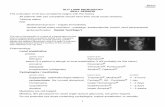
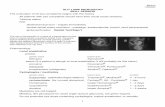
Slit lamp ..
-
Upload
rakesh-jaiswal -
Category
Health & Medicine
-
view
793 -
download
43
Transcript of Slit lamp ..
Slide 1
DR RAKESH JAISWAL
SLIT LAMP EXAMINATIONS
IntroductionAn instrument consisting of a high-intensity light source that can be focused to shine as a slit.
Used in conjunction with a microscope.
The lamp facilitates an examination which looks at anterior segment, or frontal structures, of the human eye, which includes the EyelidCorneaScleraConjunctivaIrisAnterior chamberNatural crystalline lens and Anterior vitreous.
Important historical landmarksDe Wecker 1863 devised a portable ophthalmomicroscope .
Albert and Greenough 1891,developed a binocular microscope which provided stereoscopic view.
Gullstrand ,1911 introduced the illumination system which had for the first time a slit diapharm in it
Therefore Gullstrand is credited with the invention of slit lamp.
TYPESThere are 2 types of slit lamp biomicroscope
1)Zeiss slit lamp biomicroscope
2)Haag streit slit lamp biomicroscope
In Zeiss type light source is at the base of the instrument while in Haag streit type it is at the top of the instrument.
Zeiss slit lamp biomicroscopeHaag streit slit lamp biomicroscope
PRINCIPLE A "slit" beam of very bright light produced by lamp. This beam is focused on to the eye which is then viewed under magnification with a microscope
How to start?Focus the eye piece
Adjust the headrest
Position the fixation target
Decrease the room illumination
Start with diffuse illumination
Use appropriate magnification
Basic slit lamp examination
Patient positioning:Head support unitAdjust height of table or chairAdjust height of chin rest such that patients lateral canthus is aligned with the mark.Adjust ocular eyepieces.
Power upFixationMagnification : begin with 6x -10x magnificationFocusingSpecial proceduresProtocol and documentation
MAGNIFICATIONS
Low magnification:7X - 10X : General eyeLids.Bulbar conjunctiva/sclera.Cornea/limbus.Tears.Anterior chamber/iris/crystalline lens.
Medium magnification:20X - 25X : Structure of individual layersEpithelium/epithelial breakdown.Stroma.Endothelium.Contact lens fit/lens condition.
High magnification:30X - 40X : DetailsEpithelium vacuoles microcysts dystrophies.Stroma striae folds.Endothelium Polymegathism guttata blebs cell density.
InstrumentationOperational components of slit lamp biomicroscope essentially consist of:
Illumination system
Observation system
Mechanical system
Illumination system
It consist of:
A bright ,focal source of light with a slit mechanismProvides an illumination of 2*10^5 to 4*10^5 lux.The beam of light can be changed in intensity,height,width,direction or angle and color during the examination with the flick of lever.
14
Condensing lens system:Consist of a couple of planoconvex lenses with their convex surface in apposition.Slit and other diapharm:Height and width of slit can be varied by using knobs.
Projection lens:Form an image of slit at eye.Advantages,1.keeps the aberration of lens down.2.increase the depth of focus of slit.
Reflecting mirrors and prismsFiltersYellow barrier filter Red free filterNeutral density filter Cobalt blue filterdiffuser
Observation system(microscope)
Observation system is essentially a compound microscope composed of two optical elements1.an objective ,2.an eyepieceIt presents to the observer an enlarged image of a near object.The objective lens consists of two planoconvex lenses with their convexities put togetherproviding a composite power of +22D. Microscope is binocular i.e. it has two eyepieces giving binocular observer a sterescopic view of eye.
The eye piece has a lens of +10D.
To overcome the problem of inverted image produced by compound microscope ,slit lamp microscope uses a pair of prisms b/w the objective and eyepiece to reinvert the image.
Most slit lamp provide a range of magnification from 6x to 40x
Mechanical systemJoystick arrangementMovement of microscope and illumination system towards and away from the eye and from side and side is achieved via joystick arrangement.Up and down movement arrangementObtained via some sort or screw devices.Patient support arrangementVertically movable chin rest and the provision to adjust height of table.
Fixation target:A movable fixation target greatly faciliates the examination under some conditions.Mechanical coupling :Provides a coupling of microscope and the illumination system along a common axis of rotation that coincides their focal planes.This ensures that light falls on the point where the microscope is focusedHas advantages when using the slit lamp for routine examination of anterior segment of eye.
Magnification control :Including two or pair of readily changeable objective lenses and two sets of eyepieces.
An on and off switch and illumination control .
Magnificationmay be changed byflipping a lever...
