Science Advances - Supplementary Materials for · 2019-08-05 · The includes: Supplementary Method...
Transcript of Science Advances - Supplementary Materials for · 2019-08-05 · The includes: Supplementary Method...

advances.sciencemag.org/cgi/content/full/5/8/eaaw8337/DC1
Supplementary Materials for
Nitrogen cluster doping for high-mobility/conductivity graphene films with
millimeter-sized domains
Li Lin, Jiayu Li, Qinghong Yuan, Qiucheng Li, Jincan Zhang, Luzhao Sun, Dingran Rui, Zhaolong Chen, Kaicheng Jia, Mingzhan Wang, Yanfeng Zhang, Mark H. Rummeli, Ning Kang, H. Q. Xu*, Feng Ding*,
Hailin Peng*, Zhongfan Liu*
*Corresponding author. Email: [email protected] (Z.L.); [email protected] (H.P.); [email protected] (F.D.);
[email protected] (H.Q.X.)
Published 9 August 2019, Sci. Adv. 5, eaaw8337 (2019) DOI: 10.1126/sciadv.aaw8337
The PDF file includes:
Supplementary Method Fig. S1. Procedure for the growth of Nc-G films. Fig. S2. The continuous nucleation of nitrogen-doped graphene. Fig. S3. The effect of the oxygen-assisted etching-regrowth cycle on suppression of nucleation. Fig. S4. Structural characterization of as-grown large Nc-G single crystals. Fig. S5. Raman characterizations of Nc-G grains. Fig. S6. The isotopic labeling experiment to visualize the growth kinetics of millimeter-sized Nc-G grains. Fig. S7. The reported nμ and σ values function as μ in Nc-G of this work (red) and previous CVD doping strategies (navy blue). Fig. S8. STM images of the clustered nitrogen dopants in graphene lattice. Fig. S9. The STM and STS characterization of single-substitutional nitrogen-doped graphene. Fig. S10. Calculated dissociation energy of C-C-N. Fig. S11. STS measurements of Nc-G films. Fig. S12. N atoms prefer to stay on the edge of a C-N cluster. Fig. S13. C-N clusters without N atoms at the center are more stable. Fig. S14. C-N cluster with flat structure is more stable. Fig. S15. C-N cluster with high ratio of N atoms at the edge is more stable. Fig. S16. A series of triangular shaped C-N clusters with N edges have very low formation energies. Fig. S17. The doping efficiency of Nc-G film. Fig. S18. The large-scale conductivity and transmittance of Nc-G films. Fig. S19. Potential application of Nc-G films. Fig. S20. High electrostatic potential and quasi-bound states induced by nitrogen clusters. Legend for movie S1

Table S1. Mobilities and sheet resistance of previously reported intrinsic graphene. Table S2. Mobilities, conductivity, and stability of previously reported doped graphene. References (45–62)
Other Supplementary Material for this manuscript includes the following: (available at advances.sciencemag.org/cgi/content/full/5/8/eaaw8337/DC1)
Movie S1 (.mp4 format). Demonstration of a flexible touch screen device made from Nc-G film.

Fig. S1: Procedure for growth of Nc-G
The detailed procedure for growing Nc-G, including the annealing, passivation, nucleation,
etching, regrowth, and cooling steps, is shown in fig. S1A and in the section: Materials and
methods (below). Note that two strategies were used during growth to decrease nucleation
density of Nc-G. First, after the annealing step, oxygen was introduced into the chemical vapor
deposition (CVD) chamber to passivate active sites on the Cu foil, in accordance with previous
report (18). Second, after the first nucleation, a selective etching of those nuclei with small
domain size was carried out by introducing oxygen for a second time, which would also
passivate the remaining active sites. Nucleation during the regrowth step that followed was
thereby greatly suppressed (fig. S1B).
Fig. S1. Procedure for the growth of Nc-G films. Drawing of the growth procedure (A), and a
schematic illustration of the oxygen-assisted etching-regrowth cycle for growing Nc-G with
millimeter-sized domain (B). Note that the growth procedure shown in (A) is for a continuous
Nc-G film on Cu foil. The configuration of the graphene (square or hexagonal) is determined by
the lattice orientation of the underlying Cu substrate.

