Segundo Cuaderno de Embriología Clínica, versión inglesa
-
Upload
asebir -
Category
Health & Medicine
-
view
2.369 -
download
7
description
Transcript of Segundo Cuaderno de Embriología Clínica, versión inglesa

CLINICAL EMBRYOLOGY PAPERS
II. ASEBIR criteria for the morphological evaluation of human oocytes, early embryos
and blastocysts. (2 nd edition)

ASEBIR CRITERIA FOR THE MORPHOLOGICAL EVALUATION OF HUMAN
OOCYTES, EARLY EMBRYOS AND BLASTOCYSTS.
ASEBIR working committee Coordinators
Manuel Ardoy
Gloria Calderón
Contributors Gemma Arroyo
Jorge Cuadros María José Figueroa
Raquel Herrer
Juan Manuel Moreno
Águeda Ortiz Fernando Prados Luz Rodríguez
Josep Santaló María José de los Santos
Jorge Ten
María José Torelló
Published by:
Asociación para el Estudio de la Biología de la Reproducción (ASEBIR). C/ Cronos 20, Edificio 4, 1º, 6ª 28037 Madrid Tel: 91 367 89 94 www.asebir.com · [email protected]
Design and layout: GÓBALO Gráfica · Web · Multimedia · Consultoría C/ Nogal 1, 1º 31 28250 · Torrelodones · Madrid · Tel Fax.: 91 859 57 22 www.gobalo.es · [email protected] Legal Deposit: M68262010

CONTENTS
LIST OF COMMITTEE MEMBERS . . . . . . . . . . . . . . . . . . ………………. . . . .1
PRESENTATION . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5
INTRODUCTION . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .. . . . . . .7
1. MORPHOLOGICAL EVALUATION AT EACH STAGE . . . . . . . . . . .. . . . .9 1.1 Morphological evaluation of the Oocyte . . . . . . . . . . . . . . . . . . . . . . . . . . . . …. . . . . . . . . . .9
1.1.1 Parameters evaluated at the oocyte stage. . . . . . . . . . . . . . . . . . . . . . ……… . . . . . .9 1.1.2 Oocytary parameters included in the classification system. . . . . . . . ………….. .. . . .16 1.1.3 Recommendations. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . ….... . . . . . . . . . .16
1.2 Morphological evaluation of the zygote. D+1 . . . . . . . . . . . . . . . . . . …. . . . . . . . . . . . . . . .17 1.2.1 Postinsemination observation time interval in D+1. . . . . . . . ………………….. . .17 1.2.2 Parameters evaluated at the D+1 stage. . . . . . . . . . . . . . . . . . . . . . ………….. . . .18 1.2.3 D+1 stage parameters included in the classification system. . . . . . . ………………. .24 1.2.4 Recommendations. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . ……. . . . . . . 24
1.3 Morphological evaluation at D+2 and D+3. . . . . . . . . . . . . . . . . . . . . . . . . . . . . ……... . . .25 1.3.1 Observation intervals at D+2 and D+3. . . . . . . . . . . . . . . . . . . . . . . . . …….. . . . .25 1.3.2 Parameters evaluated at the D+2 and D+3 stages. . . . . . . . . . . . . . . . ……... . . . .25 1.3.3 D+2 and D+3 stage parameters included in the classification system. ………...…... . .35 1.3.4 Recommendations. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . ….. . . .36
1.4 Morphological evaluation at the morula and blastocyst stages. D+4, D+5 and D+6 . . …... . .37 1.4.1 Postinsemination observation interval at D+4, D+5 and D+6 . . . . . . . . ……….. . .37 1.4.2 Parameters evaluated at the D+4, D+5 and D+6 stages . . . . . . . . . . . . . . ... . . . .37 1.4.3 D+4, D+5 and D+6 stage parameters included in the classification system………….. . .41 1.4.4 Recommendations. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . …... . . . . . . .42
2. GRADING SYSTEM FOR EMBRYO QUALITY. . . . . . . …. ………….. . . .43
2.1 Grading embryo quality at D+2 and D+3 transfers . . . . …………………….……. . .44 2.2 Grading embryo quality at D+5 and D+6 transfers . . …………………………. . . .45 2.3 Grading systems proposed for daytoday work. . . . . . . . . . . . ………………... . . . .46 2.4 Disagreements with the grading system. . . . . . . . . . . . . . . . . . . . . . ………. . . . . . . .49
3. GENERAL RECOMMENDATIONS. . . . . . . .. . . . . . . . . . . . . . . . . . . . . . . ..49
REFERENCES . . . . . . . . . . . . . . . . . . . ...................................... . . . . . . . . . . . .50

PRESENTATION
The ASEBIR committee for: “The definition of morphological evaluation criteria and their categorization, from Oocyte to Blastocyst” was created in 2004 to try to respond to the need for the unification of embryo evaluation criteria.
The lack of consensus in this key aspect of clinical embryology poses common problems, such as the inability to generate multicentre studies with a consistent evaluation of embryo quality, difficulties in the interpretation of clinical reports from other labs and the inability to compare bibliographical data.
The goal of the committee was to set out a proposal for morphological evaluation criteria and their categorization into scales for each of the various embryo stages.
In order to perform this work the committee has relied on three main tools: The exhaustive review of relevant literature including direct consultation with some of the authors; a multicentre survey based on this review and the actual experience of the members of the committee.
The result of this work was the creation of an embryo classification in categories which, together with the doubts about the essential parameters, are subject to future revision by the ASEBIR Interest Group “Embryo Quality”.
Lastly we should mention that we have considered it essential to include pictures with the morphological descriptions, it is of interest to note that these have been produced by members of the commission itself, meaning that the consensus on them is unanimous. We hope that this fact explains the variation in the quality and presentation of the photographs.

INTRODUCTION
Scientific evidence indicates that gestation in human largely depends on both endometrial receptivity and embryo quality; both aspects are far from being well studied.
In dealing with embryo quality the first decision to be made relates to the selection of the working tool. Twentynine years after the birth of the first baby by in vitro fertilization, the most common method for evaluating embryo quality is still the use of morphological parameters. Other criteria such as metabolic consumption, the study of aneuploides and other resources are proving to be effective and evidently have a promising future. However, morphology continues to be the most widespread and effective system in use (Gardner et al., 2001; Haggarty et al., 2006; Hamamah, 2005; Leese et al., 1993; Leese, 1995; Munne and Wells, 2002; Scott, 2003).
We will also need to decide which developmental stages should be chosen for the morphological classification of embryos. The stage usually chosen is that of the embryo during the second or third day of culture (D+2 or D+3) or the blastocyst stage (D+5 or D+6). However, recent studies have shown that evaluation during the other days of culture: oocyte (D+0), zygote and first cell division (D+1) and morula (D+4), can also be useful in the classification of embryos and so they are also considered in this study.
At each stage of the embryo development we analysed the morphological parameters extracted from the literature. The final classification system includes all parameters that are obviously related to the probability of successful embryo implantation. Some of the parameters that were excluded from the classification system have been proposed for use in helping to choose between several embryos falling within the same category. Lastly, certain morphological parameters require further study before they can be included in the decision making system.
Once all the morphological data has been collected we need to decide how they should be weighted within the final embryo classification system. We have two basic options in this respect: Evaluation either by category or by scoring. This committee has opted for the first alternative because it is a broader method and because of the difficulties in agreeing on suitable weighting levels for each of the parameters, although in the future we will definitely be concentrating our efforts on this task (Balaban et al., 2006).

