Subcutaneous epidermal inclusion cysts: Ultrasound (US) and MR imaging findings
-
Upload
hee-kyung-kim -
Category
Documents
-
view
216 -
download
3
Transcript of Subcutaneous epidermal inclusion cysts: Ultrasound (US) and MR imaging findings
SCIENTIFIC ARTICLE
Subcutaneous epidermal inclusion cysts: Ultrasound (US)and MR imaging findings
Hee Kyung Kim & Sung Moon Kim & Sang Hoon Lee &
Judy M. Racadio & Myung Jin Shin
Received: 29 September 2010 /Revised: 10 November 2010 /Accepted: 15 November 2010 /Published online: 4 December 2010# ISS 2010
AbstractObjective To describe the characteristic US and MRfindings of subcutaneous epidermal inclusion cysts.Materials and methods Seventy-nine patients with subcu-taneous epidermal inclusion cysts underwent US (n=70),MR (n=7), or both (n=2). On US, the margin, shape,echogenicity, through-transmission, wall, internal debrisand vascularity were evaluated. On MR, the shape, wall,signal intensity, internal debris, and enhancement patternwere evaluated.Results On US, characteristic findings were well circum-scribed (n=69, 96%), ovoid-shaped (n=56, 78%), hetero-geneously and mildly echogenic (n=66, 92%), increasedthrough-transmission (n=66, 92%) and low echoic rim (n=48, 67%). Internal debris was seen in 31 cases (43%) andoften contained linear echogenic reflections (n=12, 17%),dark clefts (n=13, 18%), or a mixture (n=5, 7%). Mostmasses showed no Doppler flow (n=70, 97%). On MR, all
cases demonstrated a well-demarcated oval-shaped masswith a surrounding rim. On T1-weighted image (WI), themass showed slightly high T1 signal in 4/9 (44%) and iso-signal in 5/9 (56%). On T2WI, the mass showed high signalin 6/9 (67%), intermediate in 2/9 (22%), and a targetappearance in 1/9 (11%). Internal linear dark T2 signaldebris was observed in 4/9 (44%). All lesions showedperipheral rim enhancement without central enhancement.Conclusions On US, subcutaneous epidermal inclusioncysts are usually well-circumscribed, oval-shaped, mildlyechogenic masses with occasional linear anechoic and/orechogenic reflections, increased through-transmission,hypoechoic rim and no Doppler flow. On MR, anintermediate to high T2 signal mass with occasional lowsignal debris and no central enhancement can strengthen thediagnosis.
Keywords Ultrasound .MR . Epidermal inclusion cyst
Introduction
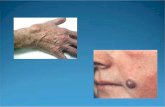
Subcutaneous epidermal inclusion cysts are relativelycommon lesions comprising about 85-90% of all excisedsubcutaneous cysts [1]. These cysts usually arise on hair-bearing areas including the scalp, face, neck, trunk,extremities, and scrotum [2–7].
These small, but palpable, superficial lesions in thesubcutaneous tissue rarely require radiologic evaluation andare most often diagnosed and treated by excision. Therehave been a few radiologic studies of epidermal inclusioncysts in the head and neck, testis, and breast [3–8].However, theses studies were based on small sample sizesand a limited number of ultrasound (US) studies. Therefore,the aim of this study was to evaluate the sonographic and
H. K. Kim : S. M. Kim : S. H. Lee :M. J. Shin (*)Department of Radiology and Research Institute,University of Ulsan College of Medicine, Asan Medical Center,86, Asanbyeongwon-gil, Songpa-gu,Seoul, Koreae-mail: [email protected]
Present Address:H. K. Kim : J. M. RacadioDepartment of Radiology,Cincinnati Children’s Hospital Medical Center,3333 Burnet Avenue,Cincinnati, OH 45229, USA
Present Address:S. M. KimDepartment of Radiology, University of Michigan Hospitals,1500 E Medical Center Dr.,Ann Arbor, MI 48109, USA
Skeletal Radiol (2011) 40:1415–1419DOI 10.1007/s00256-010-1072-4
MR imaging features of subcutaneous epidermal inclusioncysts in a larger number of patients.
Patients and methods
Patients
This study was approved by the Institutional Review Board(IRB). For the limited and anonymous review of patientdata required for this study, the IRB did not requireinformed patient consent.
This study included 79 adult patients (54 male, 25female, age range 2 to 74 years with a mean of 40 years±16 years) who underwent US (n=70), MR (n=7) or bothUS and MR exams (n=2) for the evaluation of a superficialpalpable mass and pathologically confirmed as epidermalinclusion cysts after surgical excision.
