Stereochemistry Constitutional Isomers: same molecular formula, different connectivity....
-
Upload
godwin-booker -
Category
Documents
-
view
246 -
download
1
Transcript of Stereochemistry Constitutional Isomers: same molecular formula, different connectivity....

Stereochemistry
Constitutional Isomers: same molecular formula, different connectivity.
Stereoisomers: same molecular formula, same connectivity, different arrangement of atoms in space.
Consider the following molecules:
cis-1,2-dichlorocyclopentane trans-1,2-dichlorocyclopentane
What is their relationship?
H
H
Cl
Cl
Cl
H
Cl
H

These cis/trans isomers exist because…?
What about? C C
H3C
H CH2F
CH2Cl
C C
H3C
H CH2Cl
CH2F
which is cis and which is trans?

Substituents on each end are prioritized using the Cahn-Ingold-Prelog convention and the geometry is labelled as Z or E…
C C
Y
W X
Z
C C
higher priority
higher priority
C C
higher priority higher priority
Z isomer E isomer
Z – Zusammen (together) E – entgegen (across)

The Cahn-Ingold-Prelog Rules for Determining Substituent Priority:
Substituents are evaluated by comparing atoms that are equidistant from the point of interest.1. The higher the atomic number, Z, the higher the priority.2. For atoms with the same Z, the heavier isotope has higher priority (13C vs 12C, 2H(D) vs 1H).3. If the atoms attached are the same, then move out to the next ‘shell’ of atoms connected to each of the identical atoms and list them in decreasing atomic number. The group with an element having a higher Z at the first point of difference has the higher priority.4. If all the atoms in the second shell are identical, go to the next shell and repeat the process until the first point of difference is found.5. If a substituent contains π–bonds, interpret them as multiple σ–bonds between the same atoms (e.g. an aldehyde has a carbon which is bonded to 2 oxygen atoms and one hydrogen atom.

C
CH2F
CH2CH2Br
C
C
C
H OCH3
OCH3
HH
OCH3
C
CF3
CH2SH
Use the Cahn-Ingold rules to assign priority to the following groups…

What about multiple bonds (Rule #5 in the Cahn-Ingold Rules)?
C N
COH
O
C N
N00
N00
C000
C000
C
O
O0
O H
C000

C
C
C
CH3 CH3
CH3
C
H
Use the Cahn-Ingold Rules to assign priority to the following groups:

How do you know when molecules are the same?
Molecules are identical if, in equivalent conformations, they are superimposable.
C C
H3C
HH
CH2CH3
C C
H
HH3C
CH2CH3
C C
H
HH3C
CH2CH3
C C
H3C
HH
CH2CH3
If the connectivity of two molecules differs, then they are constitutional isomers. If the connectivity is the same but the molecules are not superimposable, then they are stereoisomers.
C
CH3Cl
H3CO
HC
CH3
Cl
CH3
OH

C
H
ClCH2CH3
CH3C
H
ClH3CH2C
H3C Are molecules A and B the same?
C
H
ClH3CH2C
H3C
A B
B
Configuration: the arrangement in space of the substituents around a given asymmetric atom.
Stereocenter (asymmetric, chiral, or stereogenic):
any tetrahedral atom,
frequently carbon, which has 4
different substituents.
Chirality and Enantiomers

Since these two molecules are nonsuperimposable mirror images of each other, they are called enantiomers and are said to be chiral.
Chirality (n): is a property of objects such that they are nonsuperimposable on their mirror image.
Chiral (adj): an object is chiral if it is nonsuperimposable on its mirror image.
Enantiomers: are stereoisomers that are nonsuperimposable mirror images of one another.
C
H
ClCH2CH3
CH3C
H
ClH3CH2C
H3C
Recall:
Achiral (adj): an object is achiral if it is superimposable on its mirror image. If an object has a plane of symmetry it is not chiral.