Morphological Classification (Oocyte and Embryos). 9
1. MORPHOLOGICAL EVALUATION AT EACH STAGE 1.1 Morphological evaluation of the Oocyte
The oocyte maturation includes changes at the cytoplasm and at the nucleus, these are not always simultaneous nor do they occur at the same level of maturation. Oocytes having metaphase II nuclear maturation may present deficiencies in cytoplasmic maturation that would compromise the correct development of the embryo (Scott, 2003).
The review of the literature has shown us that a mature oocyte may present alterations or dimorphisms, which allow us to perform a morphological evaluation:
1.1.1 Parameters evaluated at the oocyte stage.
a) Cytoplasmic morphological alterations: Organelle clumping/localised granulation at the centre of the oocyte. Smooth endoplasmic reticulum clusters. Vacuoles. Cytoplasmic inclusions.
b) Extracytoplasmic morphological alterations: Exudates in the perivitelline space Anomalies in the zona pellucida Enlarged perivitelline space. Alterations to the first polar body: Fragmentation and size.
c) The cumuluscorona radiataoocyte complex
Organelle clumping/localised granulation at the centre of the oocyte
This is a common alteration of the cytoplasm of the oocyte, when viewed under the microscope it can be described as an individual dark mass with an excessively high granular content normally located at the centre of the oocyte (Kahraman et al., 2000; Meriano et al., 2001; Van Blerkom and Henry, 1992). Other apparent differences in cytoplasmic texture and density have also been described. Extensive cytoplasm granularity due to extensive vesiculization, may either be homogeneous, affecting the whole of the oocyte (Meriano et al., 2004; Van Blerkom and Henry, 1992); see image 1.

10 __________________Morphological Classification (Oocyte and Embryos).
Image 1.Localized granulation at the centre of the oocyte.
Smooth endoplasmic reticulum clusters (sERCs)
When viewed under an optical microscope this appears as a type of individual cytoplasmic inclusion that is slightly elliptical in shape and of a similar size to a pronucleus (Meriano et al., 2001; Serhal et al., 1997; Van Blerkom and Henry, 1992); see image 2.
Examined by transmission electron microscopy it has been confirmed that this dimorphism is the result of a massive accumulation of tubulartype smooth endoplasmic reticulum clusters (Otsuki et al., 2004).
Image 2. The presence of sERCs (R) and small vacuoles (V) in the oocyte.
Vacuoles
Vacuoles are different from aggregations of sER in that they are a type of cytoplasmic inclusion surrounded by a membrane and are filled with fluid, they are clearly distinguishable under the microscope (Otsuki et al., 2004; Van Blerkom,

Morphological Classification (Oocyte and Embryos). 11
1990); see image 3. Their number and size may vary considerably between different oocytes and even within each individual one (Van Blerkom and Henry, 1992). These vacuoles arise through the fusion of preexisting vesicles derived from Golgi apparatus and/or the smooth endoplasmic reticulum (El Shafie et al., 2000) or spontaneously (Van Blerkom, 1990).
Image 3. The presence of vacuoles in the oocyte.
Cytoplasmic inclusions
These include several types of cytoplasmic characteristics considered to be small areas of necrosis, these will include refractile bodies of ~10 μm in diameter and are composed of lipids and dense granules which may either be clumped together or isolated (Serhal et al., 1997, Suzuki et al., 2004) and also nonrefractile pyknotic bodies which normally appear as a horseshoe shape (Meriano et al., 2001); see image 4.
Image 4. The presence of inclusions. pyknotic body (PC), necrotic body (NC), refractile bodies (I).

12 __________________Morphological Classification (Oocyte and Embryos).
Exudates in the perivitelline space
The presence of granulation or detritus in the perivitelline space is considered to be an extracytoplasmic anomaly. This detritus is related to a deterioration of the internal zona pellucida, and any increase may affect the fertility of the oocyte after conventional IVF has been performed (Veeck, 1999b). Others authors observed that the presence of detritus may be a sign of an “overdose” of gonadotropins used in the stimulation protocols, although does not appear to be related to the quality of the embryo or rates of fertility, division, pregnancy or implantation (HassanAli et al., 1998). As such, this dimorphism may be a phenomenon related to the physical maturation of the oocyte (Balaban and Urman, 2006); see image 5.
Image 5. Detritus in the perivitelline space.
Anomalies in the zona pellucida
The zona pellucida undergoes changes in the thickness and appearance of the bilayer from D+1 to D+2; this is not noticeable at D+0. But other anomalies: a non circular profile, bulging, septation etc. remain during development and can be more easily detected at the time of microinjection when turning the oocyte; see images 6 to 9.
Image 6. Noncircular zona pellucida.

Morphological Classification (Oocyte and Embryos). 13
Image 7. Dark zona pellucida.
Image 8. Thick zona pellucida.
Image 9.Septated area

14 __________________Morphological Classification (Oocyte and Embryos).
Enlarged perivitelline space
This dimorphism can be clearly seen through a microscope due to the enlargement of the perivitelline space and makes the oocyte appear to be “floating” within the interior of the zona pellucida, see image 10. If the oocyte also presents a large first polar body this may be associated with a reduction in the embryo quality at D+2 (Xia, 1997).
Image 10. Enlarged perivitelline space.
Alterations of the first polar body (1 st PB):
It seems that the morphology of the 1st PB changes within hours of invitro culture and, as such, may vary depending on the observation time (Balaban et al., 1998). In fact, a positive correlation has been detected between the percentage of fragmentation of the 1st PB and the time passed between denudation and ICSI (Ciotti et al., 2004); as well as an increase in the fragmentation of the 1st PB over time (Verlinsky et al., 2003).
Some authors have suggested that the morphology of the 1st PB may be related to pregnancy and implantation rates (Ebner et al., 1999, 2000, 2002).
On the other hand, a variation in the size of the 1st PB (large or small) may be treated as an anomalous condition of the oocyte (Veeck, 1999b). The determining factor is probably the position of the meiotic spindle in relation to the surface of the oocyte. These oocytes may therefore present chromosomal abnormalities (Veeck, 1999b); see images 11 to 13.

Morphological Classification (Oocyte and Embryos). 15
Image 11. Different sizes of polar bodies. A, large; B, normal; C, small.
Image 12. Double polar body.
Image 13.Fragmented polar body.
The cumuluscorona radiataoocyte complex
The level of maturity of the complex has been used as an indicator of the oocyte maturity, although synchrony between both is questionable, above all in stimulated cycles (Hartshorne et al., 1999; Tarin and Pellicer, 1992). This may be due to differences in the sensitivity of the cells of the cumuluscorona complex and the nucleus of the oocyte to induced maturation through

16 __________________Morphological Classification (Oocyte and Embryos). gonadotropins and other follicular factors (Balaban and Urman, 2006).
1.1.2 Oocytary parameters included in the classification system.
The review of the literature undertaken reveals a large disparity in results (De Sutter et al., 1996; Loutradis et al., 1999) probably due to the fact that the studies are retrospective, are not standardised and, in their majority, are based on an insufficient number of cases. Table I shows a summary of the correlation of oocyte morphology with various parameters.
The current state of scientific knowledge does still not allow us to establish that the morphological parameters analyzed have a direct relationship, by themselves, with the most significant reproductive variables. For this reason we can not include oocyte quality as a relevant parameter in the categorization of the future embryo; although it is becoming more evident that it should be included in the future as more data is published.
Table I. The correlation of oocyte morphology with several parameters, according to the literature consulted. Yes, there is correlation; No, there is no correlation; NE, correlation not evaluated.
1.1.3 Recommendations
Based on the recent review of Balaban and Urman (2006) we can conclude that:
The results of the effect of extracytoplasmic alterations are controversial and there appears to be no relationship between these and the rate of implantation of the