Methods
Using a 5 to 12-MHz linear array probe, US was performedwith an HDI 5000 scanner (Advanced Technology Labora-tories, Bothell, WA). All examinations were reviewed bytwo radiologists (SMK, musculoskeletal fellowship trainingwith 12 years of experience; HKK, musculoskeletalfellowship training with 1 year of experience) by consen-sus. All US exams included gray-scale sonograms of thesoft tissue mass in transverse and longitudinal planes andcolor-Doppler images.
MR was performed using a 1.5-T Magnetom visionscanner (Siemens, Erlangen, Germany). Spin echo T1-weighted (TR/TE=400-500 ms/17-20 ms) and turbospinecho T2-weighted (3,000-3,500/90-100) axial, coronal,and sagittal images were obtained with 1-2 excitations anda 154 × 256 acquisition matrix. After intravenous injectionof contrast material (0.1 mmol/kg gadopentetate dimeglumine(Magnevist, Schering, Berlin, Germany)), axial, coronal, andsagittal T1-weighted images (400-500 ms/17-20 ms) with fatsaturation were obtained. MR images were retrospectivelyreviewed by the same radiologists by consensus.
On US exams, imaging findings were analyzed andevaluated for the following characteristics: location, margin(circumscribed or not circumscribed), shape (round, ovoid,lobulated, irregular, flat), size (the longest diameter in threedimensions), internal echogenicity, posterior echo (in-creased through-transmission, posterior acoustic shadow),wall, and associated internal debris and vascularity. On MRexams, the following characteristics were evaluated; shape,wall, signal intensity, internal debris, and enhancementpattern. The pathologic records were reviewed focusing onthe presence of rupture.
Results
All 79 lesions were located within the subcutaneous tissue.The overlying skin was intact in 71/72 cases; one cyst had afistulous tract to the skin.
Sonographic features
The locations of the lesions were the head and neck in 12patients [cheek (n=6), eyelid (n=2), lip (n=1), forehead (n=1), nose (n=1), scalp (n=1)], upper extremities in 23 patients[hand (n=12), arm (n=7), shoulder (n=4)], lower extremitiesin 17 patients [leg (n=10), foot (n=7)], back in six patients,buttock in 15 patients [gluteal fold (n=11), outer portion ofcoccyx (n=4)], and the chest wall in two patients. On US, 69lesions were well circumscribed (69/72, 96%). The shapes ofthe lesions were ovoid (n=56, 78%), lobulated (n=8, 11%),round (n=3, 4%), irregular (n=3, 4%), and flat (n=2, 3%).The maximum diameters of the lesions varied from 5 to80 mm (mean 22.3 mm). Internal echogenicity was eitherheterogeneously and mildly echogenic (n=66, 92%) (Fig. 1),homogeneously echogenic (n=3, 4%), or anechoic (n=3,4%). The cysts demonstrated increased through transmission(n=66, 92%), posterior shadowing (n=1, 1%), or noposterior echo (n=5, 7%). A surrounding low echoic rimwas observed in 48 cases (67%). Associated internal debriswas seen in 31 cases (43%); this debris included internallinear echogenic reflections (n=12, 17%) (Fig. 2), dark clefts(n=13, 18%), a combination of linear echogenic reflectionsand dark clefts (Fig.3) (n=5, 7%), or alternate layering withhyperechoic and hypoechoic rings (n=1, 1%). In all cases,the internal debris was mobile showing a “swirl” appearancewhen pressed with the probe. Among the 72 cysts, 13 weredescribed as “ruptured” in the pathologic reports. The lesionsthat were noted to have an irregular shape and a margin thatwas not well circumscribed on US (n=3) were ruptured. Theother ten cases of ruptured cyst had an ovoid (n=5), flat (n=2), or lobulated appearance (n=6). Color Doppler studiesshowed no vascular flow signal in 70 cases (97%). In twocases (3%), vascular flow was seen at the peripheral portionof the mass, but was not seen in the majority of the mass;both of those cysts were ruptured.
MR imaging features
All nine lesions were well-demarcated oval-shaped masseswith surrounding T2 low signal rim. None of the lesionsshowed infiltration into surrounding tissue. In one case (n=1, 10%), an epidermal inclusion cyst in the distal phalanx,there was bony erosion with sclerotic remodeling; this massshowed alternating concentric rings of low and high signalintensity (“target” appearance) on T2-weighted image(Fig 4).