Enantiomers have identical physical properties in all respects (thus difficult to separate from each other), except they rotate plane-polarized light in opposite directions. Substances that rotate plane-polarized light are said to be optically active.
2-butanol: One molecule rotates plane polarized-light clockwise (+) while the other rotates plane-polarized light counter clockwise (-). Therefore, these two molecules would be named (+)-2-butanol and (-)-2butanol.
Thus a 50/50 mixture of two enantiomers (called a racemic mixture) will not rotate plane polarized light.
C
H
OHH3CH2C
H3C C
H
HOCH2CH3
CH3

Absolute configuration: the actual arrangement in space of the substituents around a given asymmetric atom that can be designated R or S. Also applies to E/Z.
Note how the ‘optical activity’ of molecules (+ vs -) says nothing about the absolute configuration around the chiral centre(s).
Thus a 50/50 mixture of two enantiomers (called a racemic mixture) will not rotate plane polarized light.

The Cahn-Ingold-Prelog Convention is used to assign the absolute configuration of a chiral centre.
•we assign priorities (1-4) to the substituents of a chiral atom.•orient the molecule such that the lowest priority substituent points away from you.•if the remaining substituents are arrayed 1-2-3 in clockwise order, then the center has R configuration.•if the remaining substituents are arrayed in a counter clockwise order, then the center has S configuration.
C
C
1
2 3
4
1 2
3
4
C12
3
4
12
3
4
=
=
R
S

Any molecule with a single asymmetric carbon atom must be chiral.
C
H
ClCH3
CH2CH3C
H
ClCH2CH3
CH3
There are only 2 possible configurations for chiral centres…
These two configurations cannot be interchanged without breaking bonds.
What happens when we switch two groups on an asymmetric carbon atom?
C
H
ClCH3
CH2CH3

Assign the configuration to all stereocentres:
C
CH2OH
HH3CCl
CH3
Cl
O
BrH
OO
Cl
H O
Cl
H

Can molecules without asymmetric atoms be chiral?
C C C
H
Cl
H
ClC C C
H
Cl
H
Cl
OH
HO
HO
OH

Atoms other than carbon...
NH3CH2C CH3
PhN
H3CH2C CH3
Ph
NH3CH2C Ph
CH3
CH3CH2Br
NH3CH2C CH3
Ph
CH2CH3
Br
PH3CH2C CH3
Ph
SH3CH2C
PhO

Molecules with two chiral centres…
H3C
C C
HO
BrH
HCH3
CH3
CC
OH
Br H
HH3C
H3C
C C
HO
HBr
HCH3
CH3
CC
OH
HBr
HH3C
Stereoisomers that are not enantiomers are called…
Diastereomers have different physical properties and can easily be separated from one another. A molecule can have a maximum of 2n possible stereoisomers (n = number of stereocenters).
Diastereomers: are stereoisomers that are nonsuperimposable, non-mirror images to each other.
1 2
3 4

H
Br
IH
ClBr
F CH3
For the following molecule, draw all stereoisomers and indicate which are enantiomers and which are diastereomers.

Recall 1,2-dichlorocyclopentane. How many stereocentres does 1,2-dichlorocyclopentane have? Draw all the stereoisomers for 1,2-dichlorocyclopentane.

Fischer Projections: a short hand notation for showing absolute configurations. In no way do these diagrams attempt to show the actual shape of molecules.
CH OH
CHO
CH2OH
CHO
CH2OH
H OH
90°
CHOHOH2C
H
OH
==
CHOHOH2C
H
OH
180°
CHO
CH2OH
HHO=C HHO
CHO
CH2OH
=
A 90° or 270° rotation of a Fischer projection generates…
A 180° rotation of a Fischer projection generates…
Use models to convince yourself this is true!

Fischer projections were developed to aid in drawing molecules (sugars, actually) with many chiral centres. It is easy to spot enantiomers and superimposable molecules when drawn as these projections.
CHO
HO H
HO H
CH2OH
CHO
OHH
OHH
CH2OH CHO
HO H
HO H
CH2OH
CHO
HO H
H OH
CH2OH

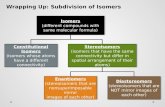
StereochemistreeStereochemistree
Is the connectivitythe same?
Yes No
Constitutional isomersAre theysuperimposable?
Identicalmolecules
They are stereoisomers.Are they mirror imagesof each other?
Enantiomers Diastereomers
No
Yes
Identical after rotation about single bondsto equivaent conf ormations?
Yes
No
Conformers
NoYes
Do they have the samemolecular f ormula?
Yes No
Different molecules