Morphological Classification (Oocyte and Embryos). 17
resulting embryos. These oocytes, instead of being anomalous, may be considered to be phenotypical deviations resulting from the heterogeneity of the extracted oocytes.
Based on the published data it would appear to be more evident that severe cytoplasmic alterations, such as the presence of accumulations of smooth endoplasmic reticulum, the severe accumulation of centralised organelles/granulation and excessive vacuolization may prejudice the embryo development and so their implantation potential. As such, these severe cytoplasmic abnormalities will need to be taken into account when establishing the differences between embryos of the same morphological quality.
We recommend the use of some of these parameters to establish the differences between embryos of the same morphological quality:
Unfavourable:
Accumulations of the sER Central granularity
We also recommend the observation of the zona pellucida at this stage.
1.2 Morphological evaluation of the zygote. D+1
Visualization of the zygote has often been considered to be a useful tool for detecting defects or alterations of fertilisation. However, the process of fertilisation involves an ordered series of morphological changes that significantly affect the appearance of the cell. These changes are gaining more and more relevance in the final evaluation of the embryo quality. What is more, certain publications have advocated decisionmaking based solely on this stage, either due to observations from their own studies or the legal requirements of each country.
1.2.1 Postinsemination observation time interval in D+1.
Fertilisation involves a sequence of morphological changes. This fact implies that the time of observation of the pronuclei is crucial in the correct evaluation of the zygote.
The use of incorrect time ranges in the evaluation of fertilisation may cause several problems:

18 __________________Morphological Classification (Oocyte and Embryos).
At the pronuclear level: Nonvisualization of the pronuclei, size alterations, incorrect evaluation of juxtaposition.
Modified sizes of nucleolar precursor bodies.
Variation of the appearance of the cytoplasm.
Usual interval time for observing pronuclei vary between 16 and 22 hours from inseminationmicroinjection. This interval may mean that observations performed at between 20 and 22 hours run the risk of the pronuclei having already disappeared or their sizes changed; for this reason we recommend to decrease the range from 16 hours to a maximum of 19 hours for ICSI.
1.2.2 Parameters evaluated at the D+1 stage.
a) Morphological observations: Polar bodies (PB) (number and appearance) Pronuclear number Appearance of the pronuclei (PN):
Symmetry, synchrony and location Nucleolar precursor bodies (NPBs) (number, symmetry and polarization)
Cytoplasmic halo (presence and appearance) b) Early cleavage
Polar bodies
Number. There is no evidence that the number of polar bodies should be included in morphological evaluation. However, it is essential that they be considered when evaluating the ploidy in conjunction with the number of pronuclei; see image 14.
Image 14. Number of polar bodies at D+1.
Appearance. The appearance of a polar body usually refers to the alterations in their size or to fragmentation. There is currently no evidence to support the

Morphological Classification (Oocyte and Embryos). 19
inclusion of this parameter in morphological evaluation; see image 15.
Image 15. A, fragmented bodies; B, separated bodies.
Pronuclear number
The joint evaluation of the number of pronuclei, the number of polar bodies and the insemination technique being used (conventional or ICSI) will help us to decide what to do when, at D+1, we have zygotes with the morphology shown in images 16 and 17.
Image 16. A, 1 pronucleus + 2 polar bodies; B, 1 pronucleus + 1 polar body; C, 2 pronuclei + 1 polar body.
Image 17.A, 2 pronuclei + 2 polar bodies; B 3 pronuclei; C, micronuclei.

20 __________________Morphological Classification (Oocyte and Embryos).
Appearance of the pronuclei
The literature we reviewed contained several pronuclear parameters that unify the appearance of the pronuclei and the nucleolar precursor bodies (NPBs). Figures 1 and 2 show two common examples of pronuclear grading based on appearance (Scott, 2000; Tesarik and Greco, 1999). However, there is no relevant literature available to enable us to evaluate each individually; see images 18 to 23.
Figure 1. The pronuclear grading system proposed by Tesarik and Greco (1999).
Figure 2. The pronuclear grading system proposed by Scott et al.(2000).
Image 18. Separated pronuclei.

Morphological Classification (Oocyte and Embryos). 21
Image 19.A, different pronuclei; B, only one nucleolar precursor body (NPB) in a pronucleus.
Image 20. A, only two precursors (NPBs) in a pronucleus; B, polarization of the NPBs only in one pronucleus.
Image 21. A, peripheral pronuclei; B, asymmetric NPBs, the arrows indicate some of the inequalities.

22 __________________Morphological Classification (Oocyte and Embryos).
Image 22. A, polarized NPBs only in one of the pronuclei; B, polarized NPBs in both pronuclei. p: polarized, d: dispersed.
Image 23. A, atresic oocyte at D+1 after microinjection; B, presence of micronucleus indicated by the arrow.
No consensus was found in the literature when attempting to correlate the various pronuclear patterns with implantation rates, although there are three morphological characteristics which do appear to be associated to low implantation rates or with bad embryo morphology.
A single nucleolar precursor body in one pronucleus Separated pronuclei Pronuclei of unequal size
Cytoplasmic halo
This is the presence of a lighter cortical area in a part or in all of the cytoplasm. Several publications have come to the conclusion that this is a positive characteristic as long as it is not excessive. Zygotes with halo will tend to develop into higher quality embryos (60.9%) than zygotes without a halo (52.2%) with a significant statistical difference (Balaban and Urman, 2006; Payne et al., 1997; Salumets et al., 2001; Zollner et al., 2002); see images 24 and 25.

Morphological Classification (Oocyte and Embryos). 23
Image 24. A, zygote without a halo; B, zygote with an incomplete halo partially present around the cell; C; zygote with a complete halo.
Image 25. A, zygote with an excessive halo; B, zygote with a correct halo.
Early cleavage
An observation performed between 25 and 27 hours after insemination; see image 26. This parameter has been analysed in many studies but never by itself; it has always been studied in conjunction with the morphology of the zygote (Chen and Kattera, 2006), mononucleation at D+2 (Ciray et al., 2005) or the morphology at D+3(Neuber et al., 2003).
Image 26. An embryo with early division at 26 hours.

24 __________________Morphological Classification (Oocyte and Embryos).
These studies attempt to correlate early division with morphology at D+2 and D+3 (Ciray et al., 2006; Lundin et al., 2001), development up to blastocyst (Neuber et al., 2003), viability (Salumets et al., 2003; Shoukir et al., 1997), the implantation rate (Ciray et al., 2006; Rienzi et al., 2005) and the abortion rate (VanMontfoort et al., 2004). The usefulness of this observation is not unanimous. Most publications recommend the use of early cleavage as a “secondary parameter” to distinguish between embryos of a similar quality.
The following morphological parameters have been analysed: Occurrence of first cell division. Similarity of blastomeres. Presence of fragmentation. Multinucleation.
Ciray et al. (2006) reached the following conclusion: If we use early cleavage to decide at the time of transfer, we will select the embryo which, 26 hours after insemination, presents two blastomeres of the same size having no fragmentation and no multinucleation.
1.2.3 D+1 stage parameters included in the classification system
As with D+0, the review of the published data we undertook revealed a large variation in results. The current state of knowledge does not allow us to establish that the morphological parameters analyzed have an independent and unquestionable relationship to the implantation rate.
Quality at D+1, as well as the D+0 parameters, is likely to be included in the future as more data are published.
1.2.4 Recommendations
We recommend the use of some of the parameters analysed but not included in the classification system as a useful means to differentiate between embryos with the same morphological quality:
Favourable: The presence of a halo. Early cleavage.
Unfavourable: A single nucleolar precursor body. Separated pronuclei. Pronuclei of unequal size.

