Sepsis
-
Upload
maryko-awang-herdian -
Category
Documents
-
view
214 -
download
0
description
Transcript of Sepsis
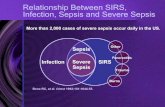
INTRODUCTIONSepsis is a clinical syndrome characterized by systemic inflammation due to infection. There is a continuum of severity ranging from sepsis to severe sepsis and septic shock. Over 1,665,000 cases of sepsis occur in the United States each year, with a mortality rate up to 50 percent [1]. Even with optimal treatment, mortality due to severe sepsis or septic shock is approximately 40 percent and can exceed 50 percent in the sickest patients [2-5].In this topic review, the management of severe sepsis and septic shock is discussed. Definitions, diagnosis, pathophysiology, and investigational therapies for sepsis, as well as management of sepsis in the asplenic patient are reviewed separately. (See "Sepsis and the systemic inflammatory response syndrome: Definitions, epidemiology, and prognosis" and "Pathophysiology of sepsis" and "Investigational and ineffective therapies for sepsis" and "Clinical features and management of sepsis in the asplenic patient".)THERAPEUTIC PRIORITIESThe early administration of fluids and antibiotics are the cornerstone of management for patients with severe sepsis and septic shock.Therapeutic priorities for patients with severe sepsis or septic shock include:Early initiation of supportive care to correct physiologic abnormalities, such as hypoxemia and hypotension [6-9].Distinguishing sepsis from systemic inflammatory response syndrome (SIRS) (table 1) because, if an infection exists, it must be identified and treated as soon as possible (table 2). This may require appropriate antibiotics as well as a surgical procedure (eg, drainage).EARLY MANAGEMENTThe first priority in any patient with severe sepsis or septic shock is stabilization of their airway and breathing. Next, perfusion to the peripheral tissues should be restored and antibiotics administered [9,10].Stabilize respirationSupplemental oxygen should be supplied to all patients with sepsis and oxygenation should be monitored continuously with pulse oximetry. Intubation and mechanical ventilation may be required to support the increased work of breathing that typically accompanies sepsis, or for airway protection since encephalopathy and a depressed level of consciousness frequently complicate sepsis [11,12].The choice and use of sedative and induction agents (eg, etomidate, ketamine) used to intubate patients with severe sepsis or septic shock are discussed separately. Other aspects of intubation and mechanical ventilation are similarly described elsewhere. (See "Sedation or induction agents for rapid sequence intubation in adults" and "Advanced emergency airway management in adults" and "Rapid sequence intubation in adults" and "The decision to intubate" and "The difficult airway in adults".)Chest radiographs and arterial blood gas analysis should be obtained following initial stabilization. These studies are used in combination with other clinical parameters to diagnose acute respiratory distress syndrome (ARDS), which frequently complicates sepsis. (See "Acute respiratory distress syndrome: Clinical features and diagnosis in adults" and "Mechanical ventilation of adults in acute respiratory distress syndrome".)Assess perfusionOnce the patient's respiratory status has been stabilized, the adequacy of perfusion should be assessed. Hypotension is the most common sign but critical hypoperfusion can also occur in the absence of hypotension, especially during early sepsis. Clinical signs of impaired perfusion include the following: Hypotension Hypotension is the most common indicator that perfusion is inadequate (eg, systolic blood pressure [SBP] 90 per min, obtundation or restlessness, and oliguria or anuria.