1416 Skeletal Radiol (2011) 40:1415–1419
The internal signal intensity on T1-weighted images ofthe masses was either high (n=4, 44%) or iso-signal to theunderlying muscles (n=5, 56%). On T2-weighted images,the internal signal of the mass was high (n=6, 67%),intermediate (n=2, 22%), or had a target appearance (n=1,11%).
Moderate linear dark debris was seen in four cases(44%), which was best visualized on T2-weighted imagesand was located in the non-dependent portion of the mass(Fig 2).
All masses showed thin peripheral enhancement, butnone showed central mass enhancement on post-contrastT1-weighted images with fat suppression.
Discussion
Epidermal inclusion cysts are also called epidermal cysts,epidermoid cysts, and infundibular cysts [1]. Proposedcauses of epidermal inclusion cysts include congenitalfactors, squamous metaplasia of the columnar epithelialcells, downward growth of the epidermal cells withinflammation after obstruction of the hair follicle, andgrowth of implanted fragments of the epidermis within thedermis after trauma [2]. Epidermal inclusion cysts areunilocular cysts without septation, encapsulated withfibrous tissue and lined by a thin layer of squamous cells.They usually grow very slowly over years or decades [1, 2].They form through an accumulation of cutaneous productswithin an enclosed space; the debris consists of keratin,protein, cholesterol, and cell membrane lipids [1, 2]. Theradiologic findings of epidermal inclusion cysts are depen-dent on the maturation of the cyst, its compactness and theamount of keratin it contains [2–9].
Previous studies have described diverse sonographicfindings. Denison et al. described eight cases of epidermalinclusion cysts in the breast, all of which appeared on US ascircumscribed hypoechoic masses with through-transmission [3]. Yasumoto et al. reported on the sono-graphic appearance of epidermoid cysts in the head andneck; they were mostly hyperechoic with slight or noposterior sound enhancement [4]. In our study, mostepidermal inclusion cysts were well-circumscribed masses;these findings are consistent with previous studies. Most ofthe epidermal inclusion cysts (92%) had a heterogeneously
Fig. 1 A 44-year-old male with a subcutaneous epidermal inclusioncyst on the right buttock. Sonogram shows a circumscribed mildlyechogenic mass
Fig. 2 A 71-year-old femalewith a subcutaneous epidermalinclusion cyst on the rightbuttock. a Sonogram showsa mildly echogenic masswith scattered internal linearechogenic reflections (arrows)in the mass and increasedthrough-transmission. b AxialT1-weighted MR image showsslightly increased signal intensi-ty of the mass compared to themuscle. c Axial T2-weightedMR image shows a high signalintensity mass with internallow signal intensity debris.d Contrast-enhancedT1-weighted axial MR imagewith fat saturation showsperipheral rim enhancement(arrow)
Skeletal Radiol (2011) 40:1415–1419 1417
and mildly echogenic appearance. Dogra et al. describedthat increased echogenicity in epidermal inclusion cystsresults from high acoustic impedance of the keratin debris[5]. The diverse echogenicity reported in other studies mayhave been due to interobserver variance and lack ofstandardization for echogenicity. Consistent with a previousstudy [4], we found that most of the cysts demonstratedincreased through-transmission. However, increasedthrough-transmission is not a consistent finding, as itsprominence is dependent on the type of US equipment, thelocation and size of the cyst, and the amount or type ofcontained fluid [6]. It is important to note that whileincreased through-transmission can occur in benign cysts, itcan also be seen in any homogeneous solid mass such aslymphoma or hemangioma [6].
In our study, when internal debris was present (41% ofcases), it characteristically contained linear echogenicreflections and/or dark clefts, which might be specificdiagnostic features on US as suggested on MR [7]. In otherpathologically proven studies, it was shown that thefloating linear echogenic reflections were created bylayered and aggregated keratin debris, while the disperseddark clefts occurred in cyst-like spaces and areas lackingkeratin [5, 8]. Langer et al. described a lack of internalvascularity as another feature that can differentiate epider-
mal inclusion cysts from most solid testicular masses [9];this was confirmed in our study.
The MR features of unruptured epidermal inclusion cystshave been described in other studies as a well defined masswith high T2 signal background with internal low T2, highT1 signal foci, and enhancement of a thin wall [10, 11].Yang et al. described the findings of presacral epidermalinclusion cysts as a high T2 signal mass with internal lowT2 signal foci in the nondependent portion of the mass(confirmed to be aggregates of keratinous material onpathologic exam) [7].