Morphological Classification (Oocyte and Embryos). 25
We also recommend that the results on table II be followed for deciding whether or not to continue embryo culture based on the number of polar bodies and pronuclei, bearing in mind that digyny involves the risk of triploidy and that parthenogenesis does not produce gestation.
PNPB relationship IVF ICSI
2 PN + 1 PB Discard*
1 PN + 2 PB** If it develops, 80% diploidy
If it develops, 20% diploidy
1 PN + 1 PB Discard
> 2PN Discard
2 PN + 2 PB Continue
Table II. Culture recommendations based on the number of polar bodies and pronuclei. * If you find 2 PN + 1 PB, the zygote should be discarded because the two possibilities are: parthenogenesis or a high likelihood of digyny with the associated risks for the foetus (Carceller et al., 2004). ** Continue up to laboratory decision.
1.3 Morphological evaluation at D+2 and at D+3.
Evaluation at these stages has traditionally formed the basis for determining embryo quality. However, while there is a certain amount of agreement on what constitutes a good embryo and what constitutes a bad one, it is very difficult to find a consensus between laboratories when attempting to evaluate intermediate quality embryos. A recent survey of IVF clinics in Spain demonstrated a large disparity in the criteria used for evaluating embryos. This disparity was more pronounced in the area of intermediatequality embryos (Bruna et al., 2005). The absence of a set of common criteria affects both the evaluation parameters and the cutoff points between categories; it also affects the algorithms used to define the morphological quality category of the embryo.
1.3.1 Observation interval at D+2 and D+3.
The recommended observation intervals are: D+2: 4447 hours postinsemination. D+3: 6771 hours postinsemination.
1.3.2 Parameters evaluated at the D+2 and D+3 stages.
Cell number and rate of division. The percentage and type of cell fragmentation. Inequalities in the sizes of blastomeres.

26 __________________Morphological Classification (Oocyte and Embryos).
Irregular shape of the blastomere Visualisation of the nucleus and level of multinucleation. Acytoplasmic ring. The presence of vacuoles. Zona pellucida. Level of early compaction/adhesion. Pitting.
Cell number and rate of division.
There are very few studies which provide a clear system for assigning different implantation rates for each number of cells or rate of division. In general, the literature we studied does not discriminate between this parameter and others which may clearly influence the potential for implantation: Multinucleation, fragmentation cutoff point, exclusion of observation at D+2 etc. (De Placido et al., 2002; Fisch et al., 2001; Neuber et al., 2003; Racowsky et al., 2003).
Based on the main publications and the results of the national multicentre survey organized by the ASEBIR committee, we can outline a classification system according to the number of cells at D+2 and the division rates for D+2 to D+3 (Desai et al., 2000; Hardarson et al., 2001; Van Royen et al., 2001); see images 27, 28 and 29.
Image 27. Cell number, cell studies from 2 to 5 blastomeres.

Morphological Classification (Oocyte and Embryos). 27
Image 28. Cell number, cell studies from 6 to 8 blastomeres.
Image 29. Cell number, cell studies from 9 to 10 blastomeres.
Transfer on D+2:
Using the current studies it is possible to group embryos with similar implantation rates according to the number of cells observed. From the best to the worst implantation rate:
4 cells. 2 or 5 cells. 3 or 6 cells. 1 cell or more than 6 cells.
While there are no significant differences between 2 and 5 cells, the presence of 5 cells tends to produce a better implantation rate. This tendency can also apply to the group of 3 or 6 six cells, being 3 cells slightly better than 6 cells on day 2.
Transfer on D+3:
The evaluation of the embryo on D+3 is dependent on the division rate between D+2 and D+3.

28 __________________Morphological Classification (Oocyte and Embryos).
Using current studies it is possible to group embryos as a function of the implantation rates as shown in table III.
Table III. Grouping of embryos according to cell division, from best to worst implantation rates.
Percentage and type of cell fragmentation
The presence of cell fragmentation is common in human embryos and does not always correlate to a low implantation rate.
As long as the level of fragmentation does not exceed 20% (Hardarson et al., 2001; Van Royen et al., 1999; Ziebe et al., 1997) or even 25% (Alikani et al., 2000; Racowsky et al., 2003), implantation of the embryo will not be compromised. Common observation methods for fragmentation levels do not allow us to detect differences smaller than 10 percentage points and so both these cutoff points (20% and 25%) are equally acceptable.
As well as the percentage of fragments, their size and distribution may also have a correlation with the probability of implantation (Alikani et al., 2000). When there is a predominance of large fragments, which may also be confused with small cells, spread throughout the embryo, there is a low probability of implantation. This is known as a “Type IV” fragmentation pattern (Alikani and Cohen, 1995) and is associated with a very low probability of implantation.
The evaluation of this parameter can be done both at D+2 and at D+3 and it can be used to divide embryo quality into four groups according to the percentage of fragmentation, see images 30 to 32:
≤ 10% 11 25% 26 35% >35%

Morphological Classification (Oocyte and Embryos). 29
Image 30. Embryos with 4 and 8 blastomeres with 10% fragmentation.
Image 31. Embryos with 15 – 25% fragmentation
Image 32. A, embryo with 35% fragmentation; B, embryo with type IV fragmentation.

30 __________________Morphological Classification (Oocyte and Embryos).
Inequalities in the sizes of blastomeres
When a zygote undergoes the first mitotic cell divisions, the cells not always cleave symmetrically and synchronically. An embryo undergoing synchronic and symmetric cleavage will present 2, 4, 8 or 16 cells of a similar size.
Inequalities in cell size are usually associated with a clear reduction in the ability for implantation (DePlacido et al., 2002;Hnida et al., 2004; Steer et al., 1992; Van Blerkom et al., 2000; Veeck, 1999a).
According to Hardarson, a 4 cell embryo with asymmetric cell division is one in which the difference between the diameters of the largest and smallest blastomeres is larger than 20% the diameter of the smallest. These unevenly sized blastomeres are related to a reduction in the implantation potential of the embryo. See figure 3 and image 33 (Hardarson et al., 2001).
Figure 3. Diameter ratios according to Hardarson.
Image 33. A, a ratio of 16% between blastomeres; B, a ratio of 24% between blastomeres.

Morphological Classification (Oocyte and Embryos). 31
When cell division is not synchronous, meaning that the embryos do not have 2, 4, 8 or 16 cells, it is evident that cells from two different cell cycles are coexisting. This implies that when we see embryos with 3, 5, 6, 7, 9 etc. blastomeres, we should also expect to see two different sizes of cells (Scott, 2003).
In an embryo with 3 cells, one of these must correspond to a blastomere of a 2 cell embryo which has not yet divided and which should be clearly larger than the other two cells, the sizes of these should be as expected for blastomeres at the 4 cell stage.
If cytokinesis has been symmetrical, 3 of the cells in a 5 cell embryo should have the size of the 4 cell stage and 2 of them the size of the 8 cell stage. This means we should expect to see 3 large cells and 2 small ones.
This means that inequalities in the sizes of the blastomeres should be compatible with a positive classification when they appear in the following way:
3 Cells 2 small + 1 large 5 Cells 2 small + 3 large 6 Cells 4 small +2 large 7 Cells 6 small + 1 large
Other size combinations imply asymmetric division with an anomalous distribution of cytoplasm between the resulting cells.
Irregular shape of the blastomere
A blastomere with an irregular shape may either have a physiological alteration or be undergoing division (Goyanes et al., 1990). It is difficult to evaluate the relationship of this characteristic with the potential for implantation and it has therefore not been included as a criterion for assigning embryo category. See image 34.
Image 34. Irregular shape.