These findings may be modified by preexisting disease or medications. As examples, older patients, diabetic patients, and patients who take beta-blockers may not exhibit an appropriate tachycardia as blood pressure falls. In contrast, younger patients frequently develop a severe and prolonged tachycardia and fail to become hypotensive until acute decompensation later occurs, often suddenly. Patients with chronic hypertension may develop critical hypoperfusion at a higher blood pressure than healthy patients (ie, relative hypotension). Elevated lactate An elevated serum lactate (eg, >1 mmol/L) can be a manifestation of organ hypoperfusion in the presence or absence of hypotension and is an important component of the initial evaluation [9]. A serum lactate level 4 mmol/L is consistent with, but not diagnostic of, severe sepsis. Additional laboratory studies that help characterize the severity of sepsis include a low platelet count, and elevated international normalized ratio, creatinine, and bilirubin. Values for laboratory parameters that suggest severe sepsis are described separately. (See "Sepsis and the systemic inflammatory response syndrome: Definitions, epidemiology, and prognosis", section on 'Severe sepsis'.)Other Tests that combine output from many organs (eg, arterial lactate) may obscure the presence of significant ischemia in an individual organ [13]. Gastric tonometry indirectly measures perfusion to the gut by estimating the gastric mucosal PCO2. It can be used to detect gut hypoxia by calculating the gastric to arterial PCO2 gap [13-15]. But, gastric tonometry is not widely available and it is uncertain whether it can successfully guide therapy. Additional studies and clinical experience are needed.Establish venous accessVenous access should be established as soon as possible in patients with suspected sepsis. While peripheral venous access may be sufficient in some patients, particularly for initial resuscitation, the majority will require central venous access at some point during their course. A central venous catheter (CVC) can be used to infuse intravenous fluids, medications (particularly vasopressors), and blood products, as well as to draw blood for frequent laboratory studies. In addition, this access can be used for hemodynamic monitoring by measuring the central venous pressure (CVP) and the central venous oxyhemoglobin saturation (ScvO2). While in the past, a major purpose of a CVC was the measurement of ScVO2 and CVP, evidence from randomized trials on the value these targets to follow therapeutic effect is conflicting [16-18]. (See 'Goals of initial resuscitation' below and "Complications of central venous catheters and their prevention".)We believe that pulmonary artery catheters (PACs) should not be used in the routine management of patients with severe sepsis or septic shock. PACs can measure the pulmonary artery occlusion pressure (PAOP) and mixed venous oxyhemoglobin saturation (SvO2). In theory, this may be helpful to guide circulatory resuscitation. However, the PAOP has proven to be a poor predictor of fluid responsiveness in sepsis and the SvO2 is similar to the ScvO2, which can be obtained from a CVC [19,20]. PACs increase complications and have not been shown to improve outcome [21-23]. (See "Pulmonary artery catheterization: Indications and complications".)Interventions to restore perfusionThe rapid restoration of perfusion is predominantly achieved by the administration of intravenous fluids, usually crystalloids. Modalities such as vasopressor therapy, inotropic therapy, and blood transfusion are added, depending on the response to fluid resuscitation, evidence for myocardial dysfunction, and presence of anemia. (See "Treatment of severe hypovolemia or hypovolemic shock in adults".)Intravenous fluidsIn patients with sepsis, intravascular hypovolemia is typical and may be severe, requiring rapid fluid resuscitation. VolumeThe optimal volume of resuscitative fluid is unknown. As examples, several studies of early goal directed therapy (EGDT) reported mean infusion volumes that ranged from 3 to 5 liters [16-18]. The volume of fluid that was administered within the initial six hours of presentation was targeted to set physiologic endpoints (eg, mean arterial pressure). Thus, rapid, large volume infusions of intravenous fluids are indicated as initial therapy for severe sepsis or septic shock, unless there is coexisting clinical or radiographic evidence of heart failure. Suggested targets for fluid resuscitation are discussed separately. (See 'Goals of initial resuscitation' below.) Fluid therapy should be administered in well-defined (eg, 500 mL), rapidly infused boluses [9]. Volume status, tissue perfusion, blood pressure, and the presence or absence of pulmonary edema must be assessed before and after each bolus. Intravenous fluid challenges can be repeated until blood pressure and tissue perfusion are acceptable, pulmonary edema