Solution Stoichiometry and Types of Reactions...4.4. Types of Chemical Reactions • Molecular...
Transcript of Solution Stoichiometry and Types of Reactions...4.4. Types of Chemical Reactions • Molecular...

Solution Stoichiometry and Types of Reactions
Chapter 4

Copyright © Houghton Mifflin Company. All rights reserved. 4 | 2
4.1 Water – A Most Versatile Solvent
• A soluteconsists of atoms, molecules or ions that dissolve in
the solvent.• A solvent
is the substance that dissolves the solute.• A solution
is a homogenous mixture made by dissolving a solute in a solvent.
• If a solute is dissolved in water, it forms an aqueous solution

Copyright © Houghton Mifflin Company. All rights reserved. 4 | 3
Solution

Copyright © Houghton Mifflin Company. All rights reserved. 4 | 4
Hydration Process
• Water molecules surround the ions.
• Note the positions of the positive and negative charges.

Copyright © Houghton Mifflin Company. All rights reserved. 4 | 5
Electrolytes
• Strong Electrolytes– Good conductors of electricity in solution.– Strong acids, strong bases, soluble ionic compounds.– Completely ionized in solution.
• Weak Electrolytes– Poor conductors of electricity in solution.– Partially ionized in solution. CH3COOH, NH3
• Non-electrolytes– Do not conduct electricity in solution.– Do not form ions in solution.
: C6H12O6,

Copyright © Houghton Mifflin Company. All rights reserved. 4 | 6
Electrolytes
• A strong electrolyte is completely dissociated in water.KCl(s) KCl(aq) K+(aq) + Cl−(aq)
• A weak electrolyte is partiallydissociated in water.NH3(aq) + H2O(l) NH4
+(aq) + OH−(aq)
• Non-electrolytes do not form ions.C12H22O11(s) C12H22O11(aq)
MSOffice4

Copyright © Houghton Mifflin Company. All rights reserved. 4 | 7
Electrolytes

Copyright © Houghton Mifflin Company. All rights reserved. 4 | 8
Electrolytes

Copyright © Houghton Mifflin Company. All rights reserved. 4 | 9
Sample Problem
Classify each of the following compounds as strong electrolyte, weak electrolyte or non-electrolyte.
• CaCl2• C6H12O6 (glucose)
• HF
• HNO3

Copyright © Houghton Mifflin Company. All rights reserved. 4 | 10
4.2. The Concentration of Solutions
• The concentration of a solution is the amount of solute per volume of solution.
moles of soluteMolarity ( ) = liter of solution
M

Copyright © Houghton Mifflin Company. All rights reserved. 4 | 11
Sample Problem
What is the molarity of 100 mL solution containing 15.6g of NaOH?
( ) 1 mol NaOH15.6g NaOH 0.390 mol NaOH40.0g NaOH
0.390 mol NaOH 3.90 NaOH1 L100 mL
1000 mL
=
=
M = M

Copyright © Houghton Mifflin Company. All rights reserved. 4 | 12
Sample Problem
How many moles of HCl are contained in 250 mL of a 0.600 molar solution?
( ) =
1 L 0.600 mol HCl250. mL 0.150 mol HCl1000 mL 1 L

Copyright © Houghton Mifflin Company. All rights reserved. 4 | 13
Other Concentration Units

Copyright © Houghton Mifflin Company. All rights reserved. 4 | 14
Other Concentration Units
ppm ppb ppt

Copyright © Houghton Mifflin Company. All rights reserved. 4 | 15
Sample Problem
What is the molarity of a solution that is 4.0 ppb Cr6+?
6+ 6+8 6+
6 6+
4.0 g4.0 ppbL soln.
4.0 g 1 g Cr 1mol Cr 7.7 10 CrL soln. 10 g 52.0 gCr
−
µ=
µ = × µ M

Copyright © Houghton Mifflin Company. All rights reserved. 4 | 16
Diluting Solutions
moles in the concentrated solution = moles in the dilute solution
Cinitial × Vintial = Cfinal × Vfinal

Copyright © Houghton Mifflin Company. All rights reserved. 4 | 17
Sample Problem
How many mL of 15.0 M nitric acid solution should be dilute to make 250. mL of a 0.50 M solution?
×=
×=
=
final finalinitial
initial
C VVC
0.50 250. mL15.0
8.3 mL
MM

Copyright © Houghton Mifflin Company. All rights reserved. 4 | 18
4.3. Stoichiometric Analysis of Solutions
TitrationControlled addition of a solution of known
concentration to react with a solution of unknown concentration.
Equivalence pointThe point when all reactants are completely
consumed.End point
Close to the equivalence point marked by an indicator.

Copyright © Houghton Mifflin Company. All rights reserved. 4 | 19
Titration

Copyright © Houghton Mifflin Company. All rights reserved. 4 | 20
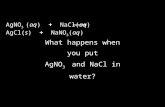
4.4. Types of Chemical Reactions
• Molecular equation (components written as compounds):
AgNO3(aq) + NaCl(aq) → AgCl(s) + NaNO3(aq)
• Complete Ionic Equation (electrolytes written as ions):Ag+(aq) + NO3
−(aq) + Na+(aq) + Cl−(aq) →AgCl(s) + Na+(aq) + NO3
−(aq)
• Net ionic equation (ions participating in the reactionAg+(aq) + Cl−(aq) → AgCl(s)
(Na+ and NO3− are spectator ions.)

Copyright © Houghton Mifflin Company. All rights reserved. 4 | 21
4.5. Precipitation Reactions

Copyright © Houghton Mifflin Company. All rights reserved. 4 | 22
Precipitation Reactions

Copyright © Houghton Mifflin Company. All rights reserved. 4 | 23
Solubility Rules
• How can a precipitation reaction be predicted ?

Copyright © Houghton Mifflin Company. All rights reserved. 4 | 24
Sample Problem
How much AgCl will be formed from mixing 1.50 L of 0.500 M AgNO3 with 1.75 L of 0.300 M NaCl?
( )
( )
+
-
0.500 mol Ag1.50 L 0.750 mol Ag1L
0.300 mol Cl1.75 L 0.525 mol Cl
+
−
=
=

Copyright © Houghton Mifflin Company. All rights reserved. 4 | 25
Sample Problem (cont)
The net ionic equation is:
Ag+(aq) + Cl−(aq) → AgCl(s)
Thus Cl- is the limiting reactant and 0.525 mol AgCl will be formed. In grams,
( ) 143.4g0.525 mol AgCl 75.3g AgCl1mol
=

Copyright © Houghton Mifflin Company. All rights reserved. 4 | 26
4.6. Acid-Base Reactions

Copyright © Houghton Mifflin Company. All rights reserved. 4 | 27
Acid-Base Reactions
Drain decloggers –contain s.base

Copyright © Houghton Mifflin Company. All rights reserved. 4 | 28
Acid-Base Reactions
• Neutralization reaction for strong acids and strong bases:
H+(aq) + OH-(aq) → H2O(l)

Copyright © Houghton Mifflin Company. All rights reserved. 4 | 29
Sample Problem
What volume of 0.200 M HCl solution is need to neutralize 25.0 mL of a 0.450 MKOH solution?
The balanced molecular equation is:
HCl(aq) + KOH(aq) → KCl(aq) + H2O(l)

Copyright © Houghton Mifflin Company. All rights reserved. 4 | 30
Sample Problem (cont)
( )
( )
-
-
- +
++
0.450 mol KOH 1mol OH 1 L25.0 mL1 L 1 mol KOH 1000 mL
0.01125 mol OHat neutralization, mol OH mol H
1mol HCl 1 L 1000 mL0.01125 mol H1 mol H 0.200 mol HCL 1 L
56.3 mL HCl
=
=
=

Copyright © Houghton Mifflin Company. All rights reserved. 4 | 31
4.7. Oxidation-Reduction Reactions
Zn(s) + Cu2+(aq)→ Zn2+(aq) + Cu(s)
oxidation reaction (loss of electrons)
Zn → Zn2+ (aq) + 2e-
reduction reaction (gain of electrons)
Cu2+ (aq) + 2e- → Cu

Copyright © Houghton Mifflin Company. All rights reserved. 4 | 32

Copyright © Houghton Mifflin Company. All rights reserved. 4 | 33
Sample Problem
Assign oxidation numbers to all atoms in the following:
CO2, K2Cr2O7, PCl5
CO2 – oxygen is -2×2 = -4, making C +4.
K2Cr2O7 – oxygen -2×7 = -14.
K (group 1A) is +1×2 = +2
2Cr must equal +12 = -14+2
each Cr = +6
PCl5 – Cl (group VIIA) = -1×5 = -5
P = +5

Copyright © Houghton Mifflin Company. All rights reserved. 4 | 34
Zinc and Iodine

Copyright © Houghton Mifflin Company. All rights reserved. 4 | 35
Dry Ice and Magnesium

Copyright © Houghton Mifflin Company. All rights reserved. 4 | 36
Reaction of Phosphorous