32 __________________Morphological Classification (Oocyte and Embryos).
Visualisation of the nucleus and the level of multinucleation.
The presence of two or more nuclei or of micronuclei in a cell has a direct correlation with an increase in the rate of chromosomal anomalies (Hardarsonetal., 2001). The presence of multinucleated blastomeres implies a low implantation rate and increases the rate of abortion, although children have been born from multinucleated blastomeres (Jackson et al., 1998; Meriano et al., 2004; Pelinck et al., 1998;VanRoyen et al., 2003); see image 35.
Image 35. A, micronucleation; B, binucleation. The arrows indicate the positions of the nuclei.
Observation of the nuclei becomes more difficult as the number of cells or the fragmentation increases. In some cases the embryo will need to be rotated for us to be able to evaluate multinucleation and single nucleation.
Acytoplasmic ring
This is characterized by the apparent contraction of the cytoplasm leaving a large translucent ring with no organelles close to the edge of the blastomere. The presence of an acytoplasmic ring in the embryo has been related to a process of cellular lysis, see image 36. (V eeck, 1999b).
Image 36. Acytoplasmic ring (arrows)

Morphological Classification (Oocyte and Embryos). 33
The presence of vacuoles
This is associated with a reduction in the developmental potential. However there is no evidence in the literature relating the exact number of vacuoles, their size or distribution in the blastomeres, with implantation rates. Vacuoles with diameters of less than 5 μm may not compromise the development of the embryo (Veeck, 1999b); see image 37.
Image 37. A, presence of large vacuoles; B, a vacuole of approximately 5 µm (arrow).
Zona pellucida
Abnormalities of the zona pellucida are associated with a low implantation rate due to the lack of hatching (Calderón et al., 2002; Gabrielsen et al., 2001; Ruiz et al., 1999; Veeck, 1999b).
A healthy zona pellucida may be described as having a round silhouette and a thickness of approximately 17 μm, in addition to this it should not be homogeneous, it should not have a double layer due to the partitioning of the inner zone, there should be no bulges and it should be translucent in appearance (Gabrielsen et al., 2001; Goyanes et al., 1990; Veeck, 1999b); see images 38 and 39.
Image 38. A, septated zona pellucida (arrow); B, noncircular zona pellucida; C, homogeneous zona pellucida, the thickness of the zona pellucida are similar.

34 __________________Morphological Classification (Oocyte and Embryos).
Image 39. A, thick zona pellucida; B, dark zona pellucida.
In order to estimate the thickness of the zona pellucida we can take measurements using a monitor connected to an inverted microscope and a ruler; calibration will need to be performed first.
Level of early compaction/adhesion.
Early compaction is a sign of embryo activation which involves intercellular contact, the formation of intercellular bonds and the start of embryonary polarization (Gardner, 1989; Veeck, 1999c). We can distinguish between two levels of compaction; see image 40:
The initiation of adhesion: The stage at which the cells can be individually identified, although the membranes are adjacent.
Compaction: The stage at which it is difficult to distinguish individual blastomeres.
Image 40. A, an embryo without evident signs of the initiation of cellular adhesion; B, an embryo with signs of the initiation of cellular adhesion; C, a compacted embryo.

Morphological Classification (Oocyte and Embryos). 35
The references we reviewed did not provide sufficient evidence to allow us to include this parameter in an embryo classification system, possibly as it is highly influenced by the culture protocol and medium used, although some recommendations can be suggested based on the level of adhesion and the observation day (Desai et al., 2000; Wiemer et al., 1996).
Pitting.
Similarly to cell adhesion the appearance of this morphological parameter indicates that cytoplasmic activation has taken place and it should occur at the moment of physiological embryo activation at D+3. It appears as an orange peel effect which should not be confused with extensive vacuolization (Desai et al., 2000; Wiemer et al., 1996); see image 41.
Image 41. Embryo with pitting.
1.3.3 D+2 and D+3 stage parameters included in the classification system.
In order to be able to decide which morphological parameters could be included in an embryo classification system we looked for bibliographical references which confirmed the relationship between these parameters and the implantation rate. Despite the fact that the review produced very variable results, some morphological characteristics have a clear relationship with the implantation rate and can be included in the classification system. Some other parameters may also be used in proposing recommendation for embryo selection.
Parameters included in the classification system:
Cell number in transfer at D+2 according to the system shown.

36 __________________Morphological Classification (Oocyte and Embryos).
Cell division rate in transfers at D+3 according to the system shown.
Cell fragmentation according to the following grading system, from best to worst implantation rate:
≤10% 11 25% 26 35% >35% or presence of type IV fragmentation as per Alikani.
Blastomere asymmetry, from best to worst implantation rate: Equal or similar blastomeres. Uneven blastomeres with differences according to the Hardarson criteria.
Multinucleation, from best to worst implantation rate: Absence of multinucleated blastomeres. Presence of multinucleated blastomeres.
Acytoplasmic ring at D+3, from best to worst implantation rate: Absence of acytoplasmic ring. Presence of acytoplasmic ring.
Vacuoles, from best to worst implantation rate: Absence of vacuoles. Few vacuoles, diameters less than 5 μm. Abundant vacuoles.
Zona pellucida, from best to worst implantation rate: Normal zona pellucida. Abnormal zona pellucida
The significance of alterations in the zona pellucida can be reduced if there is the possibility of performing an Assisted Hatching. It is possible that this technique can increase the implantation rate (Calderón et al.,2002).
1.3.4 Recommendations.
We recommend the use of some of the following parameters to make a more refined selection between embryos with similar morphological quality.

Morphological Classification (Oocyte and Embryos). 37
Favourable: If you do not see2, 4, 8 or 16 cells during observation, there should be 2 different cell sizes corresponding to asynchronous division. Presence of mononucleation in all cells (Moriwaki et al., 2004) Initiation of adhesion as long as it appears at D+3 when the embryo has 7 or 8 cells. Evaluate according to culture protocol. The presence of pitting at D+3.
Unfavourable: Very advanced compaction at D+3. Evaluate depending on culture protocol. Initiation of adhesion or compaction at D+2. Evaluate depending on culture protocol. 3 cells of the same size at D+2.
1.4 Morphological evaluation at the morula and blastocyst stages. D+4, D+5 and D+6.
Transfer during the blastocyst stage is associated with higher implantation rates as in some cases it can allow for better embryo selection (Gardner et al., 2000). The embryo presents a complex structure at the blastocyst stage due to the large number of cells and their organization. Several authors have stated which are the key parameters for morphologically optimal blastocysts and which correlate with higher implantation rates (Balaban et al., 2000; Dokras et al., 1993; Gardner and Schoolcraft, 1999; Racowsky et al., 2003; Richter et al., 2001; Shoukir et al., 1998).
1.4.1 Postinsemination observation intervals at D+4, D+5 and D+6.
D+4: 9498 hours after insemination. D+5: 112120 hours after insemination. D+6: 136140 hours after insemination.
1.4.2 Parameters evaluated at the D+4, D+5 and D+6 stages.
Morula compaction. Embryo development. Blastocyst organization. Blastocoel expansion. Zona pellucida. Trophectoderm. Inner cell mass. Fragmentation. Vacuolization.