ensues, or fluid fails to augment perfusion. Careful monitoring is essential because patients with sepsis typically develop noncardiogenic pulmonary edema (ie, ARDS). Once patients with ARDS have been fluid resuscitated a liberal approach to intravenous fluid administration has been shown to prolong the duration of mechanical ventilation, compared to a more restrictive approach that also typically requires large doses of furosemide [24]. Thus, while the early, aggressive fluid therapy is appropriate in severe sepsis and septic shock, fluids may be unhelpful or harmful when the circulation is no longer fluid-responsive. (See "Acute respiratory distress syndrome: Supportive care and oxygenation in adults", section on 'Fluid management'.)Choice of fluidEvidence from randomized trials and meta-analyses have found no convincing difference between using albumin solutions and crystalloid solutions (eg, normal saline, Ringers lactate) in the treatment of severe sepsis or septic shock, but they have identified potential harm from using pentastarch or hydroxyethyl starch rather than a crystalloid solution [25-30]: Crystalloid versus albumin: In the Saline versus Albumin Fluid Evaluation (SAFE) trial, 6997 critically ill patients were randomly assigned to receive 4 percent albumin solution or normal saline for up to 28 days [25]. There were no differences between groups for any endpoint, including the primary endpoint, mortality. Among the patients with severe sepsis (18 percent of the total group), there were also no differences in outcome. In another multicenter open-label randomized trial of patients with severe sepsis or septic shock, the addition of albumin to crystalloid did not improve survival compared to crystalloid alone (31 versus 32 percent) [26]. Crystalloid versus hydroxyethyl starch: In the Scandinavian Starch for Severe Sepsis and Septic Shock (6S) trial, 804 patients with severe sepsis were randomly assigned to receive either 6 percent hydroxyethyl starch or Ringers acetate at a volume of up to 33 mL/kg of ideal body weight per day [27]. When assessed 90 days after randomization, mortality was increased in the hydroxyethyl starch group (51 versus 43 percent) and more patients in the hydroxyethyl starch group had required renal replacement therapy at some time during their illness (22 versus 16 percent). Crystalloid versus pentastarch: The Efficacy of Volume Substitution and Insulin Therapy in Severe Sepsis (VISEP) trial compared pentastarch to modified Ringer's lactate in patients with severe sepsis and found no difference in 28-day mortality [28]. The trial was stopped early because there was a trend toward increased 90-day mortality among patients who received pentastarch.In our clinical practice, we generally use a crystalloid solution instead of albumin solution because of the lack of clear benefit and higher cost of albumin. We believe that giving a sufficient quantity of intravenous fluids rapidly and targeting appropriate goals is more important than the type of fluid chosen. We do not use hydroxyethyl starch or pentastarch. These choices are consistent with the Society of Critical Care Medicine guidelines [9]. (See "Treatment of severe hypovolemia or hypovolemic shock in adults", section on 'Choice of replacement fluid'.) VasopressorsVasopressors are second line agents in the treatment of severe sepsis and septic shock; we prefer intravenous fluids as long as they increase perfusion without seriously impairing gas exchange [31]. However, intravenous vasopressors are useful in patients who remain hypotensive despite adequate fluid resuscitation or who develop cardiogenic pulmonary edema.In most patients with severe septic shock, we prefer to use norepinephrine (table 3) [7,9,32]. However, we find phenylephrine (a pure alpha-adrenergic agonist) to be useful when tachycardia or arrhythmias preclude the use of agents with beta-adrenergic activity (eg, norepinephrine). Choosing a vasopressor agent is discussed in greater detail elsewhere. (See "Use of vasopressors and inotropes", section on 'Choice of agent in septic shock'.) Additional therapiesThere is conflicting evidence on the use of additional therapies, such as inotropic therapy or red blood cell transfusion. Such therapies are targeted at increasing the cardiac output to improve tissue perfusion and thereby raise the central venous (superior vena cava) oxyhemoglobin saturation toward normal (ScvO2 70 percent). We prefer that their use be limited to those with refractory shock in whom the ScvO2 remains 7 g/dL) is derived from direct and indirect evidence from randomized studies of patients with septic shock:One multicenter randomized study of 998 patients with septic shock reported no difference in 28 day mortality between patients who were transfused when the hemoglobin was 7 g/dL (restrictive strategy) and patients who were transfused when the hemoglobin was 9 g/dL (liberal strategy) [35]. The restrictive strategy