Changing filters.
biomicroscope
Patient positioningAlignment mark
Microscope and light source rotate indepedently
Filters used in slit lamp biomicroscopy
Cobalt blue filter
Used in conjunction with fluorescein stainDye pods in area where the corneal epithelium is broken or absent.The dye absorbs blue light and emits green.Uses:Ocular stainingRGP lenses fittingTear layer
Red free(green)filter:
Obscure any thing that is red hence the red free light , thus blood vessels or haemorrhages appears black. This increases contrast ,revealing the path and pattern of inflammed blood vessels.Fleischer ring can also be viewed satisfactorily with the red green filter.
Illumination techniquesIncludesDiffuse illuminationDirect illuminationParallilepipedOptic sectionConical(pinpoint)TangentialSpecular reflectionIndirect illuminationRetro-illuminationSclerotic scatterTransilluminationProximal illumination
Diffuse illumination
Angle between microscope and illumination system should be 30-45 degree.Slit width should be widest.Filter to be used is diffusing filter.Magnification: low to mediumIllumination: medium to high.
Optics of diffuse illuminationDiffuse illumination with slit beam and background illumination
Applications:
General view of anterior of eye: lids,lashes,sclera,cornea ,iris, pupil,Gross pathology and media opacitiesContact lens fitting.
Direct illumination
Involves placing the light source at an angle of about 40-50 degree from microscope.
This arrangement permits both light beam and microscope to be sharply focused on the ocular tissue being observed.
Wide beam direct illumination is commonly used as a preliminary technique to evaluate large area.
Parallelepiped:
Constructed by narrowing the beam to 1-2mm in width to illuminate a rectangular area of cornea.
Microscope is placed directly in front of patients cornea.
Light source is approximately 45 degree from straight ahead position.
Applications:Used to detect and examine corneal structures and defects.Used to detect corneal striae that develop when corneal edema occurs with hydrogel lens wear and in keratoconus.Higher magnification than that used with wide beam illumination is preferred to evaluate both depth and extent of corneal ,scarring or foreign bodies.it is particularly suitable for assessment of cataracts,scars,nerves,vessels etc.
Conical beam(pinpoint)
Produced by narrowing the vertical height of a parallelepiped to produce a small circular or square spot of light.Light source is 45-60 degree temporally and directed into pupil.Biomicroscope: directly in front of eye.Magnification: high(16-25x)Intensity of light source to heighest setting.
Focusing:Beam is focused between cornea and anterior lens surface and dark zone between cornea and anterior lens observed.Principle is same as that of beam of sun light streaming through a room ,illuminating airborne dust particles.This occurance is called tyndall phenomenon.Most useful when examining the transparency of anterior chamber for evidence of floating cells and flare seen in anterior uveitis.
Tyndall phenomenon
Cells, pigment or proteins in the aqueous humour reflect the light like a faint fog. To visualise this the slit illuminator is adjusted to the smallest circular beam and is projected through the anterior chamber from a 42 to 90 angle. The strongest reflection is possible at 90.
Optic section
Optic section is a very thin parallelepiped and optically cuts a very thin slice of the cornea.Axes of illuminating and viewing path intersect in the area of anterior eye media to be examined e.g. the individual corneal layers.Angle between illuminating and viewing path is 45 degree.Slit length should be kept small to minimize dazzling the patient.
With narrow slit the depth and portion of different objects(penetration depth of foreign bodies, shape of lens etc) can be resolved more easily.With wider slit their extension and shape are visible more clearly.Magnification: maximum.Examination of AC depth is performed by wider slit width .1-.3mm .
Used to localize:Nerve fibers Blood vesselsInfiltratesCataractsAC depth.
Optical section of lens1.Corneal scar with wide beam illumination 2.optical section through scar indicating scar is with in superficial layer of cornea.
Tangential illuminationThis technique is used to observe surface textureMedium wide beam of moderate height is used.Microscope is pointing straight ahead.Tangential light(projected from a oblique angle) creates shadowsMagnification of 10x,16x,or 25x are used.
Observe:Anterior and posterior corneaIris is best viewed without dilation by this method.Anterior lens (especially useful for viewing pseudoexfolation).
Example of tangential illumination (iris).
Specular reflection
Established by separating the microscope and slit beam by equal angles from normal to cornea.Position of illuminator about 30 degree to one side and the microscope 30 degree to otherside.Angle of illuminator to microscope must be equal and opposite.Angle of light should be moved until a very bright reflex obtained from corneal surface which is called zone of specular reflection.
Irregularities ,deposits ,or excavasation in these smooth surface will fail to reflect light and these appears darker than surrounding.Under specular reflection anterior corneal surface appears as white uniform surface and corneal endothelium takes on a mosaic pattern.Used to observe:Evaluate general appearance of corneal endotheliumLens surfaces Corneal epithelium
Schematic of specular reflection.
Reflection from front surfaceendothelium
Indirect illumination
The beam is focused in an area adjacent to ocular tissue to be observed.Main application:Examination of objects in direct vicinity of corneal areas of reduced transparency e,g, infiltrates,corneal scars,deposits,epithelial and stromal defectsIllumination:Narrow to medium slit beamDecentred beamMagnification: approx. m=12x (depending upon object size)
Retroillumination
Retroillumination is used to evaluate the optical qualities of a structureThe light strikes the object of interest from a point behind the object and is then reflected back to the observerA vertical slit beam 1-4mm wide can be used.Purpose:Place object of regard against a bright background allowing object to appear dark or black.
Used most often in searching for keratic precipitates and other debris on corneal endothelium.
The crystalline lens can also be retroilluminated for viewing of water clefts and vacuoles of anterior lens and posterior subcapsular cataract
Direct retroillumination from iris:
Used to view corneal pathology.A moderately wide slit beam is aimed towards the iris directly behind the corneal anomaly.Use magnification of 16x to 25x and direct the light from 45 degree.Microscope is directed straight ahead .
Schematic ofdirect retroillumination fromthe iris. direct retroillumination from the iris.
Indirect retroillumination from iris:Performed as with direct retroillumination but the beam is directed to an area of the iris bordering the portion of iris behind pathology.It provides dark background allowing corneal opacities to be viewed with more contrast.Observe:Cornea, angles.
Retroillumination from fundus(red reflex photography)In this technique, we are seeking to visualize media clarity and opacities.The light is directed so that it strikes the fundus and creates a glow behind the abnormalityThe defect creates a shadow in the lightThe slit illuminator is positioned in an almost coaxial position with the biomicroscope.A wide slit beam is decentered and adjusted to a half circle by using the slit width The decentred slit beam is projected near the pupil margin through a dilated pupil.
Focus the microscope directly on the pathology using 10X to 16X magnification
Observe: cornea, lens, vitreous
Schematic ofretroillumination from the retina.Example of retroillumination from the retina.
Sclerotic scatter
A tall, wide beam is directed onto the limbal area.Such an illumination technique causes cornea to take on total internal reflection.The slit beam should be placed approximately 40-60 degree from the microscope.When properly positioned this technique will produce halo glow of light around the limbus as the light is transmitted around the cornea. Corneal changes or abnormalities can be visualized by reflecting the scattered light.
Used to observe:Central corneal epithelial edemaCorneal abrasionsCorneal opacities
Schematic ofsclerotic scatter.Example of sclerotic scatter.
Proximal illumination
This illumination technique is used to observe internal detail, depth, and density. Use a short,fairly narrow slit beam. Place the beam at the border of the structure or pathology. The light will be scattered into the surrounding tissue, creating a light background that highlights the edges of the abnormality.
Depending on the density of the abnormality, the light from behind may reflect through, allowing detailed examination of the internal structure of the pathology.Observe: corneal opacities (edema, infiltrates, vessels, foreign bodies), lens, iris
Transillumination
In transillumination, a structure (in the eye, the iris) is evaluated by how light passes through it.Iris transillumination:This technique also takes advantage of the red reflex. The pupil must be at mid mydriasis (3to 4 mm when light stimulated). Place the light source coaxial (directly in line) with the microscope..
Use a full circle beam of light equal to the size of the pupil. Project the light through the pupil and into the eye . Focus the microscope on the iris. Magnification of 10X to 16X is adequateNormally the iris pigment absorbs the light, but pigmentation defects let the red fundus light pass through..Observe: iris defects (they will glow with the orange light reflected from the fundus)
Uses of slit lamp biomicroscopy
Diagnostic:OCTFFAAnterior segment and posterior segment diseasesDry eye
Procedures:ApplanationTear evaluationPachymetryGonioscopyContact lens fittingTherapeutic:LaserFB removalepilation
Injected conjunctivaMeibomian gland openings
pinquecula,
INSTILLATION OF FLUORESCEIN
PALPEBRAL CONJUNCTIVA EXAMINATION
Meibomian gland evaluation
With the patient at the biomicroscope, use white light and medium magnification to inspect the lower eyelid margins.
Look for capping of the meibomian gland orifices (yellow mounds), notching of the eyelid margins (indentations) and frothing of the tears on the eyelid margins.
Pull the lower eyelid down and look for concretions in the palpebral conjunctiva.
With mild pressure, press on the eyelid margins near the eyelashes and watch the meibomian gland orifices.
Clear fluid should be expressed.
Capping of the orifices, a cheesy secretion on expression and frothing of the eyelid margins indicates meibomian gland dysfunction.
Van Herrick TechniqueUse to evaluate anterior chamber angle without gonioscopyMedium magnificationAngle 60 degreesNarrow beam close to limbus
Depth of anterior chamber is evaluated it to the thickness of cornea: 4. grade open anterior chamber angle 1:1 ratio 3. grade open anterior chamber angle 1:2 ratio 2. grade narrow anterior chamber angle1:4 ratio 1. grade risky narrow anterior chamber angle less than 1:4 ratio 0. grade closed anterior chamber , cornea sits on iris
Van Herrick Technique
CENTRAL RETINA PHOTOGRAPHS WITH A 90-DIOPTER LENS
THANK YOU