38 __________________Morphological Classification (Oocyte and Embryos). Morula compaction
Cell compaction is usually presented in embryos at around D+4 of culture although it initiates at around the 8 cell stage.Visually speaking it is characterized by the appearance of a compacted mass of cells.This compaction is due to the tight bonds formed between cells thus preventing to clearly distinguish their own shape. At this stage cells are no longer totipotent, corresponding to an embryo which has already become specialized. Therefore, the ability to see compaction at D+4 is a good prognosis for development. Compaction will sometimes only affect some of the cells of the embryo resulting in partial compaction.
Image 42. A, morula with partial compaction; B, morula with complete compaction.
At present, reported evidences do not allow us to draw conclusions on the possible correlation between the type of compaction and the probability of embryo implantation, although the latest studies have shown that partial compaction may imply a lower chance of implantation.
When partial compaction occurs, the proportion of cells undergoing compaction will be correlated to the size of the resulting embryo. Embryos which are smaller than usual have a lower chance of implantation (Tao et al., 2002).
Embryo development. Blastocyst organization
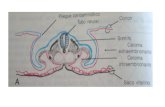
It is usually assumed that an embryo cultured in vitro reaches the blastocyst stage at D+5 or D+6. This range will vary depending on the culture system used. Embryos having a slower development rate may reach this stage at D+7 or even D+8; this situation implies a worse prognosis (Khorram et al., 2000; Richter et al., 2001; Shapiro et al., 2001). The following elements in the blastocyst should be taken into account (see image 43):

Morphological Classification (Oocyte and Embryos). 39
Blastocoele. Zona pellucida. Inner cell mass. Polar and mural trophectoderm.
Image 43. Blastocyst morphology.
Blastocoele expansion
The increase in the size of the blastocoele is very difficult to categorize and is very timedependent; especially due to the blastocoele collapse phases. If this event is observed, evaluation of the embryo should be postponed for several hours; this is a common phenomenon during the blastocyst stage and the morphology recovers after a short time lapse. The blastocyst is able to expand considerably in only a few hours, this will result in a corresponding thinning of the zona pellucida; see images 44 and 45.
Observing blastocoele expansion is associated with good implantation rates (Shoukir et al., 1998).
Image 44. A, early blastocyst; B, expanding blastocyst; C, expanded blastocyst.

40 __________________Morphological Classification (Oocyte and Embryos).
Image 45. A, Hatching blastocyst ; B, hatched blastocyst.
Zona pellucida
The thickness of the zona pellucida can greatly vary during blastocyst development. It will become thinner with the blastocoele expansion, reaching the minimum thickness when the blastocyst is completely expanded and hatching begins with the break of the zona pellucida. Some authors consider the thinning of the zona pellucida to be a favourable factor for implantation (Balaban et al., 2000; Racowsky et al., 2003; Yoon et al., 2001).
Trophectoderm
This structure presents a single layer of bonded cells which form the wall of the blastocoele or blastocyst cavity. The number, shape and cohesion degree of these cells will allow us to classify the blastocyst into several categories:
Homogeneous epithelium with elliptical cells. Irregular epithelium. Irregular epithelium with few cells.
Blastocysts having a suboptimal trophectoderm have good implantation rates as long as the inner cell mass has normal morphology (Kovacic et al., 2004).
Inner cell mass (ICM).
This is the most important parameter for defining the various categories of blastocyst. The inner cell mass should be oval in shape and its cells should be compacted.
The most favourable size varies between 1,900 and 3,800 μm 2 . Smaller sizes imply a lower implantation potential (Richter et al., 2001). 3,800 μm 2

Morphological Classification (Oocyte and Embryos). 41
is comparable to the size of a blastomere in a 4 cell stage embryo; see figure 4.
Figure 4. Comparison of blastomere size according to the stage of development and the size of the inner cell mass.
Fragmentation and vacuolization
Both parameters indicate the start of apoptosis, however there are no reported references directly relating them to implantation failures.
Presence of vacuoles and fragmentation are incompatible with a correct morphology of the blastocyst and those presenting these abnormalities should be assigned to the lowest category of embryo quality.
1.4.3 D+4, D+5 and D+6 stage parameters included in the classification system
Parameters included in the classification system:
Embryo development. Blastocyst organization, from best to worst implantation rate:
Blastocoele appearance at D+5. Blastocoele appearance at D+6. Blastocoele appearance at D+7 or D+8.

42 __________________Morphological Classification (Oocyte and Embryos).
Presence of two or more blastocoele cavities is related to lower implantation rates.
Blastocoele expansion:
When the blastocyst is organized, the blastocoele occupies almost the whole volume of the embryo. This parameter favourably correlates with the implantation rate.
Zona pellucida, from best to worst implantation rate:
Thinning appears at D+5. Thinning appears at D+6.
Trophectoderm, from best to worst implantation rate:
Trophectoderm has homogeneous epithelium and elliptical cells. Trophectoderm has an irregular epithelium. Trophectoderm has an irregular epithelium with few cells.
Inner cell mass, from best to worst implantation rate:
ICM sizes from1,900 – 3,800 μm 2 , with an oval compacted appearance. ICM size <1,900 μm 2 , with a compacted nonoval appearance.
Fragmentation and vacuolization:
As described.
1.4.4 Recommendations.
We recommend the use of some of the following parameters to establish differences between embryos of similar morphological quality.
Favourable: Compaction in the morula at D+4. Hatching signs.
Unfavourable: Appearance of the blastocoele at D+7 or even D+8.

Morphological Classification (Oocyte and Embryos). 43
2. GRADING SYSTEM FOR EMBRYO QUALITY. The various observations of an embryo should provide relevant information to classify it and to assign an implantation probability which will help us to reach conclusions with respect to transfer or freezing it.
From the material we have dealt with in this study we can conclude that an embryo should not be evaluated from just one isolated observation taken at a particular stage of its development. Categorization should be a result of several observations taken over the course of preimplantational development. Evaluating the quality of an embryo at a given moment of its development should take into account observations made at previous stages.
We have not included observations of the morphology of the oocyte and the zygote in our final conclusions due to a lack of consensus on the relevant characteristics influencing the quality of the embryo.
For our grading system we have divided the expected implantation potential of the embryo into 4 categories:
Category A: An embryo with optimal quality and the best implantation potential. Category B: An embryo of good quality and a high implantation potential. Category C: An acceptable embryo with an average chance of implantation. Category D: A poorquality embryo with a low chance of implantation.
Assigning an embryo to a particular category will depend on the morphological parameters which we have previously described.
This system is only suggested for the purposes of grading. The decision of what to do with an embryo of a certain category is left to the laboratory.
In order to classify the embryo we suggest you use tables IV and V which are based on observations of the embryos between D+2 and D+6.

44 __________________Morphological Classification (Oocyte and Embryos).

Morphological Classification (Oocyte and Embryos). 45
2.2 Grading embryo quality during transfers atD+5 and D+6.
The morphology of the embryo at the blastocyst stage should not be considered by itself as we have already mentioned for the D+3 stage. The final classification should depend on the observations made over the course of development. Observations of the embryo must be made within the time frames indicated in the text; see table VI.

46 __________________Morphological Classification (Oocyte and Embryos). 2.3 Grading systems proposed for daytoday work. .
Option 1. For transfers at D+2 and D+3.

Morphological Classification (Oocyte and Embryos). 47
Option 2. For transfers at D+2.

48 __________________Morphological Classification (Oocyte and Embryos).
Option 2. For transfers at D+3.

Morphological Classification (Oocyte and Embryos). 49
2.4 Controversies over the grading system. Some of the parameters reported in the literature could be compared with the partial analysis of the survey undertaken for IVF clinics. Other parameters could not be evaluated due to the disparity of the results between clinics. Others are still controversial with the evaluations reported in the literature; see table VII.
Table VII. Controversials over the grading system.
3.GENERAL RECOMMENDATIONS Participate in external quality control programs which include the morphological evaluation of embryos.
Ensure that there is a low variability between laboratories either by using an internal quality control system which includes embryo quality and/or by limiting the number of people dealing with this aspect.
Use image capture and storage systems which, as well as facilitating internal control, allow embryos to be evaluated later on without spending too much time out of the incubator.
In case that better embryo classification is needed, we recommend you opt for transferring at D+3 and that you respect the established observation times.
Use the category evaluation system described in this book although it is still open to correction.