resulted in 50 percent fewer red blood cell transfusions (1545 versus 3088 transfusions) and did not have any adverse effect on the rate of ischemic events (7 versus 8 percent).Data from randomized studies of early goal directed therapy (EGDT) that use red blood cell transfusion as part of the protocol for treating patients with sepsis are conflicting. While one trial initially reported a mortality benefit from EGDT that included transfusing patients to a goal hematocrit >30 (hemoglobin level 10 g/dL) [16], two similarly designed studies published since then reported no benefit to this strategy [17,18]. These studies are discussed below. (See 'Protocol-directed therapy' below.) In further support of a restrictive approach to transfusion in patients with septic shock is the consensus among experts that transfusing to a goal of >7 g/dL is also preferred in critically ill patients without sepsis [36-38]. The use of blood transfusions in critically-ill patients is discussed in detail separately. (See "Use of blood products in the critically ill", section on 'Red blood cells'.)Goals of initial resuscitationThe goal of fluid resuscitation is early restoration of perfusion to prevent or limit multiple organ dysfunction, as well as to reduce mortality. The term "early goal-directed therapy" (EGDT) refers to the administration of intravenous fluids within the first six hours of presentation using physiologic targets to guide fluid management. EGDT has gained widespread acceptance in clinical practice but the optimal targets are unknown. Early goal-directed therapy targetsAlthough evidence is conflicting regarding the routine measurement of early goal-directed therapy targets, we suggest measuring the following targets for fluid management in patients with sepsis: Mean arterial pressure (MAP) 65 mmHg (MAP = [(2 x diastolic) + systolic]/3) Urine output 0.5 mL/kg/hourStatic or dynamic predictors of fluid responsiveness, eg, central venous pressure (CVP) 8 to 12 mmHg when central access is available (static measurement) or respiratory changes in the radial artery pulse pressure (dynamic measurement).Central venous (superior vena cava) oxyhemoglobin saturation (ScvO2) 70 percent (when central access is available) or mixed venous oxyhemoglobin saturation (SvO2) 65 percent (if a pulmonary artery catheter is being used). Lactate clearance is not readily available at the bedside, making it a less useful target to follow acutely in EGDT. Nonetheless, it should be followed as a target in patients with sepsis to ensure a trend that demonstrates adequate clearance with therapy. The optimal physiologic target(s) of EGDT is unknown. There is also conflicting evidence on the value of measuring such targets, particularly CVP and ScVO2, which require central catheter placement [16-18]. In addition, the generalizability of a standard targeted approach to both resource-poor and resource-rich facilities is unknown. We prefer measuring MAP and urine output as universal targets that can be readily measured in all patients with sepsis, with the addition of CVP and/or ScVO2 in those in whom central access is otherwise required. This approach differs slightly from that of The Surviving Sepsis Campaign guidelines that recommend central venous access for CVP/ScvO2 measurement together with MAP and urine output in all patients with severe sepsis [9]. However, these guidelines were created before the results of two major randomized trials (ProCESS and ARISE), that showed no mortality benefit to an EGDT-based approach, were published [17,18]. An additional ongoing large randomized study (eg, Protocolised Management In Sepsis [ProMISe]) should clarify whether or not protocol-driven EGDT should be routinely applied to patients with sepsis, and also identify the optimal target(s) to be followed. Evidence that supports the use of EGDT targets is described below:CVP, MAP and urine output CVP 8 to 12 mmHg, MAP 65 mmHg, and urine output 0.5 mL/kg per hour are common EGDT targets used in clinical practice. Support for their use is derived from clinical experience and their use in the single randomized trial that studied them with and without ScvO2 [16]. They have not been compared to each other nor have they been proven to be superior to any other target or to clinical assessment.The ideal targets for MAP, CVP, and urine output are unknown. One trial that randomized patients to a target MAP of 65 to 70 mmHg (low target MAP) or 80 to 85 mmHg (high target MAP) reported no mortality benefit to targeting a higher MAP [39]. Patients with a higher MAP had a greater incidence of atrial fibrillation (7 versus 3 percent), suggesting that targeting a MAP >80 mmHg is potentially harmful. ScvO2 Evidence from randomized trials that study the value of central venous oxyhemoglobin saturation (ScvO2) report mixed results. While one early trial of patients with septic shock reported a mortality benefit to targeting ScvO2 70 percent in