Temperature measurement part i
-
Upload
burdwan-university -
Category
Engineering
-
view
127 -
download
5
Transcript of Temperature measurement part i
TEMPERATURE MEASUREMENT PART I
TEMPERATURE MEASUREMENT PART IEr. FARUK BIN POYEN, Asst. ProfessorDept. of AEIE, UIT, BU, BURDWAN, WB, [email protected]
1
Contents: Temperature ScalesFahrenheit and CentigradeKelvin and RankineReaumurInternational Practical Temperature ScaleMethods of Temperature MeasurementExpansion ThermometerBimetallic Thermometer (Expansion of Solid)Liquid in Glass Thermometer (Expansion of Liquid)Liquid in Metal Thermometer (Expansion of Liquid)Gas ThermometerFilled System ThermometerLiquid filled ThermometerMercury filled ThermometerVapour pressure Thermometer
TEMPERATURE MEASUREMENT - PART I Er. FARUK BIN POYEN2
2
Temperature Measurement:
Temperature is defined as the condition of a body by virtue of which heat is transferred to and from other bodies. The degree of hotness or coldness of a body or an environment is measured on a definite scale.
Temperature cannot be measured directly but must be measured by observing the effect that temperature variation causes on the measuring device. The methods are broadly classified into three classes. Non Electrical Electrical Radiation
TEMPERATURE MEASUREMENT - PART I Er. FARUK BIN POYEN3
Temperature Scales:
TEMPERATURE MEASUREMENT - PART I Er. FARUK BIN POYEN4
Basic Fixed Points:
Boiling Point: The temperature at which substance changes from liquid to gas.Freezing Point: The temperature at which substance changes from liquid to solid. Triple Point: A particular temperature and pressure at which three different phases of one substance can exist in equilibrium. According to Gibbs phase rule, a three phase situation in a component leaves it with no degrees of freedom. Absolute Zero: The temperature at which molecular motion completely ceases.
TEMPERATURE MEASUREMENT - PART I Er. FARUK BIN POYEN5
Important Laws in Temperature MeasurementMentioned below are few of the rudimentary laws that find applications in the measurement process of temperature. These laws show correlation between temperature and potential differences. Seebeck EffectPeltier EffectThompson EffectThermoelectric EffectLaw of Homogeneous MaterialLaw of Intermediate MetalLaw of Intermediate Temperature
TEMPERATURE MEASUREMENT - PART I Er. FARUK BIN POYEN6
Thermoelectric EffectThe thermoelectric effect is the direct conversion of temperature differences to electric voltages and vice versa. A thermoelectric device creates voltage when there is a different temperature on each side. Conversely, when a voltage is applied to it, it creates a temperature difference. At the atomic scale, an applied temperature gradient causes charge carriers in the material to diffuse from the hot side to the cold side.This effect can be used to generate electricity, measure temperature or change the temperature of objects. As the direction of heating and cooling is determined by the polarity of the applied voltage, thermoelectric devices can be used as temperature controllers.The term "thermoelectric effect" encompasses three separately identified effects: the Seebeck effect, Peltier effect, and Thomson effect. The PeltierSeebeck and Thomson effects are thermodynamically reversible.
TEMPERATURE MEASUREMENT - PART I Er. FARUK BIN POYEN7
Seebeck EffectTEMPERATURE MEASUREMENT - PART I Er. FARUK BIN POYEN8
TEMPERATURE MEASUREMENT - PART I Er. FARUK BIN POYEN9
Peltier EffectTEMPERATURE MEASUREMENT - PART I Er. FARUK BIN POYEN10
TEMPERATURE MEASUREMENT - PART I Er. FARUK BIN POYEN11
Thomson EffectTEMPERATURE MEASUREMENT - PART I Er. FARUK BIN POYEN12
Laws of ThermoelectricityLaw of Homogeneous Materials: - A thermoelectric current cannot be sustained in a single homogeneous material by the application of heat alone, regardless of how much it might vary in cross-section.Law of Intermediate Material: - The algebraic sum of thermoelectric forces in circuit composed of any number of dissimilar materials is Zero if all of the circuit is at same temperature.Law of Successive or Intermediate Temperature: - If two dissimilar homogeneous materials produce thermal (emf)1 when the junctions are T1 and T2 and produce thermal (emf)2 when the junction are at T2 and T3, the emf generated when the junction are at temperature T1 and T3 will be (emf)1 + (emf)2.
TEMPERATURE MEASUREMENT - PART I Er. FARUK BIN POYEN13
TEMPERATURE MEASUREMENT - PART I Er. FARUK BIN POYEN14
Methods of Temperature Measurement
Expansion ThermometerBimetallic Thermometer (Expansion of Solid)Liquid in Glass Thermometer (Expansion of Liquid)Liquid in Metal Thermometer (Expansion of Liquid)Gas ThermometerFilled System ThermometerLiquid filled ThermometerMercury filled ThermometerVapour pressure Thermometer
Electrical Temperature InstrumentResistance ThermometerThermocouple Thermistor ThermopilePyrometerRadiation PyrometerOptical PyrometerOther Methods of Temperature Measurement Quartz ThermometerSolid State Temperature Measurement Optical Fibre Temperature MeasurementUltrasonic Thermometer
TEMPERATURE MEASUREMENT - PART I Er. FARUK BIN POYEN15
Expansion Methods of MeasurementBimetallic Thermometer (Expansion of Solid)Liquid in Glass Thermometer (Expansion of Liquid)Liquid in Metal Thermometer (Expansion of Liquid)Gas Thermometer
TEMPERATURE MEASUREMENT - PART I Er. FARUK BIN POYEN16
Bimetallic Thermometer (Expansion of Solid)
Made up of bimetallic strips formed by joining two different metals having different thermal expansion coefficients. Basically, bimetallic strip is a mechanical element which can sense temperature and transform it into a mechanical displacement. This mechanical action from the bimetallic strip can be used to activate a switching mechanism for getting electronic output. Also it can be attached to the pointer of a measuring instrument or a position indicator. Various techniques such as riveting, bolting, fastening can be used to bond two layers of diverse metals in a bimetallic strip. However the most commonly used method is welding. Since two metals are employed to construct a bimetallic strip, hence they are named so.
TEMPERATURE MEASUREMENT - PART I Er. FARUK BIN POYEN17
Bimetallic Strip: Working PrincipleDifferent metals expand at different rates as they warm up.Two dissimilar metals behave in a different manner when exposed to temperature variations owing to their different thermal expansion rates. One layer of metal expands or contracts more than the other layer of metal in a bimetallic strip arrangement which results in bending or curvature change of the strip.One end of a straight bimetallic strip is fixed in place. As the strip is heated, the other end tends to curve away from the side that has the greater coefficient of linear expansion.
TEMPERATURE MEASUREMENT - PART I Er. FARUK BIN POYEN18
Bimetallic Strip: FeaturesRange: -103 F to 1000 F (-75 C 540 C)Advantages:Low costTough Easy installation and maintenanceGood accuracyWide temperature rangeDisadvantages: Limited to local mountingOnly indicating typeCalibration may change due to rough handlingAccuracy is not the best
TEMPERATURE MEASUREMENT - PART I Er. FARUK BIN POYEN19
Liquid-In-Glass ThermometersIt mainly comprises:A bulb which acts as a container for the functioning liquid where it can easily expand or contract in capacity.A stem, a glass tube containing a tiny capillary connected to the bulb and enlarged at the bottom into a bulb that is partially filled with a working liquid.A temperature scale which is basically preset or imprinted on the stem for displaying temperature readings.Point of reference i.e. a calibration point which is most commonly the ice point.A working liquid which is generally either mercury or alcohol.An inert gas, mainly argon or nitrogen which is filled inside the thermometer above mercury to trim down its volatilization.
TEMPERATURE MEASUREMENT - PART I Er. FARUK BIN POYEN20
Liquid-In-Glass Thermometers - PrincipleThe principle used to measure temperature is that of the apparent thermal expansion of the liquid. It is the difference between the volumetric reversible thermal expansion of the liquid and its glass container that makes it possible to measure temperature.
TEMPERATURE MEASUREMENT - PART I Er. FARUK BIN POYEN21
Liquid-In-Glass Thermometers - FeaturesRange of -200 C to 600C.Advantages:Comparatively cheaper Handy and convenient to use.They do not necessitate power supply or batteries for charging.They can be frequently applied in areas where there is problem of electricity.Very good repeatability and calibration remains unaffectedLimitations: Not very accurateShort temperature rangeWeaker and more delicate than electrical thermometersSubject to parallax error
TEMPERATURE MEASUREMENT - PART I Er. FARUK BIN POYEN22
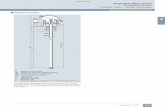
Liquid in Metal ThermometerA thermometer in which the thermally sensitive element is a liquid contained in a metal envelope, frequently in the form of a Bourdon tube.A liquid in metal thermometer in which mercury has been used as liquid and the metal is steel. This mercury-in-steel thermometer works on exactly the same principle as the liquid-in-glass thermometer. The glass bulb is replaced by a steel bulb and the glass capillary tube by one of stainless steel. As mercury in the system is not visible, a Bourdon tube is used to measure the change in its volume.
TEMPERATURE MEASUREMENT - PART I Er. FARUK BIN POYEN23
Liquid in Metal Thermometer - PrincipleWhen the temperature to be measured rises, the mercury in the bulb expands more than the bulb so that some mercury is driven through the capillary tube into the Bourdon tube. As the temperature continues to rise, increasing amounts of mercury will be driven into the Bourdon tube, causing it to bend. One end of the Bourdon tube is fixed, while the motion of the other end is communicated to the pointer which moves on a calibrated temperature scale.
TEMPERATURE MEASUREMENT - PART I Er. FARUK BIN POYEN24
Liquid in Metal ThermometerThe thermometer bulb is also placed in a protective pocket where the gas or liquid whose temperature is being measured, is at a pressure other than atmospheric. In this case the pocket prevents the bulb being subjected to this pressure and also enables the bulb to be changed without shutting down the plant. The capillary tube used in the mercury-in-steel thermometer is usually made from stainless steel, as mercury will combine with other metals. Changes of temperature affect the capillary and the mercury it contains, and hence the thermometer reading.Generally, mercury is used as a liquid. But it has its limitations, particularly at the lower end of the temperature scale. For this and other reasons, other liquids are also used sometimes in place of mercury.
TEMPERATURE MEASUREMENT - PART I Er. FARUK BIN POYEN25
Gas Filled ThermometerThe filled thermal device consists of a primary element that takes the form of a reservoir or bulb, a flexible capillary tube, and a hollow Bourdon tube that actuates a signal-transmitting device and/or a local indicating temperature dial. The filling fluid, either liquid or gas, expands as temperature increases. This causes the Bourdon tube to uncoil and indicate the temperature on a calibrated dial. The filling or transmitting medium is a vapour, a gas, mercury, or another liquid. The liquid-filled system is the most common because it requires a bulb with the smallest volume or permits a smaller instrument to be used.
TEMPERATURE MEASUREMENT - PART I Er. FARUK BIN POYEN26
Gas Filled ThermometerThe gas-filled system uses the perfect gas law, which states the following for an ideal gas: T = k P V,where: T =temperature; k = constant; P = pressure; V = volume; If the volume of gas in the measuring instrument is kept constant, then the ratio of the gas pressure and temperature is constant, so that
TEMPERATURE MEASUREMENT - PART I Er. FARUK BIN POYEN27
Filled System ThermometerFilled system thermometers consist of Bourdon tube, a capillary tube and a thermometer bulb all interconnected. The entire point is sealed after appropriate liquid filling at NTP and commonly used liquids are mercury, ethyl alcohol, xylene and toluene. Liquid expands or contracts with gain or loss of heat till measured temperature is attained leading to the expansion or contraction of the Bourdon tube which subsequently moves a pointer for indication. Types are: Gas filled (Gas Filled Thermometers)Liquid filledMercury filledVapour filled
TEMPERATURE MEASUREMENT - PART I Er. FARUK BIN POYEN28
Filled System ThermometerLiquid Filled Thermometer: They work on the principle of liquid expansion with temperature rise. The filling liquid is usually an inert hydrocarbon viz. xylene which has six times more expansion co-efficient than mercury decreasing the bulb position. One criterion to be maintained is that the pressure inside must be greater than the vapour pressure of liquid to prevent formation of bubbles inside. Solidification of liquid is also not permitted. Mercury Filled Thermometer: Similar to that of liquid filled thermometers but provides rapid response, accuracy and plenty of power. Pressure is as high as 1200 psig to as low as 400 psig. The high pressure reduces the head effect. They are normally contained in stainless steel bulb increasing the corrosion resistance. Vapour Filled Thermometer: Here the bulb is partially filled with liquid and partially with vapour. Some of the liquid vaporises during operation. The liquid inside boils and vaporises creating gas inside the system. The liquid continues to boil until pressure balance is obtained between systems and vapour pressure. Here liquid stops boiling unless temperature rises. Similarly, when temperature drops, liquid and vapour inside also cool causing some vapour to condense, bringing down the pressure inside. When pressure inside equals vapour pressure, this action stops. Due to changes in pressure, bourdon tube uncoils or tightens with increase or decrease of pressure indicating temperature on a pointer scale.
TEMPERATURE MEASUREMENT - PART I Er. FARUK BIN POYEN29
Filled System Thermometer - FeaturesSources of Error in Filled System Thermometers:Ambient temperature effectHead or elevation effectBarometric effectImmersion effectRadiation effectAdvantages of Filled System ThermometerRugged constructionLow maintenanceNo electric power requirementSatisfactory time responseLow costCapillary allows considerable separation between measurement point and temperature pointDisadvantages of Filled System Thermometers:Large bulb required for better accuracyRequires full scale replacement if found faultyAccuracy, sensitivity and span are on the lower side compared to electrical methodsNot great temperature range ability
TEMPERATURE MEASUREMENT - PART I Er. FARUK BIN POYEN30
References: Chapter 13: Temperature Measurement, Industrial Instrumentation and Control by S K Singh. Tata McGraw Hill, 3rd Edition. 2009, New Delhi. ISBN-13: 978-0-07-026222-5.Chapter 11: Temperature Measurement, Instrumentation, Measurement and Analysis. 2nd Edition, B C Nakra, K K Chaudhry, Tata McGraw-Hill, New Delhi, 2005. ISBN: 0-07-048296-9. Chapter 4: Temperature Sensors, Fundamentals of Industrial Instrumentation, 1st Edition, Alok Barua, Wiley India Pvt. Ltd. New Delhi, 2011. ISBN: 978-81-265-2882-0. Chapter 4: Temperature Measurement, Principles of Industrial Instrumentation, 2nd Edition. D. Patranabis, Tata McGaw-Hill, New Delhi, 2004. ISBN: 0-07-462334-6.
TEMPERATURE MEASUREMENT - PART I Er. FARUK BIN POYEN31