50 __________________Morphological Classification (Oocyte and Embryos).
REFERENCES
Alikani M, Cohen J. Patterns of cell fragmentation in the human embryo. J Assist Reprod
Genet, 1995;12:28S.
Alikani M, Calderón G, Tomkin G, Garrisi J, Kokot M, Cohen J. Cleavage anomalies in
early human embryos and survival after prolonged culture invitro. Hum Reprod 2000;15:26342643.
Balaban B, Urman B, Sertac A, Alatas C, Aksoy S, Mercan R. Oocyte morphology does
not affect fertilization rate, embryo quality and implantation rate after intracytoplasmic sperm injection. Hum Reprod 1998;13:34313433.
Balaban B, Urman B, Sertac A, Alatas C, Aksoy S, Mercan R. Blastocyst quality affects the success of blastocyststage embryo transfer. Fertil Steril 2000;74:282287.
Balaban B, Urman B. Effect of oocyte morphology on embryo development and
implantation. Reprod Biomed Online 2006;12:608615.
Balaban B, Yakin K, Urman B. Randomized comparison of two different blastocyst
grading systems. Fertil Steril 2006;85:559563.
Carceller Beltrán R, Sáenz Moreno I, Gracia Cervero E, Bassecourt Serra M, Ureña
Hornos T, GarcíaDihinx Villanova J, et al. Carta al Editor. Triploidía completa 69XXY. An Pediatr (Barc) 2004;61:562564.
Bruna Catalán I, Pérez Milán F, Tur Padró R, Ricciarelli E, De la Fuente Hernández A, Monzó Miralles A, et al. Embarazo múltiple derivado de FIVICSI en España: incidencia y criterios sobre la transferencia embrionaria. Rev Iberoam Fertilidad 2005;22:99110.

Morphological Classification (Oocyte and Embryos). 51
Calderón G, Prados N, Caligara C, Mantrana E, Navarro J, Pellicer A, et al. Calidad
embrionaria. Indicadores predictivos de vitalidad. En: Remohí J, Pellicer A, Simón C,
Navarro J, editors. Reproducción Humana 2º ed. Madrid: McGrawHill Interamericana;
2002. p. 463468.
Ciotti PM, Notarangelo L, MorselliLabate AM, Felleti V, Porcu E, Venturoli S. First polar body morphology before ICSI is not related to embryo quality or pregnancy rate. Hum Reprod 2004;19:23342339.
Ciray HN, Karagenc L, Ulug U, Bener F, Bahçeci M. Use of both early cleavage and day 2 mononucleation to predict embryos with high implantation potential in
intracytoplasmic sperm injection cycles. Fertil Steril 2005;84:14111416.
Ciray HN, Karagenc L, Ulug U, Bener F, Bahçeci M. Early cleavage morphology affects the quality and implantation potential of day 3 embryos. Fertil Steril 2006;85:358365.
Chen C, Kattera S. Comparison of pronuclear zygote morphology and early cleavage status of zygotes as additional criteria in the selection of day 3 embryos: a randomized
study. Fertil Steril 2006;85:347352.
Desai NN, Goldstein J, Rowland DY, Goldfarb JM. Morphological evaluation of human
embryos and derivation of an embryo quality scoring system specific for day 3 embryos:
a preliminary study. Hum Reprod 2000;15:21902196.
De Placido G, Wilding M, Strina I, Alviggi E, Alviggi C, Mollo A, et al. High outcome
predictability after IVF using a combined score for zygote and embryo morphology and
growth rate. Hum Reprod 2002;17:24022409.
De Sutter P, Dozortsev D, Qian C, Dhont M. Oocyte morphology does not correlate with
fertilization rate and embryo quality after intracytoplasmic sperm injection. Hum Reprod 1996;11:595597.

52 __________________Morphological Classification (Oocyte and Embryos).
Dokras A, Sargent IL, Barlow DH. Human blastocyst grading: an indicator of developmental potential? Hum Reprod 1993;8:21192127.
Ebner T, Moser M, Yaman C, Feichtinger O, Hartl J, Tews G. Elective transfer of embryos selected on the basis of first polar body morphology is associated with increased rates of implantation and pregnancy. Fertil Steril 1999;72:599603.
Ebner T, Yaman C, Moser M, Sommergruber M, Feichtinger O, Tews G. Prognostic value of first polar body morphology on fertilization rate and embryo quality in intracytoplasmic sperm injection. Hum Reprod 2000;15:427430.
Ebner T, Moser M, Sommergruber M, Yaman C, Pfleger U, Tews G. First polar body morphology and blastocyst formation rate in ICSI patients. HumReprod 2002;17:24152418.
El Shafie M, Sousa M, Windt ML, Kruger TF. An atlas of the ultrastructure of human
oocytes. A guide for assisted reproduction. New York: The Parthenon Publishing Group
Inc.; 2000.
Fisch JD, Rodriguez H, Ross R, Overby G, Sher G. The Graduated Embryo Score (GES)
predicts blastocyst formation and pregnancy rate from cleavagestage embryos. Hum Reprod 2001;16:19701975.
Gabrielsen A, Lindenberg S, Petersen K. The impact of the zona pellucida thicknes variation of human embryos on pregnancy outcome in relation to suboptimal embryo development.
A prospective randomized controlled study. Hum Reprod 2001;16:21662170.
Gardner RL. Cell allocation and lineage in the early mouse embryo. Ciba Found Symp
1989;144:172181; discussion 181186, 208211.
Gardner RL, Schoolcraft WB. In vitro culture of human blastocysts. En: Cansen R,
Mortimer D editors. Toward reproductive certainty: fertility and genetics beyond.
Carnforth: The Parthenon Publishing Group Inc.; 1999. p. 378388.

Morphological Classification (Oocyte and Embryos). 53
Gardner DK, Lane M, Stevens J, Schlenker T, Schoolcraft WB. Blastocyst score affects implantation and pregnancy outcome: towards a single blastocyst transfer. Fertil Steril 2000;73:11551158.
Gardner DK, Lane M, Stevens J, Schoolcraft WB. Noninvasive assessment of human
embryo nutrient consumption as a measure of developmental potential. Fertil Steril 2001;76:11751180.
Goyanes VJ, RonCorzo A, Costas E, Maneiro E. Morphometric categorization of the
human oocyte and early conceptus. Hum Reprod 1990;5:613618.
Haggarty P, Wood M, Ferguson E, Hoad G, Srikantharajah A, Milne E, et al. Fatty acid
metabolism in human preimplantation embryos. Hum Reprod 2006;21:766773.
Hamamah S. Oocyte and embryo quality: is their morphology a good criterion? J Gynecol
Obstet Biol Reprod 2005;34(7 Pt 2):5S385S41.
Hardarson T, Hanson C, Sjögren A, Lundin K. Human embryos with unevenly sized
blastomeres have lower pregnancy and implantation rates: indications for aneuploidy and multinucleation. Hum Reprod 2001;16:313318.
Hartshorne G, Montgomery S, Klentzeris L. A case of failed oocyte maturation in vivo and in vitro. Fertil Steril 1999;71:567570.
HassanAli H, HishamSaleh A, ElGezeiry D, Baghdady I, Ismaeil I, Mandelbaum J.
Perivitelline space granularity: a sign of human menopausal gonadotrophin overdose in intracytoplasmic sperm injection. Hum Reprod 1998;13:34253430.
Hnida C, Engenheiro E, Ziebe S. Computercontrolled, multilevel, morphometric analysis of blastomere size as biomarker of fragmentation and multinuclearity in human
embryos. Hum Reprod 2004;19:288293.