a protocol-based therapy, trials published since then (ProCESS and ARISE) have reported no mortality benefit [16-18]. (See 'Protocol-directed therapy' below.) Lactate clearance Although the optimal frequency is unknown, we follow serum lactate (eg, every six hours), as an additional EGDT target in patients with sepsis until the lactate value has clearly fallen.The lactate clearance is defined by the equation [(initial lactate - lactate >2 hours later)/initial lactate] x 100. The lactate clearance and interval change in lactate over the first 12 hours of resuscitation has been evaluated as a potential marker for effective resuscitation [40,41]. One trial randomly assigned 300 patients with severe sepsis to undergo resuscitation targeting either a lactate clearance 10 percent or an ScvO2 70 percent (other than these targets, the resuscitation protocols that included MAP, CVP, and urine output targets were identical) [40]. There was no difference in hospital mortality, length of stay, ventilator-free days, or incidence of multiorgan failure, suggesting that lactate clearance criteria may be an acceptable alternative to ScvO2 criteria.After the restoration of perfusion, lactate is a poor marker of tissue perfusion [42]. As a result, lactate values are generally unhelpful following restoration of perfusion, with one exception a rising lactate level should prompt reevaluation of perfusion (see "Venous blood gases and other alternatives to arterial blood gases").Other Dynamic indices have been studied as a potential target to guide fluid management in sepsis. Respiratory changes in the vena caval diameter, radial artery pulse pressure, aortic blood flow peak velocity, and brachial artery blood flow velocity are considered dynamic hemodynamic measures, whereas CVP, MAP, ScvO2 and pulmonary artery occlusion pressure are considered static hemodynamic measures [43,44]. There is increasing evidence that dynamic measures are more accurate predictors of fluid responsiveness than static measures, as long as the patients are in sinus rhythm and passively ventilated with a sufficient tidal volume [19,45,46]. For actively breathing patients or those with irregular cardiac rhythms, an increase in the cardiac output in response to a passive leg-raising maneuver (measured by echocardiography, arterial pulse waveform analysis, or pulmonary artery catheterization) is a sensitive and specific predictor of fluid responsiveness [47]. Large randomized studies will be needed proving the efficacy of assessing dynamic measurement in response to intravenous fluids before they can be routinely applied to patients for the management of sepsis.Protocol-directed therapyProtocols targeted at the use of a combination of physiologic endpoints to guide fluid management in patients with severe sepsis and septic shock are common practice [16-18,48-50]. Typically, they combine the EGDT targets (ScvO2, CVP, MAP and urine output, lactate) for fluid management with early administration of antibiotics, both within the first six hours of presentation. There is conflicting evidence regarding the value of protocol-based therapy for sepsis [16-18,50-52]:One single center randomized trial of 263 patients with severe sepsis or septic shock compared a protocol that included targeting ScvO2 70 percent, CVP 8 to 12 mmHg, MAP 65 mmHg, and urine output 0.5 mL/kg/hour to conventional therapy that targeted CVP, MAP, and urine output only [16]. Both groups initiated therapy (including antibiotics) within six hours of presentation. Mortality was lower in the group where all four targets were used (31 versus 47 percent), suggesting that targeting ScvO2, CVP, MAP, and urine output was a superior strategy. There was a heavy emphasis on the use of red cell transfusion (for a hematocrit >30) and dobutamine in order to reach the ScvO2 target in this trial. In addition, the results of this trial may not be generalizable due to the inclusion of a significant number of sick patients with liver and heart disease that may have potentially biased the outcome favorably.A multicenter randomized trial (ProCESS) of 1341 patients with septic shock reported no mortality benefit with protocol-based therapies [17]. A protocol-based therapy that used all of the EGDT targets (ScvO2, CVP, MAP and urine output; protocol-based EGDT; central access required) was compared to a protocol that used some of the EGDT targets (MAP and urine output; protocol-based standard therapy; central access not required) and to usual care (no protocol used to direct fluid management). There were no differences in 60-day mortality between the groups (21 versus 18 versus 19 percent).
A similarly designed multicenter randomized trial of 1600 patients with septic shock (ARISE) also reported no mortality benefit from EGDT [18]. Compared to usual care, the 90 day mortality of 19 percent was similar in patients who received EGDT using the traditional targets outlined in prior studies.
One explanation for the apparent negative results from ProCESS and ARISE may be that central line placement was common (>50 percent) in patients receiving protocol-based standard therapy and usual care; it is likely that CVP and ScvO2 were measured and targeted in these patients as well. Lack of benefit may also be attributed to overall better outcomes in these studies, perhaps due to early administration of antibiotics (70 to 100 percent before randomization) in all groups, and to improved clinical performance by highly trained clinicians in academic centers during an era that follows an aggressive sepsis education and management campaign.Timing and durationThe early administration of fluid appears to be more important than volume or type of fluid in reducing mortality associated with sepsis. Based upon evidence from randomized studies and meta-analyses, we agree with The Surviving Sepsis Campaign guidelines that favor the initiation of fluid resuscitation within six hours of presentation [9]. Once the targets of resuscitation are met and perfusion is restored, fluids can be reduced or stopped, and occasionally patients can be diuresed, when necessary. Resolution of severe sepsis and septic shock can take as little as a few hours or can be protracted to days or weeks. A 2008 meta-analysis of randomized trials that initiated resuscitation targeting specific physiologic endpoints reported that compared to standard care, only trials that initiated resuscitation within 24 hours of the onset of sepsis showed a mortality benefit (39 versus 57 percent, odds ratio 0.50, 95% CI 0.37-0.69) [53]. In contrast, analysis of randomized trials that initiated therapy more than 24 hours after the onset of sepsis found no difference in mortality (64 versus 58 percent for standard resuscitation, odds ratio 1.16, 95% CI 0.60-2.22).There are two possible outcomes following the interventions described above:Inadequate perfusion Despite aggressive therapy, the patient may have persistent hypoperfusion and progressive organ failure. This should prompt reassessment of the adequacy of the above therapies, antimicrobial regimen, and control of the septic focus, as well as the accuracy of the diagnosis and the possibility that unexpected complications or coexisting problems have intervened (eg, pneumothorax following CVC insertion).Adequate perfusion Patients who respond to therapy should have the rate of fluid administration reduced or stopped, and vasopressor support weaned. Patients should also continue to have their clinical and laboratory parameters followed closely. These include blood pressure, arterial lactate, urine output, creatinine, platelet count, Glasgow coma scale score, serum bilirubin, liver enzymes, oxygenation (ie, arterial oxygen tension or oxyhemoglobin saturation), and gut function (table 4). Reevaluation is indicated if any of these parameters worsen or fail to improve. CONTROL OF THE SEPTIC FOCUSPrompt identification and treatment of the primary site or sites of infection are essential [54-56]. This is the primary therapeutic intervention, with most other interventions being purely supportive. Antibiotics should be administered within the first six hours of presentation or earlier. Identification of the septic focusA careful history and physical examination may yield clues to the source of sepsis and help guide microbiologic evaluation (table 5). As an example, sepsis arising after trauma or surgery is often due to infection at the site of injury or surgery. The presence of a urinary or vascular catheter increases the chances that these are the source of sepsis.Gram stain of material from sites of possible infection may give early clues to the etiology of infection while cultures are incubating. As examples, urine should be routinely analyzed via dipstick for leukocyte esterase, Gram stained, and cultured; sputum should be examined in a patient with a productive cough; and an intra-abdominal collection in a postoperative patient should be percutaneously sampled under ultrasound or other radiologic guidance.Blood should be drawn from two distinct venipuncture sites and inoculated into standard blood culture media (aerobic and anaerobic). For patients with a vascular catheter, blood should be obtained both through the catheter and from another site [9]. (See "Blood cultures for the detection of bacteremia".)If invasive candida or aspergillus infection is suspected, serologic assays for 1,3 beta-D-glucan, galactomannan, and anti-mannan antibodies, if available, may provide early evidence of these fungal infections [9]. The limitations of these assays and their role in the diagnosis of fungal infection are discussed separately. (See "Clinical manifestations and diagnosis of candidemia and invasive candidiasis in adults", section on 'Non-culture methods' and "Diagnosis of invasive aspergillosis", section on 'Galactomannan antigen detection' and "Diagnosis of invasive aspergillosis", section on 'Beta-D-glucan assay'.) There is no single test that immediately confirms the diagnosis of severe sepsis or septic shock. However, several laboratory tests, all of which are still investigational, have been studied as diagnostic markers of active bacterial infection [6]:Elevated serum procalcitonin levels are associated with bacterial infection and sepsis [57-59]. Despite this, a meta-analysis of 18 studies found that procalcitonin distinguished sepsis from nonseptic systemic inflammation poorly (sensitivity of 71 percent and specificity of 71 percent) [58] and another meta-analysis of six trials (four in patients with sepsis and two in patients with other infections) found that using clinical algorithms based upon procalcitonin levels did not affect mortality [60].The plasma concentration of soluble TREM-1 (triggering receptor expressed on myeloid cells), a member of the immunoglobulin superfamily that is specifically upregulated in the presence of bacterial products, is increased in patients with sepsis [61-63]. In a small trial, increased TREM-1 levels were both sensitive and specific for the diagnosis of bacterial sepsis (96 and 89 percent, respectively) [61]. However, a subsequent prospective cohort study found that increased TREM-1 levels predicted sepsis with a sensitivity and specificity of only 53 and 86 percent, respectively [64]. Serial monitoring of TREM-1 may also provide prognostic information in patients with established sepsis [62,63].Increased expression of CD64 on polymorphonuclear leukocytes indicates cellular activation and has been shown to occur in patients with sepsis [65,66]. In a prospective cohort study of 300 consecutive critically ill patients, increased CD64 expression predicted sepsis with a sensitivity of 84 percent and a specificity of 95 percent [64]. In this study, the sensitivity and specificity of increased CD64 expression were superior to that of increased procalcitonin or TREM-1 levels.The combination of procalcitonin levels, TREM-1 levels, and CD64 expression appears to be superior to the use of any of these markers alone. However, evaluation of the clinical usefulness of such biomarkers is still in its early stages and should be considered preliminary. Until additional clinical investigations have been performed, we do not suggest the routine use of such biomarkers to identify sepsis.Eradication of infectionPrompt and effective treatment of the active infection is essential to the successful treatment of severe sepsis and septic shock [9]. Source control (physical measures undertaken to eradicate a focus of infection and eliminate or treat ongoing microbial proliferation and infection) should be undertaken since undrained foci of infection may not respond to antibiotics alone (table 2). As examples, potentially infected foreign bodies (eg, vascular access devices) should be removed when possible, and abscesses should undergo percutaneous or surgical drainage. Some patients require extensive soft tissue debridement or amputation; in severe cases, fulminant Clostridium difficile-associated colitis may necessitate colectomy [67].Antimicrobial regimenIntravenous antibiotic therapy should be initiated within the first six hours, after obtaining appropriate cultures, since early initiation of antibiotic therapy is associated with lower mortality [7,68]. The choice of antibiotics can be complex and should consider the patient's history (eg, recent antibiotics received [69]), comorbidities, clinical context (eg, community- or hospital-acquired), Gram stain data, and local resistance patterns [7,70,71].Poor outcomes are associated with inadequate or inappropriate antimicrobial therapy (ie, treatment with antibiotics to which the pathogen was later shown to be resistant in vitro) [72-78]. They are also associated with delays in initiating antimicrobial therapy, even short delays (eg, an hour).A prospective cohort study of 2124 patients demonstrated that inappropriate antibiotic selection was surprisingly common (32 percent) [75]. Mortality was markedly increased in these patients compared to those who had received appropriate antibiotics (34 versus 18 percent).A retrospective analysis of 2731 patients with septic shock demonstrated that the time to initiation of appropriate antimicrobial therapy was the strongest predictor of mortality [76].When the potential pathogen or infection source is not immediately obvious, we favor broad-spectrum antibiotic coverage directed against both gram-positive and gram-negative bacteria. Few guidelines exist for the initial selection of empiric antibiotics in severe sepsis or septic shock. Staphylococcus aureus is associated with significant morbidity if not treated early in the course of infection [79]. There is growing recognition that methicillin-resistant S. aureus (MRSA) is a cause of sepsis not only in hospitalized patients, but also in community dwelling individuals without recent hospitalization [80,81]. For these reasons, we recommend that severely ill patients presenting with sepsis of unclear etiology be treated with intravenous vancomycin (adjusted for renal function) until the possibility of MRSA sepsis has been excluded. Potential alternative agents to vancomycin (eg, daptomycin for non-pulmonary MRSA, linezolid, ceftaroline) should be considered for patients with refractory or virulent MRSA, or a contraindication to vancomycin. These agents are discussed separately. (See "Treatment of invasive methicillin-resistant Staphylococcus aureus infections in adults", section on 'Bacteremia' and "Treatment of hospital-acquired, ventilator-associated, and healthcare-associated pneumonia in adults", section on 'Methicillin-resistant Staphylococcus aureus'.) In our practice, if Pseudomonas is an unlikely pathogen, we favor combining vancomycin with one of the following:Cephalosporin, 3rd generation (eg, ceftriaxone or cefotaxime) or 4th generation (cefepime), orBeta-lactam/beta-lactamase inhibitor (eg, piperacillin-tazobactam, ticarcillin-clavulanate), orCarbapenem (eg, imipenem or meropenem)Alternatively, if Pseudomonas is a possible pathogen, we favor combining vancomycin with two of the following (see "Principles of antimicrobial therapy of Pseudomonas aeruginosa infections"):Antipseudomonal cephalosporin (eg, ceftazidime, cefepime), orAntipseudomonal carbapenem (eg, imipenem, meropenem), orAntipseudomonal beta-lactam/beta-lactamase inhibitor (eg, piperacillin-tazobactam, ticarcillin-clavulanate), orFluoroquinolone with good anti-pseudomonal activity (eg, ciprofloxacin), orAminoglycoside (eg, gentamicin, amikacin), orMonobactam (eg, aztreonam)Selection of two agents from the same class, for example, two beta-lactams, should be avoided. We emphasize the importance of considering local susceptibility patterns when choosing an empiric antibiotic regimen.After culture results and antimicrobial susceptibility data return, we recommend that therapy be pathogen- and susceptibility-directed, even if there has been clinical improvement while on the initial antimicrobial regimen. Gram-negative pathogens have historically been covered with two agents from different antibiotic classes. However, several clinical trials and two meta-analyses have failed to demonstrate superior overall efficacy of combination therapy compared to monotherapy with a third generation cephalosporin or a carbapenem [75,82-86]. Furthermore, one meta-analysis found double coverage that included an aminoglycoside was associated with an increased incidence of adverse events (nephrotoxicity) [85,86]. For this reason, in patients with gram negative pathogens, we recommend use of a single agent with proven efficacy and the least possible toxicity, except in patients who are either neutropenic or whose severe sepsis is due to a known or suspected Pseudomonas infection [7,84]. (See "Pseudomonas aeruginosa bacteremia and endocarditis" and "Principles of antimicrobial therapy of Pseudomonas aeruginosa infections".)Regardless of the antibiotic regimen selected, patients should be observed closely for toxicity, evidence of response, and the development of nosocomial superinfection [87]. There are no published randomized controlled trials testing safety of de-escalation of antibiotic therapy in adult patients with sepsis or septic shock [88]. The duration of therapy is typically 7 to 10 days, although longer courses may be appropriate in patients who have a slow clinical response, an undrainable focus of infection, or immunologic deficiencies [7]. In patients who are neutropenic, antibiotic treatment should continue until the neutropenia has resolved or the planned antibiotic course is complete, whichever is longer. In non-neutropenic patients in whom infection is thoroughly excluded, antibiotics should be discontinued to minimize colonization or infection with drug-resistant microorganisms and superinfection with other pathogens.ADDITIONAL THERAPIESGlucocorticoidsGlucocorticoids have long been investigated as therapeutic agents in sepsis because the pathogenesis of sepsis involves an intense and potentially deleterious host inflammatory response. Evidence from randomized trials suggest that corticosteroid therapy is most likely to be beneficial in patients who have severe septic shock (defined as a systolic blood pressure 90 per min, obtundation or restlessness, oliguria or anuria, and lactic acidosis. (See 'Assess perfusion' above.)For patients with severe sepsis and septic shock, we recommend intravenous fluids, rather than vasopressors, inotropes, or red blood cell transfusions as first-line therapy for the restoration of tissue perfusion (Grade 1B). Therapy should be initiated as early as possible, within six hours of presentation. Fluid boluses are the preferred method of administration and should be repeated until blood pressure and tissue perfusion are acceptable, pulmonary edema ensues, or there is no further response. These parameters should be assessed before and after each fluid bolus (See 'Interventions to restore perfusion' above.) For initial fluid replacement, we suggest using a crystalloid solution rather than albumin-containing solution (Grade 2B) and recommend that a hyperoncotic starch solution NOT be administered (Grade 1A). (See 'Choice of fluid' above and "Treatment of severe hypovolemia or hypovolemic shock in adults", section on 'Choice of replacement fluid'.)For patients who remain hypotensive following intravascular volume repletion, we recommend vasopressors (Grade 1B); the preferred initial agent is norepinephrine. (See 'Vasopressors' above and "Use of vasopressors and inotropes", section on 'Choice of agent in septic shock'.)For patients with severe sepsis and septic shock that are refractory to intravenous fluid and vasopressor therapy, additional therapies, such as inotropic therapy and blood transfusions, are administered based on individual assessment. We typically reserve red blood cell transfusion for patients with a hemoglobin level