In studies of intratesticular epidermal inclusion cysts, aconcentric ring (on US) and a bull’s-eye or targetappearance (on MR) have been described as importantdiagnostic features; these were shown to be caused bydense-layered debris in the center of the cyst [12, 13]. Inour study, we found two subcutaneous epidermal inclusioncysts with a concentric ring or target appearance (one caseon US and the other case on MR); both lesions were locatedin the distal tip of the finger.
This study was limited; we did not correlate the variousimaging findings with their pathologic counterparts, as thecorrelation has been well described in prior studies.
In summary, the characteristic sonographic findings ofepidermal inclusion cysts include a well-circumscribed
Fig. 4 A 32-year-old female with a subcutaneous epidermal inclusioncyst in the right index finger. a Radiograph shows bony erosion withsclerotic remodeling of the distal phalanx. b Sagittal T1-weighted MRimage shows a slightly increased signal intensity mass. c T2-weighted
sagittal MR image shows the target appearance with alternating highand low signal intensities in the mass. d Post-contrast sagittal T1-weighted MR image with fat suppression shows a thin rim ofenhancement (arrow)
Fig. 3 A 54-year-old malewith a subcutaneous epidermalinclusion cyst on the anteriorchest wall. a, b Sonogramsshow a mildly echogenic masswith scattered internal darkclefts (white arrows) andechogenic reflections(dark arrows) and increasedthrough-transmission
1418 Skeletal Radiol (2011) 40:1415–1419
mildly echogenic mass confined to the subcutaneous layer,possibly containing internal linear echogenic reflections ordark clefts, a hypoechoic rim, and an absence of flow oncolor Doppler images. MR findings of a high T2 signalmass, possibly with low-signal-intensity debris, and thin-rim enhancement on contrast-enhanced T1-weighted MRimages strengthens this preoperative diagnosis.
The authors declare that there are no conflicts of interest.
References
1. Lever WF, Lever GS. Tumor and cysts of epidermis. In: Elder D,editor. Histopathology of the skin. 8th ed. Philadelphia, JB:Lippincott, 1997, p. 685-746
2. Elder D, Elenitsas R, Jaworsky C, Johnson Jr B. Lever’shistopathology of the skin. 8th ed. Philadelphia: Lippincott-Raven; 1997. p. 695–721.
3. Denison CM, Ward VL, Lester SC, DiPiro PJ, Smith DN, MeyerJE, et al. Epidermal inclusion cysts of the breast: Three lesionswith calcifications. Radiology. 1997;204(2):493–6.
4. Yasumoto M, Shibuya H, Gomi N, Kasuga T. Ultrasonographicappearance of dermoid and epidermoid cysts in the head and neck.J Clin Ultrasound. 1991;19(8):455–61.
5. Dogra V. Testicular epidermoid cysts. Am J Roentgenol. 2002;179(4):1075–5.
6. Jackson VP, Edmond VJ. Breast ultrasonography. In: LW JVP, FuKL, Fu YS, editors. Diagnosis of disease of the breast. 2nd ed.Philadelphia: Elsevier Saunders; 2005. p. 175–92.
7. Yang DM, Yoon MH, Kim HS, Oh YH, Ha SY, Oh JH, et al.Presacral epidermoid cyst: imaging findings with histopathologiccorrelation. Abdom Imaging. 2001;26(1):79–82.
8. Eisenmenger M, Lang S, Donner G, Kratzik C, Marberger M.Epidermoid cysts of the testis: organ-preserving surgeryfollowing diagnosis by ultrasonography. Br J Urol. 1993;72(6):955–7.
9. Langer JE, Ramchandani P, Siegelman ES, Banner MP. Epider-moid cysts of the testicle: Sonographic and MR imaging features.Am J Roentgenol. 1999;173(5):1295–9.
10. Shibata T, Hatori M, Satoh T, Ehara S, Kokubun S. Magneticresonance imaging features of epidermoid cyst in the extremities.Arch Orthop Traum Su. 2003;123(5):239–41.
11. Hong SH, Chung HW, Choi JY, Koh YH, Choi JA, Kang HS.MRI findings of subcutaneous epidermal cysts: emphasis onthe presence of rupture. AJR Am J Roentgenol. 2006;186(4):961–6.
12. Brenner JS, Cumming WA, Ros PR. Testicular epidermoid cyst—sonographic and MR findings. Am J Roentgenol. 1989;152(6):1344–4.
13. Cho JH, Chang JC, Park BH, Lee JG, Son CH. Sonographic andMR imaging findings of testicular epidermoid cysts. Am JRoentgenol. 2002;178(3):743–8.
Skeletal Radiol (2011) 40:1415–1419 1419