54 __________________Morphological Classification (Oocyte and Embryos).
Jackson KV, Ginsburg ES, Hornstein MD, Rein MS, Clarke RN. Multinucleation in normally fertilized embryos is associated with an accelerated ovulation induction response and lower implantation and pregnancy rates in in vitro fertilizationembryo transfer cycles. Fertil Steril 1998;70:6066.
Kahraman S, Yakin K, Dönmez E, Samli H, Bahçe M, Cengiz G, et al. Relationship between granular cytoplasm of oocytes and pregnancy outcome following intracytoplasmic sperm injection. Hum Reprod 2000;15:23902393.
Khorram O, Shapiro SS, Jones JM. Transfer of nonassisted hatched and hatching human
blastocysts after in vitro fertilization. Fertil Steril 2000;74:163165.
Kovacic B, Vlaisavljevic V, Reljic M, CizekSajko M. Developmental capacity of different morphological types of day 5 human morulae and blastocysts. Reprod Biomed Online 2004;8:687694.
Leese HJ. Metabolic control during preimplantation mammalian development. Hum. Reprod Update 1995;1:6372.
Leese HJ, Conaghan J, Martin KL, Hardy K. Early human embryo metabolism. Bioessays
1993;15:259264.
Loutradis D, Drakakis P, Kallianidis K, Milingos S, Dendrinos S, Michalas S. Oocyte morphology correlates with embryo quality and pregnancy rate after intracytoplasmic sperm injection. Fertil Steril 1999;72:240244.
Lundin K, Bergh C, Hardarson T. Early embryo cleavage is a strong indicator of embryo
quality in human IVF. Hum Reprod 2001;16:26522657.
Mikkelsen AL, Lindenberg S. Morphology of invitro matured oocytes: impact on fertility potential and embryo quality. Hum Reprod 2001;16:17141718.

Morphological Classification (Oocyte and Embryos). 55
Meriano JS, Alexis J, VisramZaver S, Cruz M, Casper RF. Tracking of oocyte
dysmorphisms for ICSI patients may prove relevant to the outcome in subsequent
patient cycles. Hum Reprod 2001;16:21182123.
Meriano J, Clark C, Cadesky K, Laskin CA. Binucleated and micronucleated blastomeres in embryos derived from human assisted reproduction cycles. Reprod Biomed Online 2004;9:511520.
Moriwaki T, Suganuma N, Hayakawa M, Hibi H, Katsumata Y, Oguchi H, et al. Embryo
evaluation by analysing blastomere nuclei. Hum Reprod 2004;19:152156.
Munne S, Wells D. Preimplantation genetic diagnosis. Curr Opin Obstet Gynecol 2002;14:239244.
Nayudu PL, Gook DA, Hepworth G, Lopata A, Johnston WI. Prediction of outcome in human in vitro fertilization based on follicular and stimulation response variables. Fertil Steril 1989;51:117125.
Neuber E, Rinaudo P, Trimarchi JR, Sakkas D. Sequential assessment of individually cultured human embryos as an indicator of subsequent good quality blastocyst
development. Hum Reprod 2003;18:13071312.
Otsuki J, Okada A, Morimoto K, Nagai Y, Kubo H. The relationship between pregnancy
outcome and smooth endoplasmic reticulum clusters in MII human oocytes. Hum Reprod 2004;19:15911597.
Payne D, Flaherty SP, Barry MF, Matthews CD. Preliminary observations on polar body
extrusion and pronuclear formation in human oocytes using timelapse video
cinematography. Hum Reprod 1997;12:532541.
Pelinck MJ, De Vos M, Dekens M, Van der Elst J, De Sutter P, Dhont M. Embryos cultured in vitro with multinucleated blastomeres have poor implantation potential in human invitro

56 __________________Morphological Classification (Oocyte and Embryos).
fertilization and intracytoplasmic sperm injection. Hum Reprod 1998;13:960963.
Racowsky C, Combelles CM, Nureddin A, Pan Y, Finn A, Miles L, et al. Day 3 and day 5morphological predictors of embryo viability. Reprod Biomed Online 2003;6:323331.
Richter KS, Harris DC, Daneshmand ST, Shapiro BS. Quantitative grading of a human
blastocyst: optimal inner cell mass size and shape. Fertil Steril 2001; 76:11571167.
Rienzi L, Ubaldi F, Iacobelli M, Romano S, Minasi MG, Ferrero S, et al. Significance of morphological attributes of the early embryo. Reprod Biomed Online 2005;10:669681.
Ruiz A, Herrer R, Romero JLl, de los Santos MJ, Pellicer A, Remohí J. Calidad y transferencia embrionarias. En: Remohí J, Romero JL, Pellicer A, Simón C, Navarro J, editors. Manual Práctico de Esterilidad y Reproducción Humana. Madrid: McGrawHill Interamericana de España; 2000. p. 231237.
Salumets A, HydénGranskog C, Suikkari AM, Tiitinen A, Tuuri T. The predictive value
of pronuclear morphology of zygotes in the assessment of human embryo quality. Hum Reprod 2001;16: 21772181.
Salumets A, HydénGranskog C, Mäkinen S, Suikkari AM, Tiitinen A, Tuuri T. Early
cleavage predicts the viability of human embryos in elective single embryo transfer
procedures. Hum Reprod 2003;18:821825.
Scott L. Predicting Embryo Development. En: Rabe T, Dietrich K, Strowitzki T, editors.
Manual on Assisted Reproduction. Springer; 2000. p. 321338.
Scott L. The biological basis of noninvasive strategies for selection of human oocytes and embryos. Hum Reprod Update 2003;9:237249.
Serhal PF, Ranieri DM, Kinis A, Marchant S, Davies M, Kadhum IM. Oocytemorphology predicts outcome of intracytoplasmic sperm injection. Hum Reprod 1997;12:12671270.

Morphological Classification (Oocyte and Embryos). 57
Shapiro BS, Richter KS, Harris DC, Daneshmand ST. A comparison of day 5 and day 6 blastocyst transfers. Fertil Steril 2001;75:11261130.
Shoukir Y, Campana A, Farley T, Sakkas D. Early cleavage of invitro fertilized human
embryos to the 2cell stage: a novel indicator of embryo quality and viability. Hum Reprod 1997;12:15311536.
Shoukir Y, Chardonnens D, Campana A, Bischof P, Sakkas D. The rate of development
and time of transfer play different roles in influencing the viability of human blastocysts.
Hum Reprod 1998;13: 676681.
Steer CV, Mills CL, Tan SL, Campbell S, Edwards RG. The cumulative embryo score: a predictive embryo scoring technique to select the optimal number of embryos to transfer in an invitro fertilization and embryo transfer programme. Hum Reprod 1992;7:117 119.
Suzuki K, Yoshimoto N, Shimoda K, Sakamoto W, Ide Y, Kaneko T, et al. Cytoplasmic
dysmorphisms in metaphase II chimpanzee oocytes. Reprod Biomed Online 2004;9:5458.
Tao J, Tamis R, Fink K, Williams B, NelsonWhite T, Craig R. The neglected
morula/compact stage embryo transfer. Hum Reprod 2002;17:15131518.
Tarin JJ, Pellicer A. Oocyte maturation in human in vitro fertilisation programmes. Ann Acad Med Singapore 1992;21:492497.
Tesarik J, Greco E. The probability of abnormal preimplantation development can be predicted by a single static observation on pronuclear stage morphology. Hum Reprod 1999;14:13181323.
Van Blerkom J. Occurrence and developmental consequences of aberrant cellular organization in meiotically mature human oocytes after exogenous ovarian
hyperstimulation. J Electron Microsc Tech 1990;16:324346.



PUBLISHED BY:
EXECUTIVE BOARD
Mark Grossmann
Mª Victoria Hurtado de Mendoza
Nieves Cremades
Manuel Ardoy
Mª José de los Santos Begoña Arán
Jorge Cuadros
Fernando Marina
Jorge Ten
Montserrat Boada
María Bonada
Antonio Urries
With the collaboration of: