
mL volumetrie flask, 1 mL of 5% (w/v) lanthanum solution was added and
then diluted with deionized water to a volume of 50 mL. A mixed standard
was prepared containing 5.0 mg/L Ca, 0.6 mg/L Mg, 1.6 mg/L Na, 5.0 mg/L
K, 500 mgIL La and 1.2% (w/v) TCA. The filtra te was then analyzed for
sodium and potassium by atomic absorbance, all measurements were made
relative to a blank reagent containing 500 mgIL La and 1.2% TCA .
42

6. Body Condition Scoring
Cows were scored for body condition on a scale of 1 to 5 using
increments of 0.25 according to Edmonson et al. (1989) on d -10, d 0, d 14 and
d 28 relative to calving.
7. Serum Biocbemical Analysis
Frozen serum was submitted to the Animal Health Laboratory,
University of Guelph for the measurement of serum glucose, BHB, NEF A,
calcium, phosphorus, sodium, potassium, chloride and magne sium using a
serum autoanalyzer (Hitachi, model911, Roche, Laval, Quebec).
8. Antibody Production (ELISA)
To evaluate post-ealving immune response to Predef!> 2X and Flueort®,
each eow was immunized on d 0 with an intramuscular injection in the left
hind leg of 3mg/mL saline solution of Grade V chicken ovalbumin (minimum
98%; Sigma A 5503) ftom chicken egg albumin, an inert antigen to whieh
these animaIs had not been previously exposed. One ml of the chicken
ovalbumin solution was mixed immediately before injection with 1 mL of
Freund's incomplete adjuvant (PICA), therefore 2 mL of the mixed solution
was injected. Cows received a boost of the same chicken ovalbumin solution
on d 14.
Specifie antibody response to chicken ovalbumin was determined at d
1, d 7, d 14, d 21, and d 28 relative to calving. Frozen serum samples were
thawed at room temperature. Chicken ovalbumin IgG titer was determined by
ELISA. Flat-bottom 96-well plates (Immulon 2, Dynatech, Chantilly, VA)
were coated with 100 uL of chicken ovalbumin (l ug/mL) in carbonate buffer
(pH 9.6), at 4°C, ovemight. After eoating, the wells were blocked with 1%
gelatin (Sigma) in PBS containing 0.05% Tween 20 (Sigma), 200 uL per weU,
at 37°C for 1 h. After 1 wash with PBS-Tween 20 (0.05%), serum samples
were diluted 40 fold with PBS-Tween 20 (0.05%) eontaining 0.1% gelatin and
added to each weil (100 uL) in triplicate. Fetal bovine serum (FBS) was
diluted 20 fold with PBS-Tween 20 (0.05%) eontaining 0.1% gelatin and
added to 3 wells per plate as negative eontrols. The plates were then incubated
at room temperature to allow attachment of sera chieken ovalbumin
antibodies, for 1 h. Wells were washed 3 times with PBS-Tween 20 (0.05%).
Then, 100 uL/well of IgG (l :6000, HRP-goat and bovine IgG (Kirkgaard &
43

Perry Laboratories, Gaithersburg, MD» diluted in PBS Tween 20 (0.05%)
containing 0.1% gelatin was added to each weil (l00 uL) and incubated at
37°C, for 1 h. After three washings with PBS-Tween 20 (0.05%), peroxidase
activity was assayed by adding 100 uL of ABTS substrate solution (Kirkgaard
& Perry Laboratories, Gaithersburg, MD) and allowed for color development
in the dark for 30 min. Finally, the absorbance values of IgG titers were
evaluated and determined by a microplate reader at 400 nm in a Multiskan®
MCC 340 plate reader (Titertek, Alabama, USA).
9. ConA-Induced Lymphocyte Proliferation
In order to determine the proliferation of lymphocytes, blood was
collected from the coccygeal vein into 3 x 10 mL vacuum tubes (Monoject®
green heparin vacutainer stopper blood collection tubes; Sherwood Medical,
St. Louis, MO) d 0 and d 7 relative to calving. Blood samples were stored in
an insulated cooler before samples were retumed to the laboratory where fresh
blood samples were analyzed for lymphocyte proliferation.
Bovine lymphocytes were isolated from whole blood taken from
Holstein cows. Heparin-anticoagulated blood was mixed with an equal volume
of Ca2+-free Hank's balanced salt solution (HBSS, GIBCOIBRL,
Gaithersburg, MD) and added into 20 mL Ficoll-Paque solution (l.077 g/mL,
Amersham Biosciences, Piscataway, NJ) in 50 mL tubes. After centrifugation
at 3,000 x g for 40 min at 20°C, the buffy coat layer containing the
lymphocytes was removed by pipetting and placed into another 50 mL tube
and then filled with Ca2+-free Hank's balanced salt solution (HBSS,
GIBCOIBRL, Gaithersburg, MD). After centrifugation at 1,500 x g for 10 min
at 20°C, aIl remaining liquid was aspirated and discarded leaving a pellet
containing the lymphocytes. The pellet was washed twice with Ca2+ -free
Hank's balanced salt solution (HBSS, GIBCOIBRL, Gaithersburg, MD) and
centrifuged at 1,500 x g for 10 min at 20°C. The isolated lymphocytes were
resuspended (1 x 107 cells/mL) in the proliferated medium containing RPMI
(Sigma), 10% inactivated fetal bovine serum, 1 % L-glutamine, >5 mMol
HEPES, 0.05 mMol 2-mercapto-ethanol (Fischer, Pittsburgh, PA), 1%
antibiotics/antimycotic. In a 96 weIl-round bottom tissue culture plate, 100 uL
of the cell suspension were cultured in 100 uL of medium to measure the
background, unstimulated proliferation. In the same plate, 100 uL of cell
44

suspension was stimulated with a 100 uL of a 2 /lg/mL concanavalin A
solution containing ConA (Sigma) and a phosphate buffer solution. The plate
was placed in a humidified incubator with 5% CO2 for 54 h at 37°C. During
the last 18 h of culture, ceIls were pulsed with (20 /lCi/mL) eH]-thymidine
solution ([3H]-thymidine, RPMI, antibioticl antimycotic, L-glutamine and
added 20 uL in each weIl. After incubation, the ceUs were harvested with a
semi-automatic celI harvester (Skatron, Sterling, VA) and placed into 4 mL
scintillation cocktail (ICN, Montreal, Canada). The [3H]-thymidine
incorporation into ceUs (radioactivity) was measured with a liquid scintillation
counter (Beckman LS 60001C; Beckman Instruments, Inc., Columbia, MD).
Each sample was run in ten replicate wells and the values were averaged and
expressed as mean counts per minute (cpm). A stimulation index (SI) was
calculated as the mean cpm of tritium incorporated in ceUs treated with ConA
divided by the mean cpm of tritium incorporated in ceUs not treated with
ConA. Stimulation index is the ratio of mitogen-specific proliferation and
background proliferation with error of the mean (SEM). Con A is a
glycoprotein extracted from the jack bean that promotes mitosis and stimulates
T lymphocytes. The usefulness of ConA is the specifie binding action with
certain carbohydrate-containing receptors, a commonly occurring sugar, (1-
linked mannose. Since a wide variety of serum and membrane glycoproteins
have a "core oligosaccharide" structure which includes a-linked mannose
residues, many glycoproteins can be examined or purified with ConA and its
conjugates. Concanavalin A agglutinates red blood ceUs and complexes with
blood group substance (Clark and Denborough, 1971) and immunoglobulin
glycopeptides (Komfeld and Ferris, 1975). Con A is one of the most widely
used and weIl characterized lectins that is also a lymphocyte mitogen (Ruscetti
and Chervenick, 1975).
10. Serum Insulin Analysis
Frozen serum samples were analyzed for insulin by a radio-immuno
assay (RIA) procedure using a commercial kit (KTSP-l1002, Medicorp,
Montreal, Canada).
11. Statistical Analyses
This study followed a randomized double-blind placebo-controIled
design. AIl statistical analyses on serum metabolites, immune function
45

parameters, and milk composition parameters were conducted with SAS
version 8.0 for Windows using PROC MIXED. The mixed-model
methodology was used according to a repeated-measures covariance structure,
Autoregressive(l) or AR(l), as weIl as the restricted maximum likelihood
method (REML) available in the MIXED software of SAS (1995).
The mixed model applied to ail data sets included a random cow effect
and fixed effects of treatment group, parity, calving season, sampling date,
treatment-by-sampling date interaction, treatment-by-parity interaction,
treatment-by-calving season interaction, parity-by-calving season interaction,
parity-by-sampling date interaction, and calving season-by-sampling date
interaction.
Differences among treatments were compared by Least Squares Means
(SAS, 1999) and the Scheffé multiple comparison test. Statistical significance
was considered at P < 0.05. The statistical models were described as
following.
Yykl(ykm) = p. + T,.+ Pj+ CSk + SDl+ COWykm+ T;*SD1+ Pj*SD1+ CSk*SD, +
eijkl(ijkm)
Where, Yijk1(ijlan) = dependent observation
p. = overall mean
T;= fixed effect of the th treatment {i;;;:: l(control), 2 (Flucort®) or 3 (prede~
2X)}
Pr fixed effect ofthelh parity {j = 1,2,3 (or >3)}
CSk = fixed effect of the J(h calving season {k = 1 (June 22 to September 21-
summer),2 (September 22 to December 21-fall), 3 (December 22 to
March 20-winter)}
SDI ;: fixed effect of the th sampling date {l = 1 (D-10), 6 (D-5), Il (DO), 12
(Dl), 18 (D7), 25 (D14), 32 (D21), 39 (D28)}
COWij/rm = random effect of the mth animal nested in the lh treatment and in the
/h parity and in the IIh calving season <ijkm == 1, ... , 30)
T;*SDF fixed effect of interaction the th treatment and the t' sampling date
Pj*SDF fixed effect of interaction thelh parity and the lth sampling date
eSk *SDF fixed effect of interaction the ft' calving season and the th sampling
date
eijk/(ijkm) = random residual error associated with the observation from the ijkmth
46

cow on the th treatment ofthelh parity in the Iéh calving season and
ofthe th sampling date eijkl(ijkm) - N (0, R); if = random residual
variance
AlI other interactions were tested for significance (P<O.05), and were
eliminated from the model since they were not significant.
47

v. Results and discussion
This study comprised of a trial on 30 Holstein primiparous and
multiparous dairy cows, in which two veterinary drugs, Flucort® and Predefl'
2X were evaluated on blood metabolites, immune function and milk
composition in the weeks immediately pre-calving and post-calving. This is
the first work that compared Flucort® and Prede~ 2X and their effects on
immune function. The data collected from cows are particularly interesting
because there is limited literature published on the influence of Flucort® and
Prede~ 2X for this important time period after ealving.
F values of aIl effects tested in the model are represented in Table 6.
Table 6. F value of effecfs from mixed models for ail ~arameters in cows that were either treated day of calving with a Flucort ,Predef' 2X or
• 1 served as nee:atIve con trois Fvalue
Parameter T P CS SD T*P T*CS T*SD p*cs P*SD CS*SD
Glucose 0.5 0.75 0.86 <0.05 0.92 0.69 0.92 0.88 0.71 0.32
NEFA 0.35 <0.05 0.53 <0.05 0.75 0.19 0.29 0.26 0.09 0.21
BHB 0.11 0.24 0.19 <0.05 0.32 0.57 0.56 0.26 0.87 0.35
Calcium 0.65 0.07 0.92 <0.05 0.76 0.99 <0.05 0.72 <0.05 <0.05
Phosphorus 0.91 0.02 0.82 <0.05 0.71 0.51 0.26 0.81 <0.05 <0.05
Sodium 0.99 0.45 0.54 <0.05 0.27 0.39 0.51 0.85 0.34 0.99
Potassium 0.23 0.46 <0.05 0.27 0.53 0.43 0.29 0.39 0.23 0.05
Chloride 0.75 0.9 0.27 <0.05 0.28 0.28 0.75 0.31 0.11 <0.05
Magnesium 0.9 0.31 0.24 <0.05 0.99 0.93 0.049 0.87 0.71 0.11
Insulin 0.99 0.22 0.83 <0.05 0.38 0.91 0.01 0.42 <0.05 0.23
Antibody 0.83 0.14 0.59 <0.05 0.72 0.64 0.54 0.12 0.49 0.94
Lymphocyte 0.7 0.39 0.l3 0 0.42 0.67 0.53 0.96 0.5 0.03
Fat (%) 0.98 0.18 0.85 0.63 0.64 0.5 0.85 0.73 0.37 0.43
Fat (kg) 0.61 0.01 0.48 <0.05 0.32 0.41 0.73 0.24 0.l8 0.69
Protein (%) 0.4 0.l9 0.09 <0.05 0.23 0.25 <0.05 0.7 0.09 0.02
Protein (kg) 0.84 0.15 0.24 0.03 0.36 0.29 0.43 0.19 0.31 0.05
Lactose (%) 0.77 <0.05 0.08 <0.05 0.41 0.19 0.32 0.37 0.69 0.19
Lactose (kg) 0.72 0.51 0.64 <0.05 0.62 0.9 0.3 0.75 0.25 0.54
MilkNa 0.002 0.06 0.59 0.59 0.28 0.47
MilkK 0.85 0.69 0.06 0.5 0.71 0.52
SCC 0.52 0.28 0.59 <0.05 0.7 0.84 0.11 0.92 0.03 0.58
AvgMilk 0.34 <0.05 <0.05 <0.05 0.71 0.58 0.72 0.46 0.04 0.56 Yield
1 . Models usmg proc mlxed (SAS) mcluded panty, calvmg season, sample date
and the random effeet ofeow.
48

1. Flucort®
1.1. Energy Status
Blood energy metabolites included glucose, insulin, NEF A and BHB.
Ali the blood energy metaboHtes were evaluated on d-IO, d-5, dO, d 1, d 7, d
14, d 21 and d 28 relative to calving. Among the ketone bodies (BHB,
acetoacetate and acetone), only BHB was evaluated because most other
cowside tests lack sensitivity as compared to serum BHB. Testing for BHB
remains the gold standard for studying ketosis (Duffield, 1997).
1.1.1. Glucose
Statistical analysis of serum glucose levels revealed no effect of
treatment (P>0.05), however there was an effect of sampling date (P<0.05).
Furthermore, there was no significant differences (p>0.05) in serum glucose
levels among treatments. Results for overall least square means and least
square means for individual days are shown in Table 7 and 8, respectively.
49

Table 7. Overall least squares metabolites, immune function parameters in cows tbat were
means from mixed models for blood parameters and milk compositional eitber treated day of calving witb a
FI ®p df'Y2X 1 b ucort , re e or pl ace 0 contro Il
Parameter Trt OverallLSM S.E.M. Glucose (mmollL) C 3.2019 0.1690
F 2.9591 0.1690 P 3.0869 0.1733
NEFA (mmollL) C 0.4755 0.04830 F 0.4311 0.04830 P 0.5443 0.04958
BHB (IlmollL) C 700.90 234.26 F 1139.00 234.26 P 1513.69 240.22
Calcium (mmollL) C 2.5156 0.04900 F 2.5108 0.04900 P 2.4441 0.05024
Phosphorus C 2.0917 0.08423 (mmollL) F 2.0713 0.08423
P 2.0329 0.08639 Sodium (mmollL) C 143.16 1.1717
F 143.18 1.1681 P 143.38 1.1987
Potassium (mmollL) C 4.5964 0.1108 F 4.7745 0.1108 P 4.4768 0.1139
Chloride (mmollL) C 100.99 1.2121 F 99.8916 1.2091 P 100.19 1.2407
Magnesium C 1.0076 0.03493 (mmollL) F 0.9995 0.03493
P 1.0219 0.03583 Insulin (uIU/mL) C 15.0863 1.3706
F 15.0698 1.3821 P 15.2290 1.4153
Antibody (OD) C 0.8657 0.1020 F 0.8142 0.1020 P 0.7722 0.1046
Lymphocyte (SI) C 11.6433 5.8275 F 11.6078 5.9168 P 12.6123 5.6497
MilkFat(%) C 4.5035 0.4983 F 4.6083 0.4983 P 4.5023 0.5106
Milk Fat (kg) C 1.5617 0.1831 F 1.6013 0.1831 P 1.3137 0.1790
Milk Protein (%) C 3.3905 0.2482 F 3.3889 0.2482 P 3.8890 0.2543
Milk Protein (kg) C 1.1748 0.1620 F 1.1490 0.1620
50

P 1.0248 0.1584 Milk Lactose (%) C 4.2874 0.08765
F 4.3562 0.08765 p 4.2814 0.08981
Milk Lactose (kg) C 1.4566 0.08851 F l.4017 0.08851 P l.3460 0.08654
Milk sec (,000) c 108.00 50.9855 F 132.25 50.1947 p 203.90 5l.5055
Milk Sodium (mglL) C 34.3840 1.9029 F 24.4662 1.8073 p 21.6297 1.9771
Milk Potassium C 8l.5700 3.8217 (mgIL) F 79.9409 3.6296
p 78.5493 3.9708 Milk Yield (kg) C 36.8114 0.7521
F 35.4448 0.7399 p 35.5731 0.7199
1 . Models usmg proe mlxed (SAS) meluded panty, ealvmg season, sample date
and the random effeet of eow.
51

Table 8. Least squares means from mixed models for serum energy parameters in cows on D-10, D-5, DO, Dl, D7, D14, D21 and D28 relative to calving that were either treated on day of calving with a Flucort@, P d ~2X 1 b 1 re e or place 0
Serum Energy Parameter (LSM ± S.E.M.)
Day Trt Glucose NEFA BHB Insulin (mmollL) (mmollL) (mmollL) (uIU/mL)
D-10 C 3.325 0.1413a 320.15 22.23488
F 3.40723 0.0841 3 467.33 22.6638
P 3.46518 0.21593 1076.668 25.82528
D-S C 3.6357a 0.2657a 373.94 20.41998
F 3.42798 0.1875° 415.898 21.40448
P 3.41428 0.3066° 935.96° 22.86188
DO C 3.65728 0.6943b,c 669.91 20.3482A.8
F 3.38948 0.8121b,d,e 523.268 15.1735c
P 3.5239c 0.9044b,d,e 1074.328 13.0722B,b
Dl C 2.899611 0.9305A.II,a,e 758.21 10.6079A.II
F 3.0818° 0.6873B,b,d,g 566.768 18.3892B,e
p 3.1712 0.7221 b,d,g 1450.81 14.5857b
D7 C 2.9916b 0.6264b,t;g 858.65 9.4076b
F 2.4138b,d 0.5782b,d,t:i 1444.1b,c 9.4989b,d,f
P 2.624b,d 0.6411 b,d,f 1846.49d 10.5376b
D14 C 3.1528 0.541 b,t;g 759.09 11.799b
F 2.745b 0.4208b,d,f,h 1237.15c 10.4086b,f
P 2.9251d 0.5297b,f 1623.19 12.2096b
D21 C 2.896911 0.3235a,t,h 785.59A 11.8335b
F 2.5791b 0.3483b,f,h,j 1865.24B,b,c 11.8039b,f
P 2.6776b,d 0.554Sb,d,f 2098.9SB,b,d 10.0026b
D28 C 3.056811 0.281rt,h 1081.65A 14.039411
F 2.629b 0.3305b.f,h,j 2592.3B,b.d 11.2168b,f
P 2.893r 0.4799b,f,h 2003.13d 12.7372b
S.E. C 0.2541 0.08818 364.11 2.2141
F 0.2541 0.08818 364.11 2.2704
P 0.2586 0.08955 370.29 2.2938 1 .
Models UStng proe mlxed (SAS) tncluded panty, ealvtng season, sample date and the random effeet of cow. A and B, C and D differ significantly among treatments within the same day a and h, c and d, e and f, g and h, i and j differ significantly among days within the same treatment
52

The only day that Flucort® treated cows had better energy status than
control cows as indicated by the numerically higher serum glucose (P>0.05)
was on d 1. AIl cows experienced decreases in glucose levels after d 0,
however, serum glucose values for Flucort® treated cows experienced an even
further significant decrease by d 7 (P<0.05). Furthennore, glucose
concentrations on d 14, d 21 and d 28 were significantly lower than d -10, d-5
and d 0 values (P<0.05), and this is comparable to the trend observed in
control cows.
When a glucocorticoid is administered, the concentration of glucose in
the blood should increase through the synthesis of glucose from amino acids
(gluconeogenesis), a decrease in the synthesis of prote in from amino acids,
and altered lipid metabolism, thereby satisfying the systemic demand for
glucose and helping to prevent the metabolism of fats and production of
ketones. Also, peripheral utilization of glucose is reduced and liver storage of
glycogen is increased.
Butler and Elliot (1970) observed an increase (P<O.OI) in blood
glucose concentrations from the administration of Flucort®. An inhibition of
glucose utilization as a result of Flucort® administration may account for sorne
of the increase in blood glucose levels. There is evidence that when a
glucocorticoid honnone 1s administered to sheep (Butler and Elliot, 1970),
blood glucose is increased as a result of impaired glucose utilization rather
than by an increase in the rate of gluconeogenesis. It has also been reported
(Butler and Elliot, 1970) that cortisol administration to sheep markedly
inhibited peripheral glucose utilization. and had an antagonistic effect on
insulin action. Butler and Elliot (1970) further suggested that the increase in
blood glucose concentration in the lactating cow may be due to the depression
in milk production (P<0.05) and consequent reduction in lactose secretion.
However in the present study, milk yield and lactose yield were not
significantly reduced with the administration of Flucort® which may explain
the lack of increase in glucose levels. A larger dose than 10 mg/mL of
Flucort® is probably needed to induce more significant effects on milk yield,
lactose yield and glucose concentrations. However, despite the dosage, effects
of glucose would only be temporary and would have to be measured on an
53

hourly basis. Butler and Elliot (1970) demonstrated blood glucose
concentrations reached maximum levels at about 18 h after treatment of 0.7
mg of Flucort® per 100 kg body weigh and this dosage is routinely used for
treating ketotic dairy cows.
1.1.2. Insulin
Statistical analysis of insulin levels revealed no effect of treatment
(P>0.05), however there was an effect of sampling date, interaction of
treatrnent and sampling date, and an interaction of parity and sample dating
(P<0.05). Results for overall least square means and least square means for
individual days are shown in Table 7 and 8, respectively.
The only significant difference observed in serum insulin levels was on
d 1 where Flucort® treated cows experienced significantly higher (P<0.05)
levels than control cows, indicating better energy status. The only other day
that Flucort® treated cows had better energy Status than control cows as
indicated by the slightly numerically higher serum insulin (P>0.05) was on d
7. In contrast, Flucort® treated cows had numerically (P>0.05) lower insulin
values from d 14 to d 28 than control cows.
Flucort® treated cows experienced a significant decrease in serum
insulin concentration by d O. From dIto d 7, insulin levels decreased
significantly (P<0.05) to almost 50%. Insulin levels increased by the end of
the trial (P>0.05), however, post--calving values remained significantly lower
than pre-calving values (P<0.05).
The higher insulin values experienced on d 1 should have stimulated
the uptake, utilization and storage of glucose. In addition, insulin should have
facilitated the entry of glucose into muscle, adipose tissue and other tissues. If
the chain of events listed had occurred upon Flucort® administration, the
mobilization of fat would have been avoided.
1.1.3. Non-esterified fatty acids
Statistical analysis of serum NEF A levels revealed no effect of
treatment (P>O.05), however there was an effect of parity and sampling date
(P<O.05). Results for overall least square means and least square means for
individual days are shown in Table 7 and 8, respectively.
The only significant difference observed in serum NEF A levels was on
d 1 where, Flucort® treated cows experienced significantly lower (P<O.05)
54

levels than control cows, indicating a better energy status. Other days that
Flucort® treated cows experienced numerically lower NEF A values and a
better energy status than control cows were on d 0, d 7 and d 14 (P>0.05).
Furthermore, from d 0 to d l, NEFA values for Flucort® treated cows
decreased numerically, whereas NEF A values for control cows increased
significantly. NEFA values for Flucort® treated cows continued to decrease
until d 28.
The better energy status experienced by Flucort® treated cows indicate
that there is less fat mobilization in these cows (Grummer, ] 993) and therefore
less NEF A transported to the liver. Plasma NEF A concentration and
triglyceride concentration in the liver (Bertics et al., 1992) are positively
correlated. As fatty liver and ketogenesis are closely linked (Dann et al.,
1999), a reduction in plasma NEF A concentration should result in a decreased
risk of the cow developing fatty liver and ketosis which supports the
hypothesis that Flucort® increased gluconeogenic activities by inhibiting fat
mobilization.
An analysis of the frequency of cows with high NEFA (>0.7 mmol/L)
values indicative of a state of negative energy balance revealed that less cows
had higher NEF A levels than control cows after a Flucort® treatment in ail
cows (both primiparous and multiparous cows) as shown in Tables 9,10 and
Il. In this respect, treatment of Flucort® may provide potential benefits in the
reduction of fat mobilization. However, due to limited numbers of cows used
in this study, a concrete conclusion cannot he made and further experiments
are warranted.
Table 9. Frequency of cows with high NEFA levels at D-10, D-5, DO, Dl, D7 D14 D21 d D28 b t t t , , an 'Y rea men· 2roup
Ali cows - % Hi2h NEFA (NEFA Value >0.7 mmollL) Trt D-I0 D-5 DO Dl D7 D14 D21 D28
C 0 10 70 90 50 50 20 10 F 0 10 60 50 40 20 10 0 p 0 10 44.44 50 10 10 20 20 Total 0 10 58.62 63.33 33.33 26.67 16.67 10
55

Table 10 Frequency of multiparous cows with high NEFA levels at D-10, D 5 DO Dl D7 D14 D21 d D28 b t t t - , , , ' , , an >y rea men group
MULTIPAROUS - % High NEFA (NEFA Value >0.7 mmol/L) Trt D-I0 D-5 DO Dl D7 D14 D21 D28
C 0 14.29 85.71 100 57.14 57.14 28.57 14.29 F 0 14.29 71.43 71.43 57.14 28.57 14.29 0 p 0 14.29 42.86 71.43 14.29 14.29 28.57 28.57 Total 0 14.29 66.67 80.95 42.86 33.33 23.81 14.29
Table 11. Frequency of primiparous cows with high NEFA levels at D-10, D 5 DO Dl D7 D14 D21 d D28 b t t t - , , , , , an )y rea men group
PRIMIPAROUS - % High NEFA (NEFA Value >0.7 mmol/L) Trt D-I0 D-5 DO Dl D7 D14 D21 D28
C 0 0 33.33 66.67 33.33 33.33 0 0 F 0 0 33.33 0 0 0 0 0 p 0 0 33.33 0 0 0 0 0 Total 0 0 33.33 20.00 10.00 10.00 0.00 0
Analysis of the frequency of body condition score (BCS) within
treatment and within day, demonstrate that approximately 73% of the animaIs
were classified as being in fair body condition prior to calving, and 63% in
thin body condition post-calving as shown in Table 12. This indicates that fat
was mobilized from adipose tissue during the transition from late gestation to
early lactation.
T bl 12 F a e . requency 0 fDes . h' wIt ID treatment an d . h' d wIt ID ay
D-I0 DO D14 Trt Thin Fair Fat Thin Fair Fat Thin Fair Fat Thin
C 0 9 1 2 7 1 6 3 1 8
F 2 6 2 3 7 0 5 5 0 6 p 1 7 2 1 8 1 3 7 0 5 Total (%) 10 73.3 16.7 20 73.3 6.7 46.7 50 3.3 63.3 .
Thm=BCS <3.25, Falr=BCS 3.25 to 3.75, Fat=BCS >3.75
1.1.4. Il-hydroxybutyrate
D28 Fair Fat
2 0
4 0
5 0
36.7 0
Statistical analysis of serum BHB Ievels revealed no effect of treatment
(P>O.05), however there was an effect of sampling date (P<O.05). Results for
overall least square means and least square means for individual days are
shown in Table 7 and 8, respectively.
AlI cows experienced a graduai increase in BHB levels throughout the
trial. The days that Flucort® treated cows had a better energy status than
control cows were d 0 and d 1 (p>O.05). However, from dIto d 7, Flucort®
56

treated cows experienced a significant increase of serum BHB. Flucort®
treated cows had a worse energy status on d 7 and d 14 as indicated by the
numerically higher BHB values than control cows (P>0.05). The only
significant differences observed in serum BHB levels was on d 21 and d 28
where Flucort® treated cows experienced significantly higher (P<0.05) BHB
levels than control cows, indicating a worsening energy status.
The high levels of BHB indicate higher ketone bodies concentrations
post-calving in cows receiving Flucort® and an increased risk of ketosis. An
analysis of the frequency of cows with high BHB values (BHB > 1400
mmollL) (Duffield, 2000) indicative of subclinical ketosis revealed that more
cows had higher BHB levels (> 1400 JUllollL) than control cows after Flucort®
treatment in an cows (both primiparous and multiparous cows) as shown in
Tables 13, 14 and 15. In this respect, treatment of cows with Flucort® may
contribute to problems ofketosis. However, due to the limited number of cows
used in the CUITent study, a concrete conclusion cannot be made and further
experiments are warranted.
Table 13. Distribution of subclinical ketosis at D-10, D-5, DO, Dl, D7, D14, D21 d D28 b t t t an )y rea men group
Ail cows - % Ketotic (BHB Value >1400 pmollL) Trt 0-10 D-5 DO Dl D7 D14 D21 D28 C 0 0 20 10 20 10 10 40 F 0 0 0 10 40 50 50 50 p 0 10 11.11 10 30 50 40 30 Total 0 3.33 10.34 10 30 36.67 33.33 40
Table 14. Distribution of subclinical ketosis in multiparous cows at D-10, D 5 DO Dl D7 D14 D21 d D28 b t t t - , , , , , an 'Y rea men 2rOUp
MULTIPAROUS - % Ketotic (BHB Value >1400 p.mollL) Trt D-I0 O-S DO Dl D7 D14 D21 D28 C 0 0 28.57 14.29 28.57 14.29 14.29 42.86 F 0 0 0 14.29 42.86 57.14 42.86 42.86 P 0 0 14.29 14.29 42.86 42.86 42.86 28.57 Total 0 0 14.29 14.29 38.10 38.10 33.33 38.095
Table 15. Distribution of subclinical ketosis in primiparous cows at D-l0, D 5 DO Dl D7 D14 D21 d D28 b t t t - , , , , , an )y rea meoRroup
PRIMIPAROUS - % Ketotic BHB Value >1400 ~mollL) Trt D-I0 D-S DO Dl D7 D14 D21 D28 C 0 0 0 0 0 0 0 33.33 F 0 0 0 0 33.33 33.33 66.67 66.67 P 0 25 0 0 0 50 25 25 Total 0 10 0 0 10 30 30 40
57

The majority of metabolic problems associated with the transition
period occurs by d 1 post-calving. Cows that experience difficult transitions,
may be predisposed to undesirable metabolic outcomes, such as ketosis, that
occur by d 1 post-calving or will eventually take place in the following weeks
(Veenhuizen et al., 1991). If displaced abomasum is related to rumen fill, then
the extent of pre-calving DMI decrease may be an important factor
determining whether the cow develops this disorder. In the study done by
Butler and Elliot (1970), the Flucort® treatment caused a small increase
(P<O.OI) in blood ketone concentrations. This response bas also been observed
in sheep (Butler and Elliot, 1970). The response in blood ketone levels is
presumably the result of amino acid breakdown or fat mobilization.
To be sure that dry matter intake (DMI) and rumen fill was not affected
by treatment, DMI was recorded daily. Tables 4 and 5 specity the diet
composition (% of DM) of close-up rations and fresh cow ration. Statistical
analysis of DM! revealed no effect of treatment (p>0.05), and no differences
in DMI were observed among treatments (P>O.05) as shown in Table 16.
Table 16. Least squares means from mixed models for average dry matter intake in cows on D-I0, D-5, DO, Dl, week 1, week 2, week 3, and week 4 post-calving that were either treated day of calving with a Flucort®, P d f® 2X d ti t 1 1 re e or serve as nega vecon ro s
Average DMI (kg) (LSM Trt ±S.E.M.)
C F P
D-I0 23.8±1.28 21.4±1.37 21.4±O.81
D-5 22.6±1.57 21.07±1.50 20.4±1.71
DO 22.5±2.24 26.4±3.69 21.3±2.60
Dl 30.2±4.47 34.0±1.83 31.8±3.96
D7 35.0±1.45 30.8±2.00 37.4±2.75
D14 36.4±2.45 36.0±1.60 37.0±1.71
D21 37.2±2.81 36.6±1.76 37.5±1.51
D28 38.3±1.61 36.4±2.43 36.4±2.64 1 . .
Modeis usmg proc mlxed (SAS) mcluded panty, calvmg season, samphng date and the random effect of cow.
In this study, it appeared that after Flucort® treatment, less cows had
higher NEFA (>0.7 mmoIIL) Ieveis and more animaIs had higher BHB
(>1400, J..I.IllollL) leveis. High levels of BHB would be the result of fat
58

mobilization and a high concentration of NEF As released into bloodstream
causing high NEF A levels in the liver, and the eventual production of ketone
bodies. Therefore, this trend is difficult to explain and may be due to small
cow numbers. A larger sample size is needed to make a concrete conclusion.
Shpigel et al. (1996) compared the relative efficacy of 40 mg of
dexamethasone and 5 mg flumethasone alone or in combination with rapid IV
infusion of 500 mL of 50% glucose solution for treatment of ketosis in cattle.
Treatment success was defined as recovery after a single treatment without
relapse during the same lactation. Flumethasone in combination with glucose
and dexamethasone in combination with glucose were significantly more
effective than dexamethasone and flumethasone administered alone.
Flumethasone administered alone did not differ significantly from
dexamethasone administered alone. Flumethasone is approved and marketed
for the treatment of clinical ketosis in dairy cows. In this regard, the fmdings
of the current experiment of Flucort® administration are not consistent with
other studies.
1.2. Mineral status
Blood mineraI metabolites included calcium, phosphorus, potassium,
sodium, chloride and magnesium. Ail the blood minerai metabolites were
evaluated on d -10, d -5, dO, d 1, d 7, d 14, d 21 and d 28 relative to calving.
Due to the potential upset of the electrolyte balance the effects of Flucort® and
Prede~ 2X were evaluated on serum minerai parameters.
1.2.1. Calcium
Statistical analysis of serum calcium levels revealed no effect of
treatment (p>0.05), however there was an effect of sampling date, interaction
of treatment and sampling date, interaction of parity and sampling date, and
interaction of calving season and sampling date (P<0.05). Results for overall
least square means and least square means for individual days are shown in
Table 7 and 17, respectively.
59

Table 17. Least squares means from mixed models for serum minerai parameters in cows on D-lO, D-5, DO, Dl, D7, D14, D21 and D28 relative to calving that were either treated on day of calving with a Flucort®, Predetll> 2X or placebo·
Serum Mineral Parameter (LSM ± S.E.M.)
Day Trt Calcium Phosphorus Potassium (mmollL) (mmollL) (mmollL)
D-I0 C 2.588 2.35638 4.92488
F 2.54938 2.27968 5.02418
P 2.47928 2.22068 4.5316
D-S C 2.5868c 2.28768 4. 1656A ,b,c
F 2.57318 2.3439c 5.0249B,8
P 2.50288 2.26188 4.6429
DO C 2. 165D,a,e 2.0233D,c 4.7137
F 2.2153b,c 2.0246d,e 4.9338
p 2.0909b,c 1.7802b,c 4.5896
Dl C 2.1024 A,b,a,g 1.6834A,b,a,e 4.8686A ,a
F 2.3907B,b,d,e 1.3337B,b,d,t;g 5. 1 079A,c
P 2.18A,b,c 1.5372b,e 4.1278B
D7 C 2.6662t;b 1. 7571 b,a,e 4.5352
F 2.5095d,g 1.9764b,d,b,i 4.4345d
P 2.5567d 1.901 b,f,g 4.3977
D14 C 2.6493I)l 2.11011 4.5894
F 2.6386d,f,h 2. 1374h 4.7887
P 2.5761d 2.0699d,f 4.5308
D21 C 2.6632t;b 2.23571 4.4815
F 2.6115d,f 2.226h 4.7008
P 2.5759d 2.2531 d,t;h 4.6894
D28 C 2.7116b,t;b 2.27971 4.4928
F 2.5989d,f 2.24~ 4.1821b,d
P 2.5915d 2.2394d,f,b 4.3046
S.E. C 0.0646 0.1203 0.2423
F 0.0646 0.1203 0.2423
P 0.06584 0.1224 0.2457 1 . . .
Models usmg proc mlxed (SAS) mc1uded parlty, calvmg season, sample date and the random effect of cow. A and B, C and D differ significantly among treatments within the same day a and b, c and d, e and f, g and h, i and j differ significantly among days within the same treatment
60

The only significant difference observed in serum calcium levels was
on d 1 where Flucort® treated cows experienced significantly higher (P<0.05)
levels than Predef!> 2X and control cows. There were no significant
differences between pre-calving and post-calving serum calcium
concentrations (d 7, d 14, d 21 and d 28). A higher serum calcium status may
provide a beneficial effect as most fresh-cow health problems such as
displaced abomasums, metritis and clinical and subclinical milk fever are part
of one disease complex greatly dependent on the cow's calcium status (Acre,
1998).
The interaction between parity and sampling date indicated that on
sorne days older cows presented lower calcium concentrations than younger
animaIs. The relationship between age and hypocalcemia has also been weil
documented (Goff and Horst, 1997). In the present study, there were 4 first
parity heifers and 6 multiparous cows in the Predef!> 2X treatment group and
this might explain the lower serum calcium levels on d 1 compared to the
Flucort® treatment group.
1.2.2. Phosphorus
Statistical analysis of serum phosphorus levels revealed no effect of
treatment (P>0.05), however there was an effect of parity, sampling date,
interaction of parity and sampling date, and interaction of calving season and
sampling date (P<0.05). Results for overallleast square means and least square
means for individual days are shown in Table 7 and 17, respectively.
The only significant difference observed in serum phosphorus levels
was on d 1 where Flucort® treated cows experienced significantly lower
(P<0.05) levels than control cows. This was not surprising as sorne
glucocorticoids have a suppressive effect on phosphorus levels. In comparison
to control cows phosphorus levels in this experiment were relatively normal
and not affected by treatment and probably were the response to an adequate
use of anionic salts. There were no differences observed between pre-calving
(d -10, d -5) and post-calving (d 14, d 21, d 28) phosphorous levels (p>O.05).
1.2.3. Potassium
Statistical analysis of serum potassium levels revealed no effect of
treatment (P>O.05), however there was an effect of calving season (P<0.05).
No significant differences were observed in serum potassium levels between
61

Flucort® treated cows and control cows. In the Flucort® treatment cows, cows
that calved in the summer season had significantly lower potassium levels than
cows that calved in the winter season (P<O.05). In both the control and
Flucort® treatments, cows that calved in the fall season had significantly lower
potassium levels than cows that calved in the winter season (P<O.05). These
differences are probably due to the different feeds available in each season.
Results for overall least square means and least square means for individual
days are shown in Table 7 and 17, respectively.
1.2.4. Sodium
Statistical analysis of serum sodium levels revealed no effect of
treatment (P>O.05). however there was an effect of sampling date (P<O.05).
There were no differences (P>O.05) in serum sodium levels among treatments.
In comparison to control cows sodium levels in this experiment were relatively
normal and not affected by treatment and probably were the response to an
adequate use of anionic salts. Serum sodium post-calving values (d 21 and d
28) were significantly lower than pre-calving values in Flucort® treated cows
(P<O.05). which was comparable to control cows. Results for overall least
square means and least square means for individual days are shown in Table 7
and 18, respectively.
62

Table 18. Least squares means from mixed models for serum minerai parameters in cows on D-10, D-5, DO, Dl, D7, D14, D21 and D28 relative to calving that were either treated on day of calving with a Flucort®, P d ~2X 1 b 1 re e or place 0
Serum Mineral Parameter (LSM :i: S.E.M.)
Day Trt Sodium Chloride Magnesium (mmol/L) (mmol/L) (mmol/L)
D-lO C 144.808 105.068 1.07118
F 144.178 105.328 1.0192
P 145.068 105.618 1.03248
D-5 C 147.128 107.058 0.9988
F 145.92c 106.93c 1.06698
P 143.61 104.728 0.958c
DO C 147.298 107.058 1.0243
F 146.06c 104.628 1.0224
P 144.388 102.55c 1.0745d
Dl C 145.958 105.178 0.9929
F 145.198 102.90d,e 1.0009
P 147.34c 104.608 1.06518,d
D7 C 140.81 11 99.70 1411,c 0.9443 11,c
F 143.498 99.8668b,d,e 0.9424b
P 142.32d 99.3861b,e 0.9441b,e
Dl4 C 140.8611 97.415911,e 1.0381e
F 141.73d 96.2813b,d,:t;g 0.9762b
P 141.50d 96.7195b,d 1.0035
D2l C 139.2211 94.514711,11 0.948311,lI,t,g
F 139.70b,d 92.1801 b,d,:t;h 0.9864
P 142.40d 94.80 12b,d,f 1.0275
D28 C 139.2411 91.96701l,<I,t 1.043211,b
F 139.21b,d 91.0324b,d,:t;h 0.9813
P 140.39b,d 93.1059b,d,f 1.0697d,f
S.E. C 1.73 1.73 0.04467
F 1.72 1.71 0.04467
P 1.75 1.76 0.04587 . Models usmg proc mlxed (SAS) mcluded panty, calvmg season, sample date
and the random effect of cow. A and B, C and D differ significantly among treatments within the same day a and b, c and d, e and f, g and h, i and j differ significantly among days within the same treatment
1.2.5. Chloride
Statistical analysis of chloride levels revealed no effect of treatment
(P>O.05), however there was an effect of sampling date and an interaction of
calving season and sampling date (P<O.05). There were no differences
63

(P>0.05) in serum chloride levels among treatments. Results for overall least
square means and least square means for individual days are shown in Table 7
and 18, respectively.
In comparison to control cows, chloride levels in this experiment were
relatively normal and not affected by treatment and probably were the
response to an adequate use of anionic salts.
1.2.6. Magnesium
Statistical analysis of magne sium levels revealed no effect of treatment
(p>0.05), however there was an effect of sampling date and an interaction of
treatment and sampling date (P<0.05). There were no differences (p>0.05) in
serum Magnesium levels among treatments. Results for overall least square
means and least square means for individual days are shown in Table 7 and
18, respectively.
1.3. Immune Function
Cows were challenged to evaluate the effect of Flucort® and Prede~
2X on the immune system. The cows were immunized with chicken
ovalbumin on the day of calving and given a boost 2 weeks post-calving.
Immune function parameters included antibody parameters which were
evaluated on d 0, d 7, d 14, d 21 and d 28 relative to calving and analyzed by
an ELISA assay measuring the concentration of chicken ovalbumin specific
IgG (antibody) in serum; and lymphocyte proliferation which was evaluated
on d 0 and d 7 relative to calving and analyzed by an in vitro assay
determining if bovine ConA-induced lymphocyte proliferation is altered in
response to treatments.
1.3.1. Antibody production
Statistical analysis of antibody levels revealed no effect of treatment
(p>0.05), however there was an effect of sampling date (P<0.05). No
statistically significant differences (P>0.05) were observed between Flucort®
treated cows and control cows for antibody production values. Results for
overall least square means and least square means for individual days are
shown in Table 7 and 19, respectively.
64

Table 19. Least squares means from mixed models for antibody production values in eows DO, D7, D14, D21 and D28 rela.tive to ealving that were either treated day of calving with a Flucort®, Predef!> 2X or
d t , t 1 1 serve a.s nega IVe con ro s LSM±S.E.M.
Parameter Trt DO D7 Dt4 D2t D2S
S.E.M. Antibody Cl 0.4271 3 0.58803 0.8674D,c 1.1708D
,Q 1.275D,Q 0.1246
(OD-400 F 0.34553 0.41143 0.9755b,c 1.1841b,d 1.1548b 0.1246 nm)
P 0.29653 0.4608& 0.8562b,c 1.0824b,d 1.1652b,d 0.1271
1 Models usmg proe mixed (SAS) included panty, ealvmg season, sample date and the random effeet of eow. a and b, c and d differ significantly among days within the same treatment
1.3.2. Lymphocyte proliferation
Statistieal analysis of lymphocyte proliferation levels revealed no
effect oftreatment (P>O.05), there was however an effect of sampling date and
an interaction of calving season and sampling date (P<O.05). Furthermore, in
the present study, a single injection of Flucort® on the day of calving did not
affect ConA-induced lymphocyte proliferation compared to the control group
at d 0 or d 7. Differences may not have been statistieally significant due to a
large variation in lymphocyte proliferation in response to ConA among
animaIs and among replicates. However, the administration of a Flucort® to
dairy cows on the day of calving resulted in a significantly reduced d 7 blood
lymphocyte proliferation values in response to ConA compared to the
respective d 0 blood lymphocyte proliferation values and the control cows
receiving the placebo treatment (P<O.05). The decreased celI proliferation
observed in Flucort® treated cows on d 7 could also be a result of cell death.
Results for overall least square means and least square means for individual
days are shown in Table 7 and 20, respectively.
65

Table 20. Least squares means from mixed models for lymphocyte proliferation values in cows DO and D7 relative to calving that were either treated day of calving with a Flucort®, Predefi> 2X or served as negative controls1
Parameter Trt LSM±S.E.M.
DO D7 Lymphocyte proliferation C 18.22 ± 5.11 7.71 ± 3.47 (stimulation index)
F 24.10 ± 5.36a 6.32 ± 3.6b
P 19.72 ± 5.98 11.78 ± 6.08
Models usmg proc mlxed (SAS) mcluded panty, calvmg season, sample date and the random effect of cow. a and b differ significantly among days within the same treatment
This indicates that Flucort® did not induce more of an
immunosuppressive effect in cows receiving treatment on day of calving
compared to control, however Flucort® treated cows did cause more of a
significant decrease in circulating lymphocytes.
Thanasak et al. (2004) also found that dexamethasone treatment did not
induce a significant decrease in the number of circulating lymphocytes or
changes in lymphocyte subsets, considering the population of lymphocytes in
the study. Our fmdings are in accordance with the investigations of Burton and
Kehrli (1996) who demonstrated that the percentages of lymphocyte subsets
were not altered by 0.04 mglkgl d dexamethasone administered to young bulls
on three consecutive days. However, in other studies glucocorticosteroids have
been shown to cause lymphopenia and alterations of lymphocyte subsets
(Anderson et al., 1999; Doherty et al., 1995; Winnicka et al., 2000). Thus, the
differences between the results of previous studies and the present one cao
mainly be explained by the differences in the sort of corticosteroids
administered and the dosage regimen.
Nagahata et al. (1992) examined B lymphocyte populations to evaluate
host defense in dairy cows during the transition period and found no
significant changes in the number of B lymphocytes of cows from 2 weeks
before until 2 weeks after calving. Other studies of glucocorticosteroids such
as single injections with high doses or repeated treatments of dexamethasone
resulted in decreased lymphocyte proliferation and have been shown to cause
66

lymphopenia and alterations of lymphocyte subsets (Anderson et al., 1999).
On possible mechanism underlying the reduced lymphocyte proliferative
responses is the down modulation of CD 18 expression which has been shown
to act as a co-stimulatory Molecule in the process of lymphocyte proliferation
(Thanasak et al., 2004). Thanasak et al. (2004) found reduced CDl8
expression on lymphocytes in dexamethasone treated cows, however their
proliferative responses remained unaffected.
A depression in lymphocyte proliferation has been shown to be related
to increased blood glucose concentrations (Franklin et al., 1991; Thanasak et
al., 2004). In the study by Thanasak et al. (2004), although increases in blood
glucose and insulin concentration in the dexamethasone treated group
indicated that hypoglycemia was overcome, they did not affect lymphocyte
proliferation. In this study neither lymphocyte proliferation or blood glucose
concentrations was affected by Plucort® administration, however Plucort®
treated cows had significantly higher insulin concentrations on d 1. Perhaps
the therapeutic dosage of 10 mg of Flucort® is not an effective dosage to
induce significant depression in lymphocyte proliferation, and a significant
increase in blood glucose and insulin concentrations allowing cows to
overcome hypoglycemia.
1.4. Milk Status
Milk composition parameters included fat (% and yield), protein (%
and yield), lactose (% and yield), SCC, sodium and potassium. Milk
composition parameters were evaluated on d 1, d 7, d 14 and d 21 relative to
calving, with the exception of sodium and potassium which were evaluated on
d 1 by digestion and atomic absorption.
1.4.1. Milk Yield (kg)
Statistical analysis of milk yield revealed no effect of treatment
(P>0.05), however there was an effect of parity, calving season, sampling date
and an interaction of parity and sample dating (P<O.05) on milk yield. There
were no differences (P>O.05) in milk yield among treatments. Results for
overall least square means and least square means for weekly average milk
yield are shown in Table 7 and 21, respectively.
67

Table 21. Least squares means from mixed models for average milk yield in eows on week 1, week 2, week 3, and week 4 post-ealving that were either treated day of calving with a Flucort®, Prede~ 2X or served as
. 1 1 nel!atIve contro s Trt Average Milk Yield (kg) (LSM::l:: S.E.M.) S.E.M.
Weekl Week2 Week3 Week4 C 28.9072 37.8828 39.595 40.8606 1.445
F 28.6201 36.0701 38.519 38.5701 1.4387
P 27.4726 37.3306 38.8986 38.5906 1.3742 J Models usmg proc mlxed (SAS) mcluded panty, calvmg season, sample date and the random effect of cow
1.4.2. Protein
1.4.2.1. Protein (%)
Statistical analysis of protein (%) revealed no effect of treatment
(P>O.05), however there was an effect of sampling date, interaction of
treatment and sampling date, and an interaction of calving season and
sampling date (P<O.OS) on prote in %. Results for overallleast square means
and least square means for individual days are shown in Table 7 and 22,
respectively.
68

Table 22. Least squares means from mixed models for milk component parameters in cows Dl, D7, D14 and D21 relative to calving tbat were eitber treated day of calving witb a Flucort®, Prede~ 2X or served as
. 1 1 negatlve contro s Milk Parameters (LSM ± S.E.M.)
Trt Dl D7 D14 D21
S.E.M. Pa ra meter MilkFat C 4.6431a 4.1751° 4.89940 4.2963b 0.7864 (%)
F 4.9832" 3.9832b 5.4195b 4.0474b 0.7864
P 4.6032& 4.5920b 4.2354b 4.5786b 0.7997
MilkFat C 0.8852a 1.5660 1.959fD 1.8363b 0.2920 (kg)
F 0.8499& 1.3585 2.2760b 1.921 Ob 0.2920
P 0.4406a 1.4478b 1.5699b 1.7964b 0.2828
Milk C 4.4474A,a 3.3832° 2.9286° 2.8029D 0.3616 Protein
F 4.2938A.a 3.3417b 2.9820b 2.9383b 0.3616 (%)
P 6.3307B,a 3.2693b 3. 1346b 2.8213b 0.3681
Milk C 0.7809a 1.3621° 1.2680b 1.2882 0.2121 Protein
F 0.7831a 1.221 Ob 1.2346 1.3571b 0.2121 (kg)
P 0.9695 1.0083 1.1169 1.0045 0.2060
Milk C 3.7292a 4.3148b 4.5662° 4.5396° 0.1271 Lactose
F 3.9858a 4.3693b 4.4628b 4.6071 b 0.1271 (%)
P 3.6076a 4.3942b 4.6174b 4.5065b 0.1294
Milk C 0.5929a 1.5952b 1.7765b 1. 8620b 0.1171 Lactose
F 0.6418 a 1.4419 b,c 1.6288 b,e 1.8943 0.1171 (kg) b,d,f
P 0.4209 a 1.4671 b,c 1.7745 1.7215 b 0.1137 b,d
MilkSCC C 169.97A 96.3405 41.5749 124.11 67.6351
F 236.258 117.32 110.14 65.3144b 65.9659
P 437.45B,a 105.30b 141.29b 131.56b 67.1982
Milk C 34.3840A 1.9029 Sodium
F 24.4662B 1.8073 (mg/L)
P 32.62A 1.9771
Milk C 81.5700 3.8217 Potassium
F 79.9409 3.6296 (mg/L)
p 78.5493 3.9708
1 . Models usmg proc ffilxed (SAS) mcluded panty, calvmg season, sample date
and the random effect of cow. A and B differ significantly among treatments within the same day a and b, c and d, e and f, g and h, i and j differ significantly among days within the same treatment
69

AIl cows experienced a decline in milk prote in (%) with advancing
lactation, furthermore a significant decrease was observed on d 7 for aIl
treatments (P<O.05), and d 14 and d 21 values were significantly lower than d
1 values (P<O.05).
1.4.2.2. Protein Yield (kg)
Statistical analysis of protein yield (kg) revealed no effect of treatment
(P>O.05), however there was an effect of sampling date (P<0.05) on prote in
yield. There were no difIerences (p>0.05) in protein yield among treatments.
Results for overall least square means and least square means for individual
days are shown in Table 7 and 22, respectively.
Milk protein yield levels increased numerically from dIto d 7
(P>0.05) and milk protein yield on d 7 and d 21 values were significantly
higher than d 1 levels in Flucort® treated cows (P<0.05).
In a study done by Shamay et al. (2000), a single intramuscular dose of
40 mg of dexamethasone was injected into dairy cows in order to get a better
insight into the effects of corticosteroids on milk secretion and composition.
Dexamethasone caused a 45% reduction in milk yield after 24 h, full recovery
took 5 d. The concentration of total prote in increased then decreased in direct
proportion to the changes in milk yield. In the present study, as milk yield
increased, protein (%) decreased, however protein yield increased throughout
the trial, particularly between d 1 and d 7 (P<0.05). Protein yield on d 7 and d
21 were significantly higher than d 1 levels. In the study by Shamay et al.
(2000), the secretion of total protein decreased as a result of the
dexamethasone injection. In the present study, treatment had no effect on
prote in (%) or protein yield, however there was an interaction between
treatment and sampling date for protein (%) which may explain the
discrepancies.
1.4.3. Fat
1.4.3.1. Fat (%)
Statistical analysis of fat (%) revealed no effect of treatment (p>0.05)
and no significant differences (p>0.05) were observed among treatments.
Results for overall least square means and least square means for individual
days are shown in Table 7 and 22, respectively.
70

1.4.3.2. Fat Yield (kg)
Statistical analysis of fat yield revealed no effect oftreatment (p>O.05),
however there was an effect of parity and sampling date (P<O.05). There were
no differences (p>O.05) in fat yield among treatments. Results for overallleast
square means and least square means for individual days are shown in Table 7
and 22, respectively.
AH cows experienced an increase in milk fat yield with advancing
lactation and Milk fat levels on d 14 and d 21 for aH treatments were
significantly higher than their respective d 1 milk fat levels (P<0.05)
Flucort® treated cows with parity >3 had significantly higher levels of
milk fat (kg) than the frrst parity cows and cows that calved in the winter had
higher milk fat (kg) levels than cows that calved in the fall season (P<0.05).
These differences between increasing milk protein yield and increasing parity
are due to the different feeds and their respective protein content which are
available in different seasons.
In the study done by Shamay et al. (2000), the concentration of fat also
increased then decreased in direct proportion to the changes in milk yield. In
the present study fat (%) fluctuated, however fat yield (kg) increased until d 14
and then decreased by d 21 which further substantiates this observation.
1.4.4. Lactose
1.4.4.1. Lactose (%)
Statistical analysis of lactose (%) revealed no effect of treatment
(P>0.05), however there was an effect of parity and sampling date (P<0.05) on
lactose %. There were no differences (P>O.05) in lactose (%) among
treatments. Results for overall least square means and least square means for
individual days are shown in Table 7 and 22, respectively.
AlI cows experienced an increase in the percentage of milk lactose and,
d 7, d 14, and d 21 values were significantly higher than d 1 values (P<0.05).
A more increase in lactose % was observed between d 0 and d 7 for all
treatment groups (P<0.05).
1.4.4.2. Lactose Yield (kg)
Statistical analysis of lactose yield (kg) revealed no effect of treatment
(P>0.05), however there was an effect of sampling date (P<0.05) on lactose
yield. There were no differences (p>0.05) in lactose yield among treatments.
71

Results for overall least square means and least square means for individual
days are shown in Table 7 and 22, respectively.
Lactose yield from Flucort® treated cows inereased with milk yield
throughout the trial. The increase in lactose yield was particularly more
between d 14 to d 21. As expected, lactose yields for d 7, d 14, and d 21 were
significantly higher than their respective d 1 values for Flueort® treated cows
(P<O.05). The simultaneous increase in lactose and milk yield is expected as it
plays a major role in milk synthesis. It is the major osmole in milk and the
process of synthesis of lactose is responsible for drawing water into the milk
as it is being formed in the mammary gland.
1.4.5. Milk potassium (mgIL)
Sodium and potassium in milk were analyzed to see if ion transport
aeross the mammary epithelial cell was affected by treatment. Results for
overall least square means and least square means for individual days are
shown in Table 7 and 22, respectively.
Statistical analysis of milk potassium levels revealed no effect of
treatment (p>0.05). There were no differenees (p>0.05) in potassium levels
among treatments on d 1.
In the study done by Shamay et al. (2000), the concentration of lactose
and monovalent ions (sodium and potassium) was unaffeeted. AIl other results
in the study done by Shamay et al. (2000) were similar to the present study
except that of milk potassium levels (P>0.05).
1.4.6. Milk sodium (mgIL)
Statistical analysis of milk sodium levels revealed an effect of
treatment (P<0.05). Flueort® treated cows had significantly lower milk sodium
levels in comparison to the control cows (P<0.05) on d 1. Results for overall
least square means and least square means for individual days are shown in
Table 7 and 22, respeetively.
In the study done by Shamay et al. (2000), the concentration of lactose
and monovalent ions (sodium and potassium) was unaffeeted. Furthermore,
milk Na was the only parameter that responded to the treatment effects. This
would imply that the administration of Flucort® prevented the release of Na
into the mammary epithelial eeU thereby affeeting the ion transport across the
mammary epithelial celI.
72

Opening of mammary tight junctions is associated with increase in
milk sodium and chlorine, due to their leakage from the blood into the lumen,
and decrease in potassium concentration, due to its leakage from milk to blood
(Stelwagen et al., 1995). Data by Shamay et al. (2000) on monovalent ion
concentration in the milk suggest that depressed milk yield following
dexamethasone treatment cows was not associated with the disruption of the
integrity of the mammary cell tight junctions. Furthermore, dexamethasone
decreased tight junction permeability in vitro and cortisol appears to be
associated with closure of tight junction in the late pregnant mammary gland
of goats (Nguyen et al., 1998). One of the most basic principles of milk
secretion is that the total osmotic pressure of milk remains approximately
constant and equal to the blood (Holt, 1993). The output of osmotic
components, of which 60% are contributed by lactose and 40% by the
monovalent ions, determines the volume of milk (Peaker, 1977). Thus, the
reduction in milk volume following dexamethasone treatment can he fully
explained by reduction in the secretion of osmotic components. As lactose is
the main osmotic component, it is suggested that reduction in lactose secretion
induced a coordinated reduction in the output of sodium, potassium and
chlorine. However, in this study lactose percentage and yield experienced no
significant losses.
1.4.7. Somatic cell count
Statistical analysis of sec revealed no effect of treatment (p>0.05),
however there was an effect of sampling date and an interaction of parity and
sampling date (P<0.05). No significant differences (p>0.05) were observed
among treatments. Results for overall least square means and least square
means for individual days are shown in Table 7 and 22, respectively.
Numericallyon dl, Flucort® treated cows had higher sec than control
cows. As sec decreased slightly, lactose (%) increased slightly and this is
supported by Harmon (1994) who observed that elevated sec are associated
with a decrease in lactose because of reduced synthetic activity of the
mammary tissue. An analysis of the frequency of mastitis, as denoted by sec > 283,000 celIs/mL (Guidry, 1985; Reneau, 1986), indicative of cows with
subclinical mastitis, revealed greater mastitis in the flumethasone-treated cows
73

on d 1 and d 14 as shown in Table 23. However, due to limited cow numbers,
a concrete conclusion cannot be made and further studies are warranted.
Table 23. Frequency of mastitis, as denoted by sec > 283,000 cells/mL (Guidry, 1985; Reneau, 1986 , percentages of cows by treatment 2roup Trt Dl D7 D14 D21 C 14 22 0 20 F 30 20 13 10 P 78 11 20 20
2. PredefP 2X
2.1. Energy Status
Blood energy metabolites included glucose, BHB, NEF A and insulin.
AlI the blood energy metabolites were evaluated on d -10, d -5, d 0, d 1, d 7, d
14, d 21 and d 28 relative to calving. Among the ketone bodies (BHB,
acetoacetate and acetone), only BHB was evaluated because most other
cowside tests lack sensitivity as compared to serum BHB. Testing for BHB
remains the gold standard for studying ketosis (Duffield, 1997).
2.1.1. Glucose
Statistical analysis of serum glucose levels revealed no effect of
treatment (p>0.05), however there was an effect of sampling date (P<0.05).
Furthermore, there were no significant differences (P>0.05) in serum glucose
levels among treatments. Results for overall least square means and least
square means for individual days are shown in Tables 7 and 8, respectively.
The only day that Prede~ 2X treated cows had better energy status
than control cows as indicated by numerically higher serum glucose (p>0.05)
was on d 1. However, serum glucose values for Predef!> 2X treated cows
experienced a significant decrease by d 7 (P<0.05). Furthermore, glucose
concentrations on d 14, d 21 and d 28 were significantly lower than d -10, d-5
and d 0 values (P<0.05) and a similar trend was observed in control cows.
The numerical increase in glucose on d 1 has also been reported by
Convey et al (1970), in which lactating Holstein cows receiving 5 or 10 mg of
9a-fluoroprednisolone acetate (also called isoflupredone acetate or Predef!>
2X) immediately following moming milking experienced a linear increase
(P>0.05) in plasma glucose levels with time. The earliest significant increase
occurred 3 h after treatment and this agrees with the findings of Neff et al.
74

(1960), who also observed increased blood glucose 3 to 6 hr following
intramuscular administration of 10 mg of 9a-fluoroprednisolone acetate and
suggests equal availability of this steroid by intramuscular or intravenous
route. Philip et al. (1991), observed a significant increase in plasma glucose
concentration which persisted until d 5 in cows given a single intramuscular
injection of dexamethasone-21-isonicotinate of (2mg/l OOmg bodyweight).
When a glucocorticoid is administered, the concentration of glucose in
the blood should increase through the synthesis of glucose from amino acids
(gluconeogenesis), a decrease in the synthesis of proteins from amino acids,
and an altered lipid metabolism, thereby satisfying the systemic demand for
glucose and helping to prevent the metabolism of fats and production of
ketone bodies. AIso, peripheral utilization of glucose is reduced and liver
storage of glycogen is increased.
Despite other studies using the same dosage of Prede~ 2X and
observing significant increases in blood glucose, a larger dose than 10 mg/mL
of Prede~ 2X is probably needed to induce more significant effects on glucose
concentrations. Furthermore, the significant inereases that were observed in
other studies were found only within 24 h of administration. The effects on
blood glucose levels would only be temporary and would therefore have to be
measured on an hourly basis in future studies.
2.1.2. Insulin
Statistical analysis of insulin levels revealed no effect of treatment
(P>O.05), however there was an effeet of sampling date, interaction of
treatment and sampling date, and an interaction of parity and sampling date
(P<0.05). Results for overall least square means and least square means for
individual days are shown in Tables 7 and 8, respectively.
The only significant difference observed in serum insulin levels was on
d 0 where Prede~ 2X treated cows experienced significantly lower (P<0.05)
levels than control cows indicating a worse energy status. In contrast, Prede~
2X treated cows had numerically (P>O.05) higher insulin levels from dIto d
14, indieating a better energy status than control cows.
From dito d 7, insulin levels decreased significantly (P<O.05) to
almost 50% but increased towards the end of the trial (p>O.05). Post-calving
75

insulin levels however remained significantly lower than pre-calving levels
(P<0.05).
The higher insulin values experienced on d 1, d 7 and d 14 should have
stimulated the uptake, utilization and storage of glucose. In addition, insulin
should have facilitated the entry of glucose into muscle, adipose tissue and
other tissues. If the chain of events listed above had occurred upon Prede~ 2X
administration, the mobilization of fat would have been avoided.
2.1.3. Non-esterified fatty acids
Statistical analysis of serum NEF A levels revealed no effect of
treatment (p>0.05), however there was an effect of parity and sampling date
(P<0.05). Results for overall least square means and least square means for
individual days are shown in Tables 7 and 8, respectively.
There were no significant differences (p>0.05) in serum NEFA levels
between Prede~ 2X treated cows and control cows (p>0.05). Days at which
Prede~ 2X treated cows experienced numerically lower NEF A values and a
better energy status than control cows were d 1 and d 14 (P>0.05).
Furthermore, from d 0 to d 1, NEF A levels for Prede~ 2X treated cows
decreased numerically, whereas NEF A levels for control cows increased
significantly. NEF A values for Prede~ 2X treated cows continued to decrease
until d 28.
The better energy status experienced by Prede~ 2X treated cows
indicate less fat mobilization in these cows (Grummer, 1993) and therefore
less NEFA transported to the liver. Plasma NEFA concentration and
triglyceride concentration in the liver (Bertics et al., 1992) are positively
correlated. As fatty liver and ketogenesis are closely linked (Dann et al.,
1999), a reduction in plasma NEF A concentration should result in a decreased
risk of the cow developing fatty liver and ketosis. Our current findings
supports the hypothesis that, Prede~ 2X increases gluconeogenic activities
and therefore inhibits fat mobilization from the adipose tissue. FUrll and Jackel
(2005), investigated the effects of 0.02 mglkg body weight of dexamethasone
(in the form of Voren suspension) on d 7 and d 11 post-calving when high
levels of lipolysis normally occur and observed an increase in glucose and
insulin concentrations and a concomitant decrease free fatty acid
concentrations. Contrary to this fmdings, Seifi et al (2006) observed
76

significantly higher (P<0.02) NEFA concentrations 1 week after intramuscular
injection with 20 mg of isoflupredone acetate (Prede~ 2X) compared to the
control cows. In the present study, by d 21 Prede~ 2X treated cows had
numerically higher serum NEF A (P>0.05) levels and by d 28 the cows had a
worse energy status as indicated by higher serum NEF A (P>0.05)
concentrations.
An analysis of the frequency ofcows with high NEFA (>0.7 mmollL)
values indicative of a state of negative energy balance revealed that less
Prede~ 2X treatment cows had higher NEF A levels than the control cows in
both, primiparous and multiparous cows as shown in Tables 9,10 and 11. In
this respect, a treatment with Prede~ 2X may provide potential benefits in the
reduction of fat mobilization. However, due to limited numbers of cows used
in tbis study, a concrete conclusion cannot he made and further experiments
are warranted.
An analysis of the frequency of body condition score (BeS) within
treatment and within day, demonstrate that approximately 73% of the animaIs
were classified as being in fair body condition prior to calving, and 63% in
thin body condition post-calving as shown in Table 12. This indicates fat
mobilization during the transition from late gestation to early lactation.
2.1.4. p-hydroxybutyrate
Statistical analysis of serum BHB levels revealed no effect of treatment
(P>0.05), however there was an effect of sampling date (P<0.05). Results for
overall least square means and least square means for individuai days are
shown in Tables 7 and 8, respectively.
AlI cows experienced a graduaI increase in BHB levels throughout the
trial. From d 0 to d 28, Prede~ 2X treated cows had higher BHB values than
control cows but the only significant difference between the two groups was at
d 21 (P<0.05). This indicates that, Prede~ 2X cows were in a worse energy
status and that more fat was mobilized, resulting in an increase of serum
NEF A concentrations, which underwent more metabolism in the liver
resulting in more acetyl-CoA and its divergence into ketone body production
causing the eventual higher ketone concentrations post-calving in cows and
causing an increased risk of ketosis. This also may suggest that liver damage
may have occurred and that Prede~ 2X may have a lipolytic effect in vivo and
77

may aggravate or accelerate fatty degeneration of the liver. Seifi et al (2006)
also observed significantly higher (P<0.02) BHB concentrations 1 week after
intramuscular injection of cows with 20 mg of isoflupredone acetate (Prede~
2X) compared to control cows. In contrast, a study done by Philip et al.
(1991), cows given a single intramuscular injection of dexamethasone-21-
isonicotinate of (2mg/100mg bodyweight) increased plasma BHB
concentration insignificantly (P<0.05) on d 1 after treatment then showed a
graduaI and significant (p>0.05) decline until the end of the study.
An analysis of the frequency of cows with high BHB values (BHB >
1400 mmollL) (Duffield~ 2000) indicative of subclinical ketosis revealed that
more Prede~ 2X treated cows had higher BHB levels (> 1400 f.llllollL) than
control cows in both primiparous and multiparous cows as shown in Tables
13, 14 and 15. In tbis respect, treatment with Predef!> 2X may contribute to
problems of ketosis. However, due to limited numbers of cows used in the
current study, a concrete conclusion cannot he made and furtber experiments
are warranted.
To be sure that dry matter intake (DMI) was not affected by treatment,
and that DMI rumen fin, DMI was recorded on a daily basis. Tables 4 and 5
specify the diet composition (% of DM) of close-up rations and fresh cow
ration. Statistical analysis of DMI revealed no effect of treatment (p>0.05),
and no differences in DMI were observed among treatments (p>0.05) as,
shown in Table 16.
Seifi et al. (2006) observed that a treatment of 20 mg ofPrede~ 2X did
not resolve subclinical ketosis (SCK; ::: 1400 f.llllollL) among 190 cows that
were ketotic before treatment relative to control animaIs. Among 972 cows
that were not ketotic at enrollment, cows that received Prede~ 2X were 1.6
times more likely to develop subclinical ketosis 1 week after treatment.
Prede~ 2X is approved and marketed for the treatment of clinical ketosis in
dairy cows and in this regard, the findings of the current experiment are not
consistent with the approval.
In this study, it appeared that after a Prede~ 2X treatment, less cows
had higher NEFA (>0.7 mmollL) and more animaIs had higher BHB (>1400,
f.llllollL). High levels of BHB would he the result of fat mobilization and a
high concentration of NEF As released into bloodstream causing high NEF A
78

levels in the liver, and the eventual production of ketone bodies. Therefore,
this trend is difficult to explain and may be due to small cow numbers. A
larger sample size is needed to make a concrete conclusion.
2.2. Serum Mineral Status
Blood mineraI metabolites included calcium, phosphorus, potassium,
sodium, chloride and magnesium. AH the blood mineraI metabolites were
evaluated on d -10, d -5, d 0, d 1, d 7, d 14, d 21 and d 28 relative to calving
and due to the potential upset of the electrolyte balance, effects of Flucort®
and Predeflll2X were evaluated on serum mineraI parameters.
2.2.1. Calcium
Statistical analysis of serum calcium levels revealed no effect of
treatment (p>0.05), however there was an effect of sampling date, interaction
of treatment and sampling date, interaction of parity and sampling date, and
interaction of calving season and sampling date (P<0.05). Results for overall
least square means and least square means for individual days are shown in
Tables 7 and 17, respectively.
The only significant difference observed in serum calcium levels was
on d 1 where, Predef> 2X treated cows experienced signiticantly lower
calcium (P<0.05) levels than Flucort® treated cows. In a study done by Schafer
et al. (1983), 300 mg of prednisolone acetate injected intramuscularly caused a
decrease in blood plasma calcium sharply within 8 h. Seifi et al (2006)
observed significantly lower (p<O.Ol) calcium concentrations 1 week
following intramuscular injection with 20 mg of isoflupredone acetate
(prede~ 2X). AlI these observations contirm what was observed in the present
study. Furthermore, calcium concentrations of PredefJ 2X treated cows
remained lower than control cows until d 28 in the present study. The
numerically higher serum calcium concentrations observed on d 1 in Predef>
2X treated cows may provide a beneticial effect as most fresh-cow health
problems such as displaced abomasums, metritis, and clinical and subclinical
milk fever are part of one disease complex greatly dependent on the cow's
calcium status (Acre, 1998).
2.2.2. Phosphorus
Statistical analysis of serum phosphorus levels revealed no effect of
treatment (p>0.05), however there was an effect of parity, sampling date,
79

interaction of parity and sampling date, and interaction of calving season and
sampling date (P<0.05). No significant differences were observed among
treatments (p>0.05). Results for overall least square means and least square
means for individual days are shown in Tables 7 and 17, respectively.
Phosphorus levels in this experiment were relatively normal and not
affected by treatment and probably were the response to an adequate use of
anionic salts. No significant differences were observed between pre-calving (d
-10, d -5) and post-calving (d 14, d 21, d 28) levels (p>0.05). Sorne approved
glucocorticoid drugs have a suppressive effect on serum phosphorus levels. In
clinical safety studies on Prede~ 2X done for product registration, serum
phosphorus levels dropped to 60 to 70% of pretreatment levels and then rose
to 80% of those levels by 24 h. When treatment was terminated, phosphorus
levels rebounded to 40% above pretreatment levels (Dairy, Utah State
University Extension, July 1996). In the present study, the opposite was
observed and after d 1, phosphorus levels in Prede~ 2X treated cows
remained higher than control cows (not statistically significant).
2.2.3. Potassium
Statistical analysis of serum potassium levels revealed no effect of
treatment (P>O.05), however there was an effect of calving season (P<0.05).
There was a difference (P<0.05) in serum potassium levels on d 1 where,
Prede~ 2X treated cows had significantly lower potassium levels than
Flucort® treated cows and control cows (P<0.05). Results for overall least
square means and least square means for individual days are shown in Tables
7 and 17, respectively.
Sorne approved glucocorticoid drugs have decreasing effects on
potassium. Repeated or large, off-label doses of isoflupredone acetate can
upset electrolyte balance in the cow causing very low blood potassium levels
and down cows (Goff, 2001). Furthermore, in clinical safety studies on
Predet4!> 2X done for product registration. serum potassium values changed
very little with a single 10 mg injection, but dropped to 70 to 80% of
pretreatment levels with a second or successive injections. In the clinical
safety studies. doses of 100 mg of Predef!> 2X alone did not exhibit toxic signs
of hypokalemia (Dairy, Utah State University Extension, July 1996). In the
present study, potassium levels did change very little with the exception of d 1.
80

This observation was also observed by Schafer et al. (1983) where a decrease
in blood plasma potassium sharply occurred within 8 h following an
intramuscular injection of 300 mg of prednisolone acetate. In the present study
after d 1, potassium concentrations in the Predefl 2X treated cows were not
significantly different from those of the control cows. This is also confrrmed
by Seifi et al (2006), who observed that treatment had no influence on
potassium concentrations 1 week following an intramuscular injection of 20
mg of isoflupredone acetate (Predef!> 2X).
2.2.4. Sodium
Statistical analysis of serum sodium levels revealed no effect of
treatment (p>0.05), however there was an effect of sampling date (P<0.05) on
sodium levels. In comparison to control cows, sodium levels in this
experiment were relatively normal and not affected by treatment and probably
were the response to an adequate use of anionic salts. Serum sodium post~
calving values (d 21 and d 28) were significantly lower than pre-calving
values in Predefl2X treated cows (P<0.05), which was comparable to control
cows. Results for overall least square means and least square means for
individual days are shown in Tables 7 and 18, respectively.
2.2.5. Chloride
Statistical analysis of chloride levels revealed no effect of treatment
(P>0.05), however there was an effect of sampling date and an interaction of
calving season and sampling date (P<0.05) on serum chloride levels. There
were no differences (P>O.05) in serum chloride levels among treatments.
Results for overall least square means and least square means for individual
days are shown in Tables 7 and 18, respectively.
In comparison to control cows, chloride levels in this experiment were
relatively normal and not affected by treatment and probably were the
response to an adequate use of anionic salts. This is also confirmed by Seifi et
al (2006), who observed that treatment had no influence on sodium and
chloride concentrations 1 week following an intramuscular injection of 20 mg
of isoflupredone acetate (prede:t«> 2X). Prede~ 2X treated cows experienced a
graduaI decline in chloride levels and d 28 chloride values were significantly
lower than d -10 values (P<0.05). This trend was observed in aIl cows.
81

2.2.6. Magnesium
Statistical analysis of magnesium levels revealed no effect of treatment
(p>O.05), however there was an effect of sampling date and an interaction of
treatment and sampling date (P<O.05) on serum magne sium levels. There were
no differences (P>O.05) in serum magne sium levels among treatments. Results
for overall least square means and least square means for individual days are
shown in Tables 7 and 18, respectively.
Predefb 2X treated cows experienced slight fluctuations in magnesium
levels which was comparable to control cows. In a study done by Schafer et al.
(1983), 300 mg of prednisolone acetate injected intramuscularly caused a
decrease in blood plasma magnesium sharply within 8 h. This result was not
observed in the present study where d 0 and d 1 magnesium concentrations
values was much higher in PredefID 2X treated cows than control cows,
although not statistically different. There was a significant decrease (P<0.05)
in serum magnesium levels until d 7, which then significantly increased in
Predefb 2X treated cows and a similar trend was also observed in control
cows. Furthermore Goff and Horst (1998) reported a similar pattern of plasma
magnesium.
2.3. Immune Function
Cows were challenged to evaluate the effect of Flucort® and PredefID
2X on the immune system. The cows were immunized with chicken
ovalbumin on the day of calving and given a boost 2 weeks post-calving.
Immune function parameters included antibody parameters which were
evaluated on d 0, d 7, d 14, d 21 and d 28 relative to calving and analyzed by
an ELISA assay measuring the concentration of chicken ovalbumin specifie
IgG (antibody) in serum; and lymphocyte proliferation which was evaluated
on d 0 and d 7 relative to calving and analyzed by an in vitro assay
determining if bovine ConA-induced lymphocyte proliferation is altered in
response to treatments.
2.3.1. Antibody production
Statistical analysis of antibody levels revealed no effect of treatment
(p>0.05), however there was an effect of sampling date (P<0.05) antibody
levels. No statistically significant differences (P>0.05) were observed between
PredefID 2X treated cows and control cows for antibody production values.
82

Results for overall least square means and least square means for individual
days are shown in Tables 7 and 19, respectively.
2.3.2. Lymphocyte proliferation
Statistical analysis of lymphocyte proliferation levels revealed no
effect oftreatment (P>0.05), however there was an effect ofsampling date and
an interaction of calving season and sampling date (P<0.05). In addition, there
were no statistically significant differences (P>0.05) observed for ConA
induced lymphocyte proliferation values for d 0 or d 7. Results for overall
least square means and least square means for individual days are shown in
Tables 7 and 20, respectively. However this may be due to the large variation
in lymphocyte proliferation among animais and among replicates. Nagahata et
al. (1992) examined B lymphocyte populations to evaluate host defense in
dairy cows during the transition period and found no significant changes in the
number of B lymphocytes of cows from 2 weeks before until 2 weeks after
calving. Thanasak et al. (2004) demonstrated that a single injection with
dexamethasone did not affect Con A-induced lymphocyte proliferation. In
previous studies, however, single injections with high doses or repeated
treatments resulted in decreased lymphocyte proliferation (Pruett et al., 1987).
Perhaps high dosages or repeated treatments of Prede~ 2X would cause
decreased lymphocyte proliferation, but the single therapeutic dosage does not
cause decreased lymphocyte proliferation. Thanasak et al. (2004) suggested
one possible mechanism underlying the reduced lymphocyte proliferative
responses is the down modulation of CD 18 expression which has been shown
to act as a co-stimulatory molecule in the process of lymphocyte proliferation.
Studies by Thanasak et al. (2004) found no detrimental effects of
glucocorticoid injection in the post-calving period on measures on immune
function. In contrast, FürU and Jackel (2005) found that two doses of Voren
resulted in typical glucocorticoid-related changes in the differential leukocyte
count.
A depression in lymphocyte proliferation bas been shown to be related
to increased blood glucose concentrations (Franklin et al., 1991; Thanasak et
aL, 2004). In the study by Thanasak et al. (2004), although increases in blood
glucose and insulin concentration in the dexamethasone treated group
indicated that hypoglycemia was overcome, they did not affect lymphocyte
83

proliferation. In this study neither lymphocyte proliferation, blood glucose nor
blood insulin concentrations were affected by Prede~ 2X administration.
Perhaps the therapeutic dosage of 10 mg of Prede~ 2X is not an effective
dosage to induce a significant depression in lymphocyte proliferation, and a
significant increase in blood glucose and insulin concentrations allowing cows
to overcome hypoglycemia.
2.4. Milk Status
Milk composition parameters included fat (% and yield), protein (%
and yield), lactose (% and yield), sec, sodium and potassium levels in milk.
Milk composition parameters were evaluated on d 1, d 7, d 14 and d 21
relative to calving, with the exception of sodium and potassium which were
evaluated on d 1 by digestion and atomic absorption.
2.4.1. Milk Yield (kg)
Statistical analysis of milk yield revealed no effeet of treatment
(P>0.05), however there was an etfect ofparity, calving season, sampling date
and an interaction of parity and sample dating (P<0.05) on milk yield. There
were no ditferences (p>0.05) in milk yield among treatments. Results for
overall least square means and least square means for weekly average milk
yield are shown in Tables 7 and 21, respectively.
Milk yield for Predef> 2X treated cows increased throughout the trial
but remained slightly lower than control cows although not significantly
ditferent from each other. This is supported by Wagner and Apley (2003) who
reported that isoflupredone acetate (20 mg, IV) did not atfect milk production
in mastitic or healthy cows. Wagner and Apley (2003) concluded that drug
treatment effects would have to be very strong or study power (cow numbers)
would have to be quite high to detect an effeet of drug treatment on milk
production. FürU and Jackel (2005) noted only very slight changes in daily
milkyield.
2.4.2. Protein
2.4.2.1. Protein (%)
Statistical analysis of protein % revealed no etfect of treatment
(P>0.05), however there was an effeet of sampling date, interaction of
treatment and sampling date, and an interaction of calving season and
sampling date (P<0.05) on prote in %. Results for overall least square means
84

and least square means for individual days are shown in Tables 7 and 22,
respectively.
Ail cows experienced a decline in milk prote in percentage and a
significant decrease was observed on d 7 for ail treatments (P<0.05). Day 14
and d 21 values were significantly lower than d 1 values (P<0.05). Prede~ 2X
treated cows had significantly higher milk protein % than Flucort® and control
cows on d 1 (P<0.05).
Predef> 2X treated cows in parity >3 had significantly higher milk
protein % levels than cows in parity l, in addition cows that calved in the
winter season had higher milk protein % levels than cows that calved in the
summer or faH season (P<0.05). These differences are due to increasing milk
protein yields with increasing parities and the different feeds available at each
season.
2.4.2.2. Protein Yield (kg)
Statistical analysis of protein yield (kg) revealed no effect of treatment
(P>0.05), however there was an effect of sampling date (P<0.05) on prote in
yield. There were no differences (p>0.05) in protein yield among treatments.
Results for overall least square means and least square means for individual
days are shown in Tables 7 and 22, respectively.
Milk protein yield levels increased numerically from dito d 7 for
Prede~ 2X treated cows (p>0.05).
In a study done by Shamay et al. (2000), a single intramuscular dose of
40 mg of dexamethasone was injected into dairy cows in order to get a better
insight into the effects of corticosteroids on milk secretion and composition.
Dexamethasone caused a 45% reduction in milk yield after 24 h and full
recovery took 5 d. The concentration of total protein increased then decreased
in direct proportion to the changes in milk yield. In the present study, as milk
yield increased, protein % decreased. On d l, protein % of Prede:r 2X treated
cows was significantly higher than control cows and d 7, d 14, and d 21,
furthermore Predet«> 2X treated cows experienced the biggest decrease in
protein (%) by d 7 The higher protein percentage observed in Prede:r 2X
treated cows May provide a beneficial effect considering that during the fIfSt
few weeks of lactation the cow is typically in negative energy and prote in
balance and these May both impact and limit milk protein synthesis (Green et
85

al., 1999). However, the higher protein percentage may also be due to the high
protein content of colostrum and the high sec experienced on d 1. Prote in
(%) on d 7, d 14 and d 21 for Prede~ 2X treated cows were not statistically
different from control cows. In the study by Shamay et al. (2000), the
secretion of total protein decreased as a result of treatment. In the present
study, protein yield) changed slightly throughout the trial and the difference
from control cows and the difference between days were not statistically
different. Treatment had no effect on protein % or protein yield, however there
was an interaction effect between treatment and sampling date for prote in %
which may be an explanation.
2.4.3. Fat
2.4.3.1. Fat (Ofo)
Statistical analysis of fat % revealed no effect of treatment (p>0.05)
and no significant (p>0.05) differences were observed among treatments.
Results for overall least square means and least square means for individual
days are shown in Tables 7 and 22, respectively.
2.4.3.2. Fat Yield (kg)
Statistical analysis of fat yield (kg) revealed no effect of treatment
(P>0.05), however there was an effect of parity and sampling date (P<0.05) on
fat yield. There were no differences (p>0.05) in fat (% and yield) among
treatments. Results for overall least square means and least square means for
individual days are shown in Tables 7 and 22, respectively.
AIl cows experienced an increase in milk fat yield. Prede~ 2X treated
cows experienced a significant increase on d 7 (P<0.05). Milk fat levels on d
14 and d 21 for aIl treatments were significantly higher than their respective d
1 milk fat levels (P<0.05)
In the present study, fat % of Prede~ 2X treated cows tluctuated and
was not statistically different from control cows. On d l, fat % was
significantly higher than d 7, d 14, and d 21. However, fat yield increased
throughout the trial but not statistically different from control cows. On dl, fat
yield was significantly lower than d 7, d 14, and d 21. Therefore, in the first
week post-calving (d 7), Predefi> 2X treated cows experienced a significant
increase in milk fat yield. In the study done by Shamay et al. (2000), the
concentration of fat increased then decreased in direct proportion to the
86

changes in milk yield. In the present study fat % fluctuated but not in
proportion ta changes in milk yield.
2.4.4. Lactose
2.4.4.1. Lactose (%)
Statistical analysis of lactose % revealed no effect of treatment
(P>0.05), however there was an effect of parity and sampling date (P<0.05).
There was no difference (P>0.05) in lactose % among treatments. Results for
overall least square means and least square means for individual days are
shown in Tables 7 and 22, respectively.
AlI cows experienced an increase in percentage of milk lactose %, and
d 7, d 14, and d 21 values were significantly higher than d 1 values (P<0.05).
A significant increase was observed on d 7 for aIl treatments (P<0.05). First
parity Predefl 2X treated cows experienced a significantly higher lactose (%)
than cows in parity >3 and, in addition cows that calved in the summer or fall
season had significantly higher lactose (%) than cows that calved in the winter
season (P<O.05). These differences may be due ta increasing lactose yield with
increasing parities, and the different feeds available in each season.
2.4.4.2. Lactose Yield (kg)
Statistical analysis of lactose yield revealed no effect of treatment
(p>0.05), however there was an effect of sampling date (P<0.05) on lactose
yield. There were no differences (P>0.05) in lactose yield among treatments.
Results for overall least square means and least square means for individual
days are shawn in Tables 7 and 22, respectively.
Lactose (%) and lactose yield from Predefl2X treated cows increased
with milk yield throughout the trial but was not statistically different from
control cows. The increase was particularly significant for lactose yield and
lactose % between d 7 ta d 21. As expected, lactose yield for d 7, d 14, and d
21 were significantly higher than their respective d 1 values for Predefl 2X
treated cows (P<O.05). The simultaneous increase in lactose and milk yield is
expected as it plays a major raIe in milk synthesis. It is the major osmole in
milk and the process of synthesis of lactose is responsible for drawing water
into the milk as it is being formed in the mammary gland. In a study done by
Shamay et al. (2000), the concentration of lactose and monovalent ions
(sodium and potassium) was unaffected. However, in a study done by Schafer
87

et al. (1983), 300 mg of prednisolone acetate injected intramuscularly caused
an increase in Nalkg milk solids, but no significant changes in Klkg milk
solids within 8 h. In the present study, milk sodium and potassium levels in
Prede~ 2X treated cows were unaffected by treatment (P<0.05).
2.4.5. Milk potassium (mgIL)
Sodium and potassium in milk were analyzed to see if ion transport
across the mammary epithelial cell was affected by treatment. Results for
overall least square means and least square means for individual days are
shown in Tables 7 and 22, respectively.
Statistical analysis of milk potassium levels revealed no effect of
treatment (p>0.05). There were no differences (p>O.05) in potassium levels
among treatments on d 1.
2.4.6. Milk sodium (mgIL)
Statistical analysis of milk sodium levels revealed an effect of
treatment (P<0.05). PredefP 2X treated cows had significantly lower milk
sodium levels in comparison to Flucort® treated cows on d 1 (P<O.05). This
would imply that the administration ofPrede~ 2X prevented the release of Na
into the mammary epithelial cell thereby affecting the ion transport across the
mammary epithelial cell. Results for overall least square means and least
square means for individual days are shown in Tables 7 and 22, respectively.
2.4.7. Somatic cell count
Statistical analysis of sec revealed no effect of treatment (P>O.05),
however there was an efIect of sampling date and an interaction of parity and
sampling date (P<0.05) on sec. A significant difference (p>0.05) in sec was
observed on d 1 where Prede~ 2X treated cows had significantly higher
(P<0.05) sec than control cows. Results for overallleast square means and
least square means for individual days are shown in Tables 7 and 22,
respectively.
Cows treated with Predet4l> 2X experienced fluctuations in milk SCC
throughout the trial. Prede~ 2X treated cows had sec significantly higher
(P<O.05) than control cows, and numerically higher (P>O.05) than Flucort®
treated cows on d 1. Furthermore, sec on d 1 was significantly higher than d
7, d 14, and d 21. Prede~ 2X treated cows experienced a significant decrease
in milk sec by d 7, a numerical increase by d 14 and a numerical decrease by
88

the d 21. As sec decreased, lactose % increased and this is supported by
Hannon (1994) who found that elevated sec are associated with a decrease in
lactose because of reduced synthetic activity of the mammary tissue. An
analysis of the frequency of mastitis, as denoted by sec> 283,000 cells/ml
(Guidry, 1985; Reneau, 1986) indicative of cows with subclinical mastitis
revealed greater mastitis in the Prede:f 2X treated cows on d 1 and d 14, as
shown in Table 23. However, due to limited cow numbers used in the current
study, a concrete conclusion cannot be made and further studies are warranted.
3. Flucort® versus Prede~ 2X
3.1. Energy Status
There were no statistically significant differences between Prede:f 2X
and Flucort® treated cows in tenns of energy status, Le. values for glucose,
insu lin, NEF A and BHB between Prede:f 2X and Flucort® treated cows were
not significantly different. However, serum glucose values of Prede:f 2X were
numerically higher than Flucort® treated cows on aIl days throughout the trial.
Serum insulin values for Flucort® treated cows were numerically higher than
Prede:f 2X treated cows on d 1 and d 21, whereas Prede:f 2X treated cows
had numerically higher insulin values on d 1, d 14, and d 28. Serum NEFA
values for Prede:f 2X treated cows were numerically lower than Flucort®
treated cows from d 0 to d 14. Serum BHB values for Prede:f 2X treated cows
were numerically lower than Flucort® treated cows.
3.2. Serum Mineral Status
There were no statistically significant differences between Prede:f 2X
and Flucort® treated cows in tenns of serum minerai status. Phosphorus,
sodium, chloride, and magnesium values were not statistically different with
the exception of calcium and potassium on d 1.
Serum calcium values for Flucort® treated cows were numerically
higher than Prede:f 2X treated cows on d 0, dl, d 14, d 21 and d 28. Flucort®
treated cows had significantly higher calcium values than Predet«' 2X treated
cows on d 1 (P<0.05). Serum phosphorus values for Prede:f 2X treated cows
were numerically higher than Flucort® treated cows from dIto d 28. Serum
sodium values for Prede:f 2X treated cows were numerically higher than
Flucort® treated cows on d 1, d 21, and d 28. Serum sodium values for
Flucort® treated cows were numerically higher than Prede~ 2X treated cows
89

on d 0, d 7, and d 14. Serum potassium values for Flucort® treated cows were
always numerically higher than Predet«' 2X treated cows except on d 7, d 21
and d 28. PredefID 2X treated cows had statistically lower potassium values
than Flucort® treated cows on d 1. This decrease in serum potassium May be
due to the hypokalemic effect of Predet«' 2X. Serum chloride values for
Prede~ 2X treated cows were numerically higher than Flucort® treated cows
from d 1, d 21 and d 28. Serum chloride values for Flucort®treated cows were
numerically higher than Prede~ 2X treated cows from d 0, d 7 and d 14.
Serum Magnesium values for Prede~ 2X treated cows were numerically
higher than Flucort® treated cows from d 0 to d 28.
3.3. Immune Function
There were no statistical differences between Prede~ 2X and Flucort®
treated cows for parameters of immune function. Numerically, Flucort® treated
cows experienced a higher ConA~induced lymphocyte proliferation on d 0
compared to Prede~ 2X treated cows, however numerically Prede~ 2X
treated cows experienced a higher ConA·induced lymphocyte proliferation on
d 7 compared to Flucort® treated cows. Flucort® treated cows experienced the
largest decrease in ConA .. induced lymphocyte proliferation.
Numerically, Predet«> 2X treated cows experienced a higher antibody
production from d 0 to d 28 compared to Flucort® treated cows.
3.4. Milk Status
There were no statistical differences between Predef' 2X and Flucort®
treated cows. Milk fat (% and yield), milk protein yield (kg), milk lactose (%
and yield), milk somatic cell count and milk yield, with the exception of milk
protein (%) on d 1.
Statistically, milk prote in % for Prede~ 2X treated cows was higher
than Flucort® treated cows on d 1. Numerically, milk protein % for Prede~ 2X
treated cows was higher than Flucort® treated cows on d 14. Numerically, milk
protein % for Flucort® treated cows was higher than Predet«' 2X treated cows
on d 7 and d 21. The higher prote in percentage observed in Predet«' 2X treated
cows May provide a beneficial effect considering that during the first few
weeks of lactation the cow is typically in negative energy and prote in balance
and these May both impact and limit milk protein synthesis (Green et al.,
90

1999). Numerically, milk protein yield for Prede-r 2X was higher than
Flucort® treated cows on d 1, d 7 and d 14.
Numerically, milk fat % for Flucort® treated cows was higher than
Predef\' 2X treated cows on d 1 and d 14. Numerically, milk fat yield (kg) for
Flucort® treated cows was higher than Predef!> 2X treated cows on d 1, d 14
and d 21. Numerically, milk lactose (% and yield) for Flucort® treated cows
was higher than Prede-r 2X treated cows on d 1, d 14 and d 21. Numerically,
milk sec for Flucort® treated cows was lower than Predefll 2X treated cows
on dl, d 14 and d 21. .
Numerically, average milk yield for Flucort® treated cows was higher
than Predefll 2X treated cows for the fust week and fourth week post-calving,
whereas average milk yield for Predefll 2X treated cows was higher than
Flucort® treated cows for the second and third week post-calving.
Numerically, Prede~ 2X treated cows experienced higher milk sodium
and milk potassium values than Flucort® treated cows on d 1. In the present
study, there was no statistically significant difference between treatments for
milk potassium levels (P>O.05). However, Prede-r 2X treated cows had
significantly lower (P<O.05) milk sodium levels than Flucort® treated cows
91

VI. Conclusion
In conclusion, the following new discoveries were found in the present
study: (1) There were no effect oftreatments on any of the parameters except
for milk sodium on d 1. (2) There were very few statistical differences
between treatments in tenns of energy parameters. Predef!' 2X treated cows
had significantly lower insulin values on d 0 and significantly higher BHB
values on d 21. Flucort® treated cows had significantly lower NEF A values on
d l, significantly greater insulin values on d l, significantly greater BHB
values on d 21 and d 28. (3) There were very few statistical differences
between treatments in tenns of serum mineraI parameters. Predef!' 2X treated
cows had significantly higher calcium values on d 1 and significantly lower
potassium values than control and Flucort® treated cows on d 1. Flucort®
treated cows had significantly lower calcium and phosphorus levels on d 1. (4)
The administration of Flucort® or Predefl> 2X on d 0 did not cause
immunosuppression. (5) There were very few statistical differences between
treatments in tenns of milk parameters. Predef!' 2X treated cows had
significantly higher milk protein % than both control and Flucort® treated
cows. Predef!' 2X treated cows had significantly higher milk sec than control
cows.
Based on the statistically significant data in this study, the use of
glucocorticoids Flucort® and Predef!' 2X in a single intramuscular injection on
d 1 for the treatment of ketosis is not warranted. In this study there is not
enough evidence to suggest such a treatment. Glucocorticoids, although
approved for the therapy of ketosis, may not be the best treatment option for
post-calving dairy cattle. From this study, dairy producers and veterinarians
may want to rethink the way that transition dairy cattle are treated in order to
prevent such metabolic diseases as ketosis and negative energy balance.
Future experiments for the treatment of ketosis could include the
combination therapy of Flucort® and intravenous glucose; FJucort® and
endogenous dextrose; Flucort® and exogenous insulin; Predef!' 2X and
intravenous glucose; Predef!' 2X and endogenous dextrose; Predef!' 2X and
exogenous insulin; larger dosage than 10mg of Flucort® or Predef!' 2X; second
or successive doses Flucort® or Predef!' 2X; administration of treatments on
92

other days in the fresh cow period rather than the day of calving; and the effect
of treatment on ion transport across mammary epithelial ceUs. The effects of
Flucort® and Predefl> 2X is not well documented on immune function and milk
composition. It would be interesting to continue to evaluate these experimental
designs on blood metabolites, immunologie, and milk composition parameters
with a larger sample size.
93

VII. References
Acre, K. June 1998. Monitoring transition cows·Here are checks to make on close-up eows and rations. Page 451. Hoard's Dairyman.
Anderson, B.H., D.L. Watson, and I.G. Colditz. 1999. The effect of dexamethasone on sorne immunological parameters in cattle. Veto Res. Commun. 23:399-413.
Andersson, L., and U. Emanuelson. 1985. An epidemiological study of hyperketonaemia in Swedish dairy cows; determinants and the relation to fertility. Prev. Veto Med. 3:449-462.
Andersson. L. 1988. Subelinical ketosis in dairy eows. Vet. Clin. N. Amer.Food Animal Practice. 4:233-251.
Arieli, A, J. E. Vallimont, Y. Aharoni, and G. A. Varga. 2001. Monensin and growth hormone effeets on glucose metabolism in the pre-calving eow. J. Dairy Sci. 84:2770-2776.
Baird, G. D.,M. A Lomax, H. W. Symonds, and S. R. Shaw. 1980. Net hepatic and splanchnic metabolism of lactate, pyruvate and propionate in dairy cows in vivo in relation to lactation and nutrient supply. Biochem.1. 186:47-57.
Baird, G. D. 1982. Primary ketosis in the high-producing dairy cow: clinical and subclinical disorders, treatment, prevention, and outlook. J. Dairy Sei. 65:1-10.
Bareille, N., and P. Faverdin. 1996. Lipid metabolism and intake behavior of dairy cows: Effects of intravenous lipid and p-adrenergic supplementation. J. Dairy Sci. 79:1204-1220.
Bartlett, P. C., G. Y. Miller, C. R. Anderson, and J. H. Kirk. 1990. Milk production and somatic eeU count in Michigan Dairy Herds. J. Dairy Sei. 73 :2794-2800.
Bauman, D. E., and W. B. Currie. 1980. Partitioning of nutrients during pregnancy and lactation: a review of mechanisms involving homeostasis and homeorhesis. J. Dairy Sei. 63:1514-1529.
Beaudeau, F., A.Henken, C. Fourichon, K. Frankena, and H. Seegers. 1993. Associations between health disorders and culling of dairy cows: A review. Livest. Prod. Sei. 35:213-236.
Bell, A W. 1995. Regulation of organic nutrient metabolism during transition from late pregnancy to early lactation. J. Anim. Sei. 73:2804-2819.
94

Bell, A.W., and D.E. Bauman. 1997. Adaptations of glucose metabolism during pregnancy and lactation. J. Mammary Gland Biol and Neoplasia. 2:265-278.
Bell, A. W.,W. S. Burhans, and T. R Overton. 2000. Protein nutrition in late pregnancy, maternai protein reserves and lactation performance in dairy cows. Proc. Nutr. Soc. 59:119-126.
Bergman, E. N., and R N. Heitmann. 1978. Metabolism of amino acids by the gut, liver, kidneys, and peripheral tissues. Fed. Proc. 37:1228.
Bertics, S. J., R R Grummer, C. Cadorniga-Valino, and E. E. Stoddard. 1992. Effect of pre-calving dry matter intake on liver triglyceride concentration and early lactation. J. Dairy Sei. 75:1914-1922.
Blalock, J.E. 1994. The syntax of immune-neuroendocrine communication. Immunol. Today. 15:504-511.
Block, S. S., W. R Butler, R A. Ehrhardt, A. W. Bell, M. E. Van Amburgh, and Y. R Boisclair. 2001. Decreased concentration of plasma leptin in periparturient dairy cows is caused by negative energy balance. J. Endocrinol. 171:339-348.
Bremmer, D. R., L. D. Ruppert, J. H. Clark, and J. K. Drackley. 1998. Effects of chain length and unsaturation of fatty acid mixtures infused into the abomasum oflactating dairy cows. J. Dairy Sei. 81:176-188.
Burton, lL., and RJ. Erskine. 2003. Immunity and mastitis. Sorne new ideas for an old disease. The Veterinary Clinics of North America Food and Animal Praetice. 19: 1-45.
Burton, J.L., B.W. MeBride, B.W. Kennedy, J.H. Burton. T.H. Elsasser, and B. Woodward. 1992. Contact sensitivity and systemic antibody responses in dairy cows treated with recombinant bovine somatotropin. J. Dairy Sei. 75:741-755.
Burton, J. L., M. E. Kehrli, Jr., S. Kapil, and R L. Horst. 1995. Regulation of L-selection and CD 18 on bovine neutrophils by glucocorticoids: effects of cortisol and dexamethasone. J. Leukoc. Biol. 57:317-325.
Burton, J.L., and M.E. Kehrli, Jr. 1996. Effects of dexamethasone on bovine circulating T lymphocyte populations. J. Leukoc. Biol. 59:90-99.
Butler, T.M., and J.M. Elliot. 1970. Effect of diet and glucocorticoid administration on liver phosphenolpyruvate carboxykinase activity in the dairy cow. J. Dairy Sei. 53:1727pI733.
95

Butler, W. R, and R D. Smith. 1989. Interrelationships between energy balance and post-calving reproductive function in dairy eattle. 1. Dairy Sei. 72:767-783.
Cai, T., P. G. Weston, L. A. Lund, B. Brodie, D. J. McKenna, and W.C. Wagner. 1994. Association between neutrophil functions and periparturient disorders in cows. Am. J. Vet. Res. 55:934-943.
Carroll, D. 1., M. 1. Jerred, R R Grummer, D. K. Combs, R A. Pierson, and E. R Hauser. 1990. Effects of fat supplementation and immature alfalfa to concentrate ratio on plasma progesterone, energy balance, and reproductive traits of cattle. J. Dairy. Sci. 73:2855-2863.
Cer6n-Munoz, M., H. Tonhati, 1. Duarte, J. Oliveira, M. Munoz-Berrocal, and H. Jurado-Gâmez. 2002. Factors Affeeting Somatic Cell Counts and Their Relations with Milk and Milk Constituent Yield in Buffaloes. J. Dairy Sei. 85 :2885-2889.
Chew, B. P., R. E. Erb, J. F. FessIer, C. J. Callahan, and P. V. Malven. 1979. Effects of ovariectomy during pregnancy and of prematurely induced calving on progesterone, estrogens, and ealving traits. J. Dairy Sci. 62:557.
Chopra, 1. J., D. H. Solomon, U. Chopra, S. Y. Wu, D. A. Fisher, and Y. Nakamura. 1978. Pathways of metabolism of thyroid hormones. Recent Prog. Horm. Res. 14:521-567.
Clark, A., and M. Denborough. 1971. The interaction of Concanavalin A with blood-group-substanee glycoproteins from human secretions. Biochem. J. 121:811.
Comin, A., D. Gerin, A. Cappa, V. Marehi, R. Renaville, M. Motta, U. Fazzini, and A. Prandi. 2002. The effect of an acute energy deficit on the hormone profile of dominant follieles in dairy cows. Theriogenology. 58:899-910.
Convey, E.M., L.S. Miller, and H.A. Tucker. 1970. Acute effects of 9afluoroprednisolone acetate on blood leukocytes and somatic cell content of milk. J. Dairy Sci. 54:360-363.
Curtis, C. R, H. N. Erb, C. J. Sniffen, R. D. Smith, and D. S. Kronfeld. 1985. Path analysis of dry period nutrition, post-calving metabolic and reproductive disorders, and mastitis in Holstein eows. 1. Dairy Sei. 68:2347-2360.
Danfaer, A., V. Tetens, and N. Agergaard. 1995. Review and an experimental study on the physiological and quantitative aspects of gluconeogenesis in lactating ruminants. Comp. Biochem. Physiol. IIIB:201-210.
Dann, H. M., G. A. Varga, and D. E. Putnam. 1999. Improving Energy Supply to Late Gestation and Early Post-ealving Dairy Cows. J Dairy Sci. 82:1765-1778.
96

de Boer, G.,A.Trenkle, and J. W. Young. 1985. Glucagon, insulin, growth hormone, and sorne blood metabolites during energy restriction ketonemia of lactating cows. J. Dairy Sci. 68:326-337.
Delavaud, C., F. Bocquier, Y. Chilliard, D. H. Keisler, A. Gertler, and G. Kann. 2000. Plasma leptin determination in ruminants: Effect of nutritional status and body fatness on plasma leptin concentration assessed by a specific RIA in sheep. 1. Endocrinol. 165:519-526.
Delavaud, C., A. Ferlay, Y. Faulconnier, F. Bocquier, G. Kann, and Y. Chilliard. 2002. Plasma leptin concentration in adult cattle: Effects of breed, adiposity, feeding level, and meal intake. J. Anim. Sci. 80:1317-1328.
Detilleux, J. C., M. E. Kehrli, Jr., 1. R Stabel, A. E. Freeman, and D. H. Kelley. 1995. Study of immunological dysfunction in periparturient Holstein cattle selected for high and average milk production. Vet. Immunol. Immunopath. 44:251-267.
Derijk. R., E.M. Sternberg. 1994. Corticosteroid action and neuroendocrineimmune interactions. Ann. N.Y. Acad. Sei. 746:33-44.
DiCostanzo, A., J. E. Williams, and D. H. Keisler. 1999. Effects of Short- or Long-Term Infusions of Acetate or Propionate on Luteinizing Hormone, Insulin, and Metabolite Concentrations in Beef Heifers. J. Anim. Sci. 77:3050-3056.
Doherty. M.L., Bassett. H.F., Quinn. P.J., Davis. W.C., and M.L. Monaghan. 1995. Effects of dexamethasone on cell-mediated immune responses in cattle sensitized to Myeobacterium bovis. Am. J. Vet. Res. 56:1300-1306.
Dohoo, 1. R, and S. W. Martin. 1984. Subclinical ketosis: prevalence and associations with production and disease. Cano J. Comp. Med. 48:1-5.
Donkin, S. S., and L. E. Armentano. 1993. Preparation of extended in vitro cultures of bovine hepatocytes that are hormonally responsive. J. Anim. Sei. 71:2218-2227.
Drackley, 1. K. 1999. Biology of dairy cows during the transition period: The final frontier? J. Dairy Sci. 82:2259-2273.
Drackley, J. K., M. J. Richard, D. C. Beitz, and J. W. Young. 1992. Metabolic changes in dairy cows with ketonemia in response to feed restriction and dietary 1,3.butanediol. J. Dairy Sci. 75:1622-1634.
Draekley, J. K., T. R Overton, and G. N. Douglas. 2001. Adaptations of glucose and long-chain fatty acid metabolism in liver of dairy cows during the periparturient period. J. Dairy Sci. 84 (Suppl. E):ElOO-EI12.
97

Duffield, T. F., D. Sandals, K. E. Leslie, K. Lissemore, B. W. McBride, J. H. Lumsden, P. Dick, and R. Bagg. 1998. Effect ofpre-calving administration of monensin in a controlled-release capsule on post-calving energy indicators in lactating dairy cows. J. Dairy Sci. 81:2354-2361.
Duffield, T. F., K. E. Leslie, D. Sandals, K. Lissemore, B. W. McBride, J. H. Lumsden, P. Dick, and R Bagg. 1999. Effect ofpre-calving administration of a monensin controlled release capsule on cow health and reproduction. J. Dairy Sci. 82:2377-2384.
Duffield, T.F. 2000. Subclinical ketosis in lactating dairy cattle Veto Clin. N. Amer.-Food Animal Practice. 16:231-253.
Duffield, T., R Bagg, L. DesCoteaux, E. Bouchard, M. Brodeur, D. DuTremblay, G. Keefe, S. LeBlanc, and P. Dick. 2002. Pre-calving monensin for the reduction of energy associated disease in post-calving dairy eows. J. Dairy Sci. 85:397-405.
Duffield, T. F., S. LeBlanc, R Bagg, K. Leslie, J. Ten Hag, and P. Dick. 2003. Effect of a monensin controlled release capsule on metabolie parameters in transition dairy cows. J. Dairy Sei. 86:1171-1176.
Dunklee, T. S., A. E. Freeman, and D. H. Kell. 1994. Comparison of Hoisteins selected for high and average milk production. 2. Health and Reproductive Response to Selection For Milk. J. Dairy Sci. 77:3683-3690.
Ebling, F.J.P., R I. Wood, F. J. Karseh, L. A. Vannerson, J.M. Suttie, D. C. Bucholtz, R E. Schall, and D. L. Foster. 1990. Metabolic interfaces between growth and reproduction. III. Central mechanisms controlling pulsatile luteinizing hormone secretion in the nutritionally growth-limited female lamb. Endocrinology. 126:2719-2727.
Edgerton, L. A., and H. D. Hafs. 1973. Serum luteinizing hormone, prolaetin, glucocorticoid, and progestin in dairy cows from calving t 0 gestation. J. Dairy Sei. 56:451.
Edmonson, A. J., 1. J. Lean, L. D. Weaver, T. Farver, and G. Webster. 1989. A body condition scoring ehart for Holstein dairy cows. J. Dairy Sei. 72:68-78
Ehrhardt, R A., RM. Slepetis, J. Siegal-Willott, M. E. Van Amburgh, A. W. Bell, and Y. R Boisclair. 2000. Development of a specifie radioimmunoassay to measure physiological changes of circulating leptin in cattle and sheep. J. Endocrinol. 166:519-528.
Etherton, T. D., and D. E. Bauman. 1998. Biology of somatotropin in growth and lactation of domestic animais. Physiol. Rev. 78:745-761.
98

Franco. P., O. Marelli, D. Lattuda, V. Locatelli, D. Cocchi, E.E. Muller. 1990. Influence of growth hormone on the immunosuppressive effect of prednisolone in mice. Acta Endocrinologica. 173:339-344.
Franklin, S.T., J.W. Young., and B.J. Nonnecke. 1991. Effects of ketones, acetate, butyrate, and glucose on bovine lymphocyte proliferation. J. Dairy Sci. 74:2507-2514.
Fürll, M., and F. Jackel. 2005. Effects of glucocorticoids on paramteres of lipid metabolism, hepatic metabolism, haematological parameters and milk yield in high-tielding cows in early lactation. Berliner und Mucnchener Tierarztliche Wochenschrift. Schlutersche Verlagsgesellschaft mbH & Co. KG, Hannover, Germany. 118(5/6):247-254. Abstract (English)
Garnsworthy, P. C. 1988. The effect of energy reserves at calving on performance of dairy cows. Page 157 in Nutrition and Lactation in the Dairy Cow. P. C. Gamsworthy, ed. Butterworths, London, England.
Gilvert, RO., Y. T. Gr6hn, P.M. Miller, and D.J. Hoffinan. 1993. Effect of parity on periparturient neutrophil function in dairy cows. Vet. Imm. and Immunopathology. 36:75-82.
Gillund, P., O. Reksen, Y. T. Grhn, and K. Carlberg. 2001. Body condition related to ketosis and reproductive performance in Norwegian dairy cows. J. Dairy Sci. 84:1390-1396.
Glass, E. J., R A. Oliver, and R L. Spooner. 1990. Variation in T cell responses to ovalbumin in cattle: evidence for Ir gene control. Anim. Genet. 21:15-28.
Goetsch, D.D., L.E. McDonald, and G. Odell. 1959. The effects of four synthetic corticosteroids on leukocytes, blod glucose, and plasma sodium and potassium in the cow. Am. J. Veto Research. 20:697.
Goff, J. P., and R L. Horst. 1997. Physiological changes at calving and their relationship to metabolic disorders. J. Dairy Sci. 80:1260-1268.
Goff, J. P., and R. L. Horst. 1998. Use ofhydrochloric acid as a source of anions for prevention ofmilk fever. J. Dairy Sci. 81:2874-2880.
Goff, J.P. and K. Kimura. 2002. Interactions between metabolic disease and the immune system: Why cows are likely to develop mastitis at freshening. Mid-South Ruminant Nutrition Conference. Dallas/Fort Worth Airport.
Green, B. L., B. W. McBride, D. Sandals, K. E. Leslie, R. Bagg, and P. Dick. 1999. The impact of a monensin controlled-release capsule on subclinical ketosis in the transition dairy cOW. J. Dairy Sci. 82:333-342.
99

Greenfield, RB., M. J. Cecava, and S. S. Donkin. 2000. Changes in mRNA expression for gluconeogenic enzymes in liver of dairy cattle during the transition to lactation. J. Dairy Sci. 83:1228-1236.
GrtShn, Y. T., and H. N. Erb. 1989. Epidemiology ofmetabolic disorders in dairy cattle: Associations among host characteristics, disease, and production. J. Dairy Sci. 72:1876-1885.
Gruffat, D., D. Durand, Y. Chilliard, P. Williams, and D. Bauchart. 1997. Hepatic gene expression of apolipoprotein BI 00 during early lactation in underfed, high produeing dairy cows. J. Dairy Sci. 80:657-666.
Grum, D. E. 1994. Hepatic lipid metabolism and peroxisomal ~-oxidation in dairy cows. Ph.D. diss., Univ. Illinois, Urbana.
Grum, D. E., J. K. Drackley, R. S. Younker, D. W. LaCount, and l l Veenhuizen. 1996. Nutrition during the dry period and hepatic lipid metabolism ofperiparturient dairy cows. J. Dairy Sci. 79:1850-1864.
Grummer, R R, and C. L. Davis. 1984. Plasma concentrations and lipid composition of lipoproteins in lactating dairy cows fed control and high grain diets. J. Dairy Sei. 67:2894-2901.
Grummer, R R, and D. J. Carroll. 1988. A review oflipoprotein cholesterol metabolism: Importance to ovarian function. J. Anim .. Sei. 66:3160-3173.
Grummer, R R, S. J. Bertics, D. W. LaCount, J . A. Snow, M. R Dentine, and R. H. Stauffacher. 1990. Estrogen induction offatty liver in dairy cattle. J. Dairy Sei. 73:1537.
Grummer, R R 1993. Etiology of lipid-related metabolic disorders in periparturient dairy cows. J. Dairy Sci. 76:3882-3896.
Grummer, R R 1995. Impact of ehanges in organic nutrient metabolism on feeding the transition dairy cattle. J. Anim. Sei. 73:2820-2833.
Grummer, R R, P. C. Hofman, M. L. Luck, and S. J. Bertics. 1995. Effect of pre-calving and post-calving dietary energy on growth and lactation of primiparous cows. J. Dairy Sei. 78: 172.
Guidry, A. J. 1985. Mastitis and the immune system of the mammary gland. Pages 262-299 in Lactation. B. L. Larson, ed. The Iowa State University Press, Ames, lA.
Gunnink, J. W. 1984. Pre-calving leucotytie activity and retained placenta. Veto Q. 6:52-54.
Hatziolos, B.B., and lC. Shaw. 1950. An approach to the etiology of ketosis in dairy cows. l Dairy Sei. 33:387.
100

Harmon, R. 1. 1994. Symposium: Mastitis and genetie evaluation for somatie ceU count-Physiology of mastitis and factors affecting somatic cell counts. 1. Dairy Sei. 77:2103-2112.
Harris, H. W., 1. E. Gosnell, and Z. L. Kumwenda. 2000. The lipemia of sepsis: Triglyceride-rich lipoproteins as agents of innate immunity. 1. Endotoxin Res. 6:421-430.
Heinonen, K., Y. Grôhn, L. A. Lindberg, and M. Alanko. 1987. The effect of mild fat infiltration in the liver on the fertility of Finnish Ayrshire cows. Acta Veto Scand. 28:151-155.
Herdt, T. H. 1991. Relationship of fat metabolism to health and performance in dairy cattle. Bov. Pract. 26:92-95.
Heyneman, R., C. Burnevich, and R. Vercauteren. 1990. Interaction between the respiratory burst activity of neutrophil leukocytes and experimentally induced Escherichia Coli mastitis in cows. J. Dairy Sei. 73:985-994.
Higgins, R. J., and W. S. Anderson. 1983. Fat cow syndrome in a British dairy herd. Veto Rec. 113:461-463.
Hoeben, D., C. Burvenich, A.M. Massart-Leen. 1998. Glucoeorticosteroids and in vitro effects on chemiluminescence of isolated bovine blood granulocytes. Eur. J. Pharm. 354: 197-203.
Hoeben, D., E. Monfardini, G. Opsomer, C. Burvenich, H. Dosogne, A. De Kruif, and J-F. Beckers. 2000. Chemilumineseence of bovine polymorphonuc1ear leuokocytes during the periparturient period and relation with metabolic markers and bovine pregnancy-associated glycoprotein. J. Dairy Res. 67:249-259.
Holt C. 1993. Interrelationship of the concentrations of sorne ionic constituents of human milk and comparison with eow and goat milks. Comp. Biochem. Physiol. 104:35-41.
Holtenius, P., G. Olsson, M. Emanuelson, and H. Wiktorsson. 1996. Effects of different energy levels, eoneentrate/forage ratios and lipid supplementation to the diet on the adaptation of the energy metabolism at calving in dairy eows. J. Veto Med. 43:427-435.
Holter, J. B., M. J. Slotnick, H. H. Hayes, C. K. Bozak, W. E. Urban, Jr., and M. L. McGilliard. 1990. Effect of pre-calving dietary energy on condition score, post-calving energy, nitrogen partitions, and lactation production responses. J. Dairy Sei. 73:3502.
Hortet, P., F. Beaudeau, H. Seegers, and C. Fourichon. 1999. Reduction in milk yield associated with somatic cell counts up to 600,000 cells/mi in French Holstein cows without clinical mastitis. Livest. Prod. Sci. 61 :33-42.
101

Ishikawa, H. 1987. Observation of lymphocyte function in perinatal cows and neonatal calves. Jpn. J. Veto Sci. 49:469.
Ishikawa, H., T. Shirahata, and K. Hasegawa. 1994. Interferon gamma production of mitogen stimulated peripherallymphocytes in perinatal cows. 1. Veto Med. Sciences. 56:735-738.
Ingvartsen, K. L. and J. B. Andersen. 2000. Symposium: Dry matter intake of lactating dairy cattle - Integration of metabolism and intake regulation: A review focusing on periparturient animaIs. 1. Dairy Sei. 83:1573-1597.
Jochle, W. 1973. Corticosteroid-induced calving in domestic animaIs. Ann. Rev. Pharmacol. 13:33-55.
Jones, G. M. 1986. Symposium: Reducing somatic cell counts: Meeting the 1986 challenge-Impact on producer and processor. J. Dairy Sei. 69:1699-1707.
Jorritsma, R., H. Jorritsma, Y. H. Schukken, P. C. Bartlett, T. Wensing, and G. H. Wentink. 2001. Prevalence and indicators of post partum fatty infiltration of the liver in nine commercial dairy herds in The Netherlands. Livest. Prod. Sei. 68:53-60.
Jorritsma, R., T. Wensing, T. A. M. Kruip, P. L. A. M. Vos, and J. P. T. M. Noordhuizen. 2003. Metabolic changes in early lactation and impaired reproductive performance in dairy cows. Veto Res. 34:11-26.
Kadokawa, H., D. Blache, Y. Yamada, and G. B. Martin. 2000. Relationships between changes in plasma concentrations of leptin before and after calving and the timing of first post-calving ovulation in high-producing Holstein dairy cows. Reprod. Fertil. Dev. 12:405-411.
Kauppinen, K., and Y. Grohn. 1984. Treatment of bovine ketosis with invert sugar, glucocorticoids, and propylene glycol. Acta Veto Scand. 25:467-479.
Kehrli, Jr., M. E., B. J. Nonnecke, and J. A. Roth. 1989a. Alterations in bovine neutrophil function during the periparturient period. Am. 1. Veto Res. 50:207-214.
Kehrli, Jr., M. E., B. J. Nonnecke, and J. A. Roth. 1989b. Alterations in bovine lymphocyte function during the periparturient period. Am. J. Veto Res. 50;215-220.
Kehrli, Jr., M. E., J.L. Burton, B. J. Nonnecke, and E.K. Lee. 1999. Effects of stress on leukocyte trafficking and immune responses: Implications for vaccination. Advances in Veto Med. 41 :61-81.
102

Kimura, K., J.P. Goff, M.E. Jr. Kehrli, and J.A. Harp. 1999. Phenotype analysis of peripheral blood mononuclear cells in periparturient dairy cows. J. Dairy Sei. 82:315-319.
Koprowski, J. A, and H. A. Tucker. 1973. Bovine serum growth hormone, corticoids and insulin during lactation. Endocrinology. 93:645-651.
Komfeld, R., and C. Ferris. 1975. Interaction of immunoglobulin glycopeptides with Concanavalin A. J. Biol. Chem. 250:2614.
Kunz, P. L., J. W. Blum,!. C. Hart, H. Bickel, and J. Landis. 1985. Effects of different energy intakes before and after calving on food intake, performance and blood hormones and metabolites in dairy cows. Anim. Prod. 40:219.
Lacetera, N., U. Bemabucei, B. Ronchi, and A Nardone. 2001. Effects of subclinical pregnancy toxemia on immune responses in sheep. Am. J. Vet. Res. 62: 1020-1024.
Lacetera, N., D. Scalia, O. Franci, U. Bemabucei, B. Ronchi, and A. Nardone. 2004. Effects of nonesterified fatty acids on lymphocyte function in dairy heifers. J. Dairy Sei. 87:1012-1014.
Langston, V. C. 1993. Therapeutic management of inflammation. Am. J. Vet. Res. 59:37-43.
Lee, E.K., and M. Kehrli. 1998. Expression of adhesion molecules on neutrophils of Lescourret, F., and J. Coulon. 1994. Modeling the impact ofmastitis on milk production by dairy cows. J. Dairy Sci. 77:2289-2301.
Leslie, K.E., T.F. Duffield, Y.H. Schukken, and SJ. LeBlanc. 2000. The influence of negative energy balance on udder health. National Mastitis Council Regional Meeting Proceedings. Pages 25-33.
Leung, K., and A. Munck. 1975. Peripheral actions of glucocorticoids. Annu. Rev. Physiol. 37:245-272.
Li, P. S., and W. C. Wagner. 1983. In Vivo and In Vitro studies on the effect of adrenocorticotropic hormone or cortisol on the pituitary response to gonadotropin releasing hormone. Biol. Reprod. 29:25-37.
Li, Y.C., D. R. Ledoux, A.J. Bermudez, K.L. Fritsche, and G.E. Rottinghaus. 2000. Effects of Moniliformin on Performance and Immune Function of Broiler Chicks. Poultry Sci. 79:26-32.
Lin, C. Y., A J. McAllister, and A J. Lee. 1984. Multitrait estimation of relationships of first lactation yields to body weight changes in Holstein heifers. J. Dairy Sci. 68:2954.
103

Lohuis, lAC.M., W. Van Leeuwen, and lH.M. Verheijden. 1998. Effect of dexamethasone on experimental Escherichia coli mastitis in the cow. 1 Dairy Sei. 71 :2782-2789.
Lomax, M. A, and G. D. Baird. 1983. Blood flow and nutrient exehange across the liver and gut of the dairy cow. Effects oflactation and fasting. Br. J. Nutr.49:481-496.
Lucy, M. C., C. R. Staples, F.M.Michel, and W. W. Thatcher. 1991. Energy balance and size and number of ovarian foUicles detected by ultrasonography in early post-calving dairy cows. J. Dairy Sci. 74:473-482.
Mallard, B.A L.C. Wager, M.J. Ireland, and J.C.M. Dekkers. 1997. Effects of growth hormone, insulin-like growth factor-l, and cortisol on periparturient antibody response profiles of dairy cattle. Veto Imm. and Immunopath. 60: 61-76.
Mallard, B. A J. C. Dekkers, M. 1. Ireland, K. E. Lelsie, S. Sharif, C. L. Vankampen, L. Wagter, and B. N. Wilkie. 1998. Symposium: Bovine immunology. Alteration in immune responsiveness during the peripartum period and its ramification on dairy cow and calfhealth. J Dairy Sci. 81:585-595.
Manns, J. G., 1 M. Boda, and R. F. Willes. 1967. Probable role ofpropionate and butyrate in control of insulin secretion in sheep. Am. 1. Physiol. 212:756-764.
Mehrzad, J., H. Dosogne, E. Meyer, R. Heyneman, an C. Burvenich. 2001. Respiratory burst activity of blood and milk neutrophils in dairy cows during different stages oflactation. J. Dairy Res. 68:399-415.
Meister, B. 2000. Control of food intake via leptin receptors in the hypothalamus. Vit. Hormones. 59:265-304.
Morin, D.E. 2004. Beyond antibiotics -What else can we do? Pages 13-23 in NMC Annual Meeting Proceedings.
Nagahata. H., Ogawa, A., Sanada, Y., Noda, H., Yamamota. S. 1992. Peripartum changes in antibody producing capability of lymphocytes from dairy cows. Vet. Q. 14:39-40.
National Research Council. 2001. Nutritional Requirements of Dairy Cattle. 7th rev. ed. Natl. Aead. Sei., Washington, OC.
Neff. A W., N.D. Conner, and R.S. Bryan. 1960. Studies on 9afluoroprednisolone acetate, a new synthetic corticosteroid for the treatment of ketosis. J. Dairy Sci. 43:553.
104

Nielen, M., M.G.A. Aarts, A.G.M. Jonkers, T. Wensing, and Y. H. Schukken. 1994. Evaluation of two cowside tests for the detection of subclinical ketosis in dairy cows. Can. Veto J. 35: 229-232.
Nonnecke, B.J., J.L. Burton, and M.E. Kehrli. 1997. Associations between function and composition of blood mononuclear leukocyte populations from Holstein bulls treated with dexamethasone. J. Dairy Sei. 80:2403-2410.
Nguyen D.A.D., and M.C. Neville M.C. 1998. Tight junction regulation in the mammary gland. J. Mammary Gland Biol. Neoplasia. 3:233-246.
Opsomer, G., P. Mijten, M. Coryn, and A. de Kruif. 1996. Post-calving anoestrus in dairy cows: A review. Veto Q. 18:68-75.
Osmundsen, H., J. Bremer, and J. 1. Pedersen. 1991. Metabolic aspects of peroxisomal J}-oxidation. Biochim. Biophys. Acta. 1085:141-158.
Otto, K. L., J. D. Ferguson, D. G. Fox, and C. J. Sniffen. 1991. Relationship between body condition score and composition of ninth to eleventh rib tissue in Holstein dairy cowS. J. Dairy Sei. 74:852-859.
Overton, T. R. 1998. Substrate utilization for hepatic gluconeogenesis in the transition dairy cow. In Proc. Cornell Nutr. Conf. Feed Manuf., Cornell Univ., Ithaca, NY, pp. 237-246.
Overton, T. R., G. Bernai-Santos, J. W. Perfield II, and D. E. Bauman. 2001. Effects of feeding conjugated linoleic fatty acids (CLA) on metabolism and performance oftransition dairy cows. Cornell Nutr. Conf. 63:179-187.
Overton, T.R., and M. R. Waldron. 2004. Nutritional management of transition dairy cows: Strategies to optimize metabolic health. J. Dairy Sei. 87:(E. Suppl.):EI05-EI19.
Owen, J. B., R. F. E. Axford, and S. C. Bishop. 2000. Mastitis in Dairy Cattle in Breeding for Disease Resistance in Farm Animais. R. F. E. Axford, S. C. Bishop, F. W. Nicholas, and J. B. Owen, eds. CAB International.
Paape, M., J. Mehrzad, X. Zhao, J. Detilleux, and C. Burvenich. 2002. Defense of the bovine mammary gland by polymorphonuclear neutrophil leukocytes. J. Mammary Gland Biol. Neoplasia. 7:109-121.
Peaker M. 1977. The aqueous phase ofmilk: Ion and water transport. Symp. Zool. Soc. Lond. 41:113-120.
Perkins, K. H., M. J. VandeHaar, R. J. Tempelman, and J. L. Burton. 2001. Negative energy balance does not decrease expression of leukocyte adhesion or antigen-presenting molecules in cattle. J. Dairy Sei. 84:421-428.
105

Philipp, H., L. Goossens, J. Limper, and J.F. Quirke. 1991. EfIect of dexamethasone isonicotinate on milk yield in ketotis cows. Veto Record. 128:427.
Preisler, M. T., P.S.D. Weber, R. J. Tempelman, R. J. Erskine, H. Hunt, and J. L. Burton. 1999. Glucocorticoid receptor downregulation in neutrophils of periparturient cows. Am. J. Vet. Res. 61:14-19.
Preisler, M. T., P.S.D. Weber, R. J. Tempelman, R. J. Erskine, H. Hunt, and J. L. Burton. 2000. Glucocorticoid receptor expression profiles in mononuclear leukocytes ofperiparturient Holstein cows. J Dairy Sei. 83:38-47.
Pruett, J.H., W.F. Fisher, J.R. DeLoaeh. 1987. EfIect of dexamethasone on selected parameters of the bovine immune system. Vet. Res. Commun. 11:305-323.
Radostits, O. M., K. E. Leslie, and 1. Fetrow. 1994. Dairy health and production management program. Pages 97-140 in Herd Health: Food Animal Production Medicine. 2nd ed. W. B. Saunders Co., Philadelphia, PA.
Radostits, O.M., Gay, C.C., Blood, D.C., and K.W. Hinchcliff. 2000. Veterinary medicine: A textbook of the diseases of cattle, sheep, pigs, goats and horses. 9th ed. London: W.B. Saunders Company Ltd; Pages 137-145.
Reneau, J. K. 1986. Effective use of dairy herd improvement somatie cell counts in mastitis control. 1. Dairy Sci. 69: 1708-1720.
Reynolds, C. K., G. B. Huntington, H. F. Tyrrell, and P. J. Reynolds. 1988. Net metabolism of volatile fatty acids, D-BHB, nonesterified fatty acids, and blood gasses by portaldrained viscera and liver of lactating Holstein cows. J. Dairy Sei. 71:2395-2405.
Rodriguez-Zas, S. L., D. Gianola, and G. E. Shook. 2000. Evaluation of models for somatic eell score lactation patterns in Holsteins. Livest. Prod. Sei. 67:19-30.
Roth, 1. A., and M. L. Kaeberle. 1982. EfIect of glucocorticoids on the bovine immune system. JAVMA. 180:894-901.
Ruscetti, F., and P. Cherveniek. 1975. Regulation of the release of colonystimulating activity from mitogen-stimulated lymphocytes. 1. Immunol. 114:1513.
Saad, A.M., Concha, C., Astrom, G. 1989. Alterations in neutrophil phagocytosis and lymphocyte blastogenesis in dairy cows around calving. J. Veto Med. 36:337-345.
Sano, H., Nakai, M., Kondo, T. & Terashima, Y. 1991. Insulin responsiveness to glucose and tissue responsiveness to insulin in laetating,
106

pregnant, and nonpregnant, nonlactating beef cows. J. Anim. Sci. 69:1122-1127.
SAS Institute Inc., 1999. SAS/STAT User's Guide, Version 8.0. Cary, NC, USA.
Seal, C. J., and C. K. Reynolds. 1993. Nutritional implications of gastrointestinal and liver metabolism in ruminants. Nutr. Res. Rev. 6:185-208.
Seifi. H., S. LeBlanc, K. Leslie, and T. Duffield. 2006. Effect of isoflupredone acetate with or without long acting insulin on postparturient energy metabolism in lactating dairy cows. Abstract.
Schafer, M. A. Uhlig, M. Anke, and H. Kirbach. 1983. Lithium, sodium, potassium, calcium and magnesium in body fluids of dairy cows after administration of glucocorticoids. Spurenelement-Symposium. 4(Lithium): 186-192. Abstract (English)
Shafer-Weaver, K.A., G.M. Pighetti, L.M. Sordillo. 1996. Diminished mammary gland lymphocyte functions parallel shifts in trafficking patterns during the post-calving period. Proceedings of the Society for Experimental Biology and Medecine. 212:271-280.
Shafer-Weaver, K.A., and L.M. Sordillo. 1997. Bovine CD8+ suppressor lymphocyte alter immune responsiveness during the post-calving period. Veto Imm. and Immunopathology. 56:53-64.
Shafer-Weaver, K.A., C.M. Corl, and L.M. Sordillo. 1999. Shift in bovine CD4+ subpopulations increase T-helper-2 compared with T-helper-l effector ceUs during the post-calving period. J. Dairy Sci. 82:1696-1706.
Shamay, A., F. Shapiro, H. Barash, l, Bruckental, and N. Silanikove. 2000. Effect of dexamethasome on milk yield and composition in dairy cows. Annales de Zootechnie. 49:343-352.
Sheldon, 1. M., D. E. Noakes, A. N. Rycroft, D. U. Pfeiffer, and H. Dobson. 2002. Influence of uterine bacterial contamination after calving on ovarian dominant follicle selection and follicle growth and function in cattle. J. Reprod. Fertil. 123:837-845.
Shpigel, N.Y., R. Chen, Y. Avidar, and E. Bogin. 1996. Use of corticosteroids alone or in combined with glucose to treat ketosis in dairy cows. J. Am. Veto Med. Assoc. 208:1702-1704.
Sielman, E.S., N. Sattler, R W. Sweeney, RH. Whitlock, and RY., Reams. 1997. Hypokalemia syndrome in dairy cows: 10 cases (1992-1996). J. Amer. Veto Med. Assoc. 210:240-243.
107

Simmons, C. R, W. G. Bergen, M. J. Vandehaar, D. J. Sprecher, C. J. Sniffen, E. P. Stanisiewski, and H. A. Tucker. 1994. Protein and fat-metabolism in cows given somavubove before calving. J. Dairy Sci. 77:1835-1847.
Smith, K. L., D. A. Todhunter, and P. S. Schoenberger. 1985. Environmental mastitis: cause, prevalence, prevention. J. Dairy Sci. 68:1531-1553.
Spangelo, B.L., and W.C. Gorospe. 1995. Role of cytokines in the neuroendocrine-immune system axis. Front. Neuroendocrinol. 16, 1-22.
Spicer, L. J., W. B. Tucker, and G. D. Adams. 1990. Insulin-like growth factor-I in dairy cows: Relationships among energy balance, body condition score, ovarian activity, and estrous behavior. J. Dairy Sci. 73:929-937.
Spicer, L. J., R K. Vernon, W. B. Tucker, R P. Wettemann, J. F. Hogue, and G. D. Adams. 1993. Effects of inert fat on energy balance, plasma concentrations of hormones and reproduction in dairy cows. J. Dairy Sci. 76:2664-2673.
Spicer, L. J., and S. E. Echternkamp. 1995. The ovarian insu lin and insulinlike growth factor system with emphasis on domestic animaIs. Domest. Anim. Endocrinol. 12:223-245.
Spicer, L. J., and C. C. Francisco. 1997. The adipose obese gene product, leptin: evidence of a direct inhibitory role in ovarian function. Endocrinology. 138:3374-3379.
Spicer, L. J., and C. S. Chamberlain. 1998. Influence of cortisol on insulinand insulin-like growth factor 1 (IGF-I )-induced steroid production and on IGF-l receptors in cultured bovine granulose cells and thecal cells. Endocrine. 9:153-161.
Spicer, L. J., and C. C. Francisco. 1998. Adipose obese gene product, leptin, inhibits bovine ovarian thecal cell steroidogenesis. Biol. Reprod. 58:207-212.
Stafford, RO., L.E. Bames, B.J. Bowman, and M.M. Meinzinger. 1955. Glucocorticoid and mineralocorticoids activities of delta 1-fluorohydrocortisone. Proc Soc Exp Biol Med. 89:371-374.
Staple s, C. R, W. W. Thatcher, and J. H. Clark. 1990. Relationship between ovarian activity and energy status during the early post-calving period of high produeing dairy cows. J. Dairy Sei. 73:938-947.
Stelwagen. K., Farr. V.C., Davis. S.R, and C.K. Prosser. 1995. EGTAinduced disruption of epithelial cell tight junctions in the lactating caprine mammary gland. Am. J. Physiol. 269:848-855.
108

Stevenson, J. S., and E. P. Call. 1983. Influence of early estrus, ovulation, and insemination on fertility in post-ealving Holstein eows. Theriogenology. 19:367.
Stoebel, D. P., and G. P. Moberg. 1982. Effeet of adrenoeortieotropin and cortisol on luteinizing hormone surge and estrous behavior of eows. J. Dairy Sei. 65:1016-1024.
Stryer, L. 1981. Biochemistry. 2nd Ed., Freeman & Co., San Francisco.
Suriyasathapom, W., C. Heuer, E.N. Noordhuizen-Stassen, and Y.H. Schukk:en. 2000. Hyperketonemia and the impairment of udder defense: a review. Vet. Res. 31:397-412.
Thanasak, J., Jorritsma, R., Hoek, A., Noordhuizen, J., Rutten, V., and K.E. MUller. 2004. The effects of a single injection of dexamethasone-21-isonicotinate on the lymphocyte functions of dairy cows at two weeks post partum. Vet. Res. 35:103-112.
Tveit, B., F. Lingaas, M. Svendsen, and O. V. Sjaasta. 1992. Etiology of acetonemia in Norwegian cattle. 1. Effeet of ketogenic silage, season, energy level, and genetie factors. J. Dairy Sei. 75:2421-2432.
Van Kampen, C. A., and B. A. Mallard. 1997. Effeets of peripartum stress and health on circulating bovine lymphocyte subsets. Vet. Immunol. Immunopathol. 59:79-91.
Vassilopoulou-Sellin. R. 1994. Endocrine effects of cytokines. Oncology. Huntingt. 8: 43-46.
Vazquez-Anon, M., S. J. Bertics, M. Luek, and R. R. Grummer. 1994. Peripartum liver triglyceride and plasma metabolites. J. Dairy Sei. 77:1521-1528.
Veenhuizen, J. J., J. K. Drackley, M. J. Richard, T. P. Sanderson, L. D. Miller, and J. W. Young. 1991. Metabolic changes in blood and liver during development and early treatment of experimental fatty liver and ketosis in eows. J. Dairy Sei. 74:4238-4253.
Villa-Godoy, A., T. L. Hughes, R. S. Emery, L. T. Chapin, and R. L. Fogwell. 1988. Association between energy balance and luteal funetion in lactating cows. J. Dairy Sei. 71:1063-1072.
Wagner, S. A. and M. D. Apley. 2003. Pharmacodynamies of isoflupredone aeetate in an endotoxin-indueed mastitis model. J. Dairy Sei. 86:792-798.
Weinberg, E.D. 1984. Pregnancy-associated depression of cell-mediated immunity. Rev. Infect. Dis. 6. 814-831.
109

Wentink, G. H., V. P. M. G. Rutten, T. S. G. A. M. van den Ingh, A. Hoek, K. E. Müller, and T. Wensing. 1997. Impaired specifie immunoreactivity in cows with hepatic lipidosis. Vet. Immunol. Immunopathol. 56:77-83.
Winnicka. A., KlucÏnski. W., Kawiak. J., Roser. G., and J. Sikora. 2000. Effect ofBaypamun on blood leucocytes in normal and dexamethasone treated goats. J. Vet. Med. A Physiol. Pathol. Clin. Med. 47:385-394.
Zerbe, H., N. Schneider, W. Leibold, T. Wensing, T. A. M. Kruip, and H. J. Schuberth. 2000. Altered functional and immunophenotypical properties of neutrophilic granulocytes in post-calving cows associated with fatty liver. Theriogenology. 54:771-786.
110

Appendices
111