Fig. S2-3: Selective etching by oxygen to suppress graphene nucleation
After passivation of active sites on Cu foil, the nucleation density of graphene is still very high,
presumably as a result of efficient decomposition ability of acetonitrile (ACN) (fig. S2A).
Furthermore, the nitrogen-doped graphene (NG) grains on Cu foil exhibit a broad distribution of
domain sizes. In particular, the maximum domain size exceeds 200 μm, while the smallest is
around 10 μm. Such a broad distribution is attributed to difference in growth rate for each grain.
Nucleation and growth of the nitrogen-doped graphene is fueled by the active carbon species
formed by catalytic decomposition of the carbon source by Cu. Such catalytic capability of Cu is
mainly determined by the morphology of Cu foil. For instance, grain boundaries, dislocation, and
point defects on the Cu foil exhibit strong catalytic ability and thus a higher possibility for
graphene nucleation (19,45-46). Consequently, a spatial difference in catalytic ability leads to a
broad distribution of graphene grain size.
To explore the nucleation time of graphene grains, Cu foil was exposed to a constant flux of
ACN while a small amount of carbon-isotope labeled methane (13
CH4) was introduced in a
sequence of pulses. Because the growth of graphene on Cu is surface-mediated, and the Raman
modes of 12
C and 13
C are separated in wavelengths, thus, the temporal evolution, especially the
nucleation time of each grain, can be visualized by Raman maps of the isotopically labeled
nitrogen-doped grains, as described in previous reports (20). Note that, except for the
introduction of the isotopically labeled methane pulses, the growth parameters were kept the
same as in the normal nucleation of NG grains. In addition, the quantity of 13
CH4 introduced in
the pulses was very limited, to avoid an undesirable perturbation in the overall growth, and was
just sufficient to produce a detectable shift of the G or 2D band in the Raman spectrum.
Consequently, there were two characteristic Raman sources: graphene containing only 12
C,
which was formed entirely from ACN, and graphene containing 13
C and 12
C, which was grown
from a complex gas mixture consisting ACN and 13
CH4. Graphene composed of
13C and
12C
would indicate a G band and a 2D band at lower wavenumbers (20). In detail, a four-pulse

sequence was introduced during the nucleation step. As shown in the corresponding Raman maps
of the 2D band position (fig. S2B, inset), some graphene grains nucleated before the first pulses,
however, the domain size of the grains formed prior to the first pulse was quite different.
Furthermore, for some small nuclei, one or two of the labeling pulses were missing. Such an
observation suggests that the occurrence times of nucleus on the Cu substrate are quite different,
leading to a broad distribution of domain sizes; a nucleus formed earlier exhibiting a larger
domain size. In this regard, the nucleation time for each grain can be inferred from the plots of
domain size as a function of growth time during nucleation, assuming that the growth rate during
nucleation stage is constant and that there is no degradation due to the relatively lower graphene
coverage. The statistics of nucleation time of each grain are presented in fig. S2B, confirming
continuous nucleation of nitrogen-doped graphene.
During the subsequent oxygen etching step, the prominent differences in grain size predict a
highly different capability for grains to survive the etching. In this regard, grains with a larger
size will need a longer time to be totally removed by the oxygen, while smaller ones will be
removed more easily (selective etching). Thus, if etching time is carefully controlled according
to the domain size of Nc-G nuclei, only grains with larger grain size will remain after etching. In
the next step, the ACN and H2 were introduced into the system to initiate the regrowth of
remaining nitrogen-doped graphene nuclei. Furthermore, the oxygen would produce a second
passivation of the remaining active sites on the Cu foil, which is clear of graphene grains after
selective etching (18). When regrowth begins, a large quantity of active species is produced on
the Cu surface. These precursors for graphene are, nevertheless, forced to diffuse towards the
remaining nuclei and fuel the epitaxial growth from the edge, rather than producing new
nucleation centers, as a result of the constant passivation of active sites. Therefore, the overall
nucleation density is maintained during the regrowth. Consequently, the overall number of
as-formed grains is prominently reduced by etching, as evident in scanning electron microscope
(SEM) images of the graphene grains (fig. S3A). In particular, more growth-etching-regrowth

cycles can be used to further reduce the nucleation density (fig. S3B). The reduction of
nucleation density after two selective etching cycles is plotted in fig. S3C. Note that, after the
oxygen etching step, the remaining Nc-G grains exhibit a relatively narrow distribution,
confirming the selective etching effect of oxygen (fig. S3D) (18,47). Note that the average
nucleation density is 0.597 mm-2
, 0.463 mm-2
, 0.914mm-2
for graphene domains grown at 900 oC,
950 oC, 1000
oC, respectively. Although the enhanced growth temperature can result in a higher
nucleation barrier and reduced nucleation density, graphene grains grown at 1000 oC exhibit a
highest nucleation density presumably owing to the enhanced dissociation of acetonitrile, which
would produce more active carbon species for nucleation.
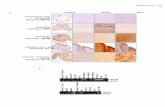
Fig. S2. The continuous nucleation of nitrogen-doped graphene. (A) SEM image of Nc-G
grains on Cu foil, formed after the first nucleation. (B) The statistics of nucleation time of the
grains, inferred from the Raman mapping results. The nucleation time is calculated from the
introduction of ACN to the presence of grains on Cu foil. Note that grains with a smaller domain
size, that is, containing less 13
C pulse, are formed after a relative longer duration, and thus
indicate a longer nucleation time. Inset: Raman map of 2D band position of the isotopically
labeled nitrogen-doped grains transferred onto SiO2/Si substrate, confirming a broad distribution
of domain size.

Fig. S3. The effect of the oxygen-assisted etching-regrowth cycle on suppression of
nucleation. SEM images of Nc-G grains on Cu foil formed after the first (A) and second (B)
oxygen-assisted etching-regrowth cycle (i.e., selective etching cycle). Note that the regrowth step
was stopped before full coverage in order to count the nucleation density. (C) Nucleation density
as a function of the number of growth-etching-regrowth cycles with 50 sccm H2 (green) and 100
sccm H2 (red). (D) Domain size distribution of the as-formed graphene nuclei after first
nucleation (green) and one growth-etching-regrowth cycle (red).

Fig. S4: Single-crystal nature of Nc-G grains with a millimeter-sized domain
Transmission electron microscopy (TEM) was performed to probe the detailed microstructure
and crystallinity of the as-synthesized graphene. The inset of fig. S4A displays an SEM image of
one graphene grain on the TEM grid. Selected-area electron diffraction (SAED) patterns were
obtained in a representative manner from 20 individual regions across the entire domain and
were extensively analyzed, as shown in fig. S4B-I. The distribution of the relative angles of
graphene lattices extracted from the SAED patterns is plotted accordingly (fig. S4A), confirming
the single-crystal character of millimeter-sized NG grains.
Fig. S4. Structural characterization of as-grown large Nc-G single crystals. (A) Histogram
of the angle distribution from the extensive SAED patterns within one millimeter-sized Nc-G

grain on a TEM grid. Inset: SEM image of the grain on the TEM grid (scale bar, 500 μm). (B-I)
Representative SAED patterns of the graphene grain collected across the graphene domain.

Fig. S5: Doping uniformity and quality of the graphitic-nitrogen doped graphene film
Raman spectroscopy was used to study the doping characteristics of Nc-G graphene, such as
doping uniformity and the crystalline quality of Nc-G graphene grains. The Nc-G single crystals
were transferred onto a SiO2/Si substrate using a “dry” transfer method, to avoid the
transfer-related doping (fig. S5A) (48). Note that, a uniform contrast of the transferred grain
indicates the monolayer nature on a large scale, which is also confirmed by Raman spectroscopy.
In contrast to pristine graphene, Raman spectrum of Nc-G (fig. S5B) exhibits a prominent D
band (~1351 cm-1
) and an accompanying D′ band (~1620 cm
-1), which stem from the elastically
scattered photoelectron generated by foreign atoms in a graphene matrix and intravalley double
resonance scattering processes, respectively (49). Furthermore, the observation of a strong 2D
band is indicative of the high quality of nitrogen doped graphene. In comparison to intrinsic
graphene, the blue shifts of the 2D and G bands were also observed, clearly revealing a shift of
the Fermi level as a result of nitrogen doping. Moreover, the corresponding Raman mapping of
defect-induced D band intensity confirms the doping uniformity (fig. S5C).
Fig. S5. Raman characterizations of Nc-G grains. (A) OM image of one Nc-G grain
transferred onto a SiO2/Si substrate. (B) Raman spectra for the Nc-G (red) and intrinsic graphene
grains (green), displaying a prominent D band in Nc-G. The ID/IG ratio ranges from 0.66 to 0.76,
which is also sensitive to the transfer process. (C) Raman map of D band intensity corresponding
to the same area as in (A).

Fig. S6: The growth rate of large Nc-G single crystals
In order to visualize the growth dynamics of the Nc-G grains (including the growth rate), Cu foil
was exposed to a constant flux of ACN and H2, along with a small amount of carbon-isotope
labeled methane (13
CH4) in a specific pulsed sequence. Figure S6A shows a schematic of the
CVD growth of isotopically labeled, millimeter-sized, nitrogen-doped graphene grains using an
oxygen-assisted growth-etching-regrowth strategy. In detail, after oxygen-assisted etching was
used to suppress the nucleation, Cu foil was subsequently exposed to ACN with a small amount
of 13
CH4 introduced in a specific sequence of pulses. The Raman G band position map (fig. S6B,
inset) clearly shows the spatial distribution of isotopic carbon atoms, in which the prominent
striped structure indicates the temporal sequence (marked as times t1, t2, t3, t4) of the 13
CH4
pulses. Therefore, in conjunction with the spatial distribution of the carbon isotopes (marked as
positions x1, x2, x3, x4), the time-dependent growth behavior can be visualized. The Nc-G grains
exhibit a concentric structure, confirming the surface-mediated growth mechanism and the
single-crystal nature of the millimeter-sized grains. The consistency of the rectangular domain
shape during the entire growth suggests the dominance of attachment-limited kinetics during
growth (50). From the time-dependent diameter of the Nc-G grain, the growth rate was
calculated to be around 160 μm/min (fig. S6B), among the best results reported with regarding to
the growth of high-quality large single-crystal graphene (19,50-52). The presence of surface
oxygen, and the higher decomposition rate of ACN, both contribute to the higher growth rate (19).
We believe two reasons that contribute to the improved growth rate. The first one is the low
dissociation energy for the reaction: C-C-NC+CN (0.42 eV), which would produce a large
number of active carbon and nitrogen species to fuel the growth, in contrast with the growth of
graphene by using methane. The second one is the presence of oxygen during the growth, which
would accelerate the growth of graphene by lowering the dissociation energy of carbon
precursors and attachment barrier of active carbon species.

Fig. S6. The isotopic labeling experiment to visualize the growth kinetics of millimeter-sized
Nc-G grains. (A) Schematic of the procedure for growing isotopically labelled millimeter-sized
Nc-G grains by introducing 13
CH4 in a specific pulsed sequence. The (t1, t2, t3, t4) denote times
when the 13
CH4-pulses were introduced. (B) The diameter of the corresponding Nc-G grains as a
function of the growth time. Inset: The Raman G13
-band position map of graphene grains in Fig.
1F. The positions (x1, x2, x3, x4) denote where the 13
CH4 pulses were detected, corresponding to
the times (t1, t2, t3, t4), from which the time evolution of the diameter can be inferred. The
domain-size scales linearly with growth time, showing a constant growth rate of 160 μm/min.

Fig. S7: Improved conductivity of Nc-G in comparison with previously reported doping
strategies.
Incorporation of heteroatoms during CVD growth aim to deliberately enhance the carrier
concentration to improve graphene conductivity (2,23,25-27,53-55). According to the equation:
𝜎 = 𝑛𝜇e (where n is the carrier concentration, μ is the carrier mobility, σ is the conductivity),
graphene conductivity is determined by the carrier mobility and carrier density (concentration) in
working devices. Thus, to present a reasonable comparison of conductivity between Nc-G and
other CVD-doping strategies, both the carrier density and mobility should be taken into
consideration. To attain this, we compared the values of 𝑛𝜇 and 𝜎 from different reports
regarding graphene doping.
We estimated the carrier concentration from the position of charge neutrality points (Dirac points)
from the transfer curve, according to the equation: 𝑛 = −α(𝑉G − 𝑉CNP), where α is related to the
gate capacity (For a 300 nm SiO2, α is 7.2 × 1010
cm-2
V-1
). Note that, as for the carrier mobility,
we directly summarized the reported values from literature, which were obtained using different
fitting models. The values of carrier density and mobility (𝑛𝜇) were usually presented for the
evaluation of conductivity and sheet resistance in applications of transparent conductive films
(13).
Consequently, the values of 𝑛𝜇, 𝜎 and 𝜇, obtained in Nc-G samples and other doping routines,
are presented in fig. S7 for comparison, indicative of significantly improvements in carrier
mobility and conductivity. Clearly, the 𝑛𝜇 value of Nc-G approaches the theoretical value. The
sheet resistance can also be estimated according to the equation: 𝑅s = (𝜎𝑑Gr.)−1, where 𝑑Gr. is
the thickness of monolayer graphene.

Fig. S7. The reported nμ and σ values function as μ in Nc-G of this work (red) and previous
CVD doping strategies (navy blue). The data points are labeled with the reference number from
whence they came in brackets. Theoretical value (orange) (carrier density and carrier mobility) is
obtained according to the previous theoretical work (11). Note that, the carrier mobility values
were obtained using different fitting models. Thus, the nμ values, obtained from literature, are
not the experimental results, which is only used for the comparison of material conductivity (13).

Fig. S8-11: Scanning tunneling microscopy (STM) characterization of Nc-G
STM/STS is an efficient technique for visualizing the atomic-level distribution of dopants in the
graphene lattice and the characterization of local electronic properties, and was used here to
probe the atomic arrangement of dopants in Nc-G films. Large-scale STM image reveals many
bright areas of 2-5 nm in diameter, which can be attributed to the clustering of graphitic-nitrogen
dopants in the hexagonal lattice (Fig. 2A and fig. S8). In sharp contrast to the randomly
distributed individual nitrogens in single-substitutional nitrogen doped graphene, as broadly
observed in previously reports (34,56) and in our results (fig. S9), the lateral dimensions of each
doping center in our Nc-G sample are much larger than that in single-substitutional nitrogen
doped samples. The STS measurements were performed to examine the effect of nitrogen
clusters on the local electronic structures (fig. S11). Similar features of the gap-like depression
near EF and near -400 meV dip relative to the tip, were seen in all of the dI/dV spectra, which
were obtained at points inside a single nitrogen cluster. The gap-like feature can be attributed to
the tunneling current decay near EF caused by graphene electron-phonon coupling (34). The -400
meV feature is associated with the position of Dirac point, confirming the n-type doping induced
by nitrogen-clusters in Nc-G film (34).
In addition, single substitutional nitrogen-doped graphene was synthesized using ammonia gas to
achieve a single-substitutional doping configuration (52). In contrast to nitrogen-cluster doping
in Nc-G films, the atomic-level structure of the ammonia-prepared sample, as shown in STM
images, is totally different, exhibiting a random distribution of single-substitutional N dopants
(fig. S9A), that is consistent with the previous report (34). In particular, the STM images show a
graphene lattice with bright points with lateral dimension less than a few atomic spacings,
indicating single substitution of carbon by nitrogen (34). A strong intervalley scattering tail was
also observed around each dopant in the enlarged STM image of one doping center (fig.S9B,
inset), consistent with previous work (34,57). We also performed detailed STS measurements of
the differential conductance dI/dV to probe the low-energy electronic structure of the

single-point doped graphene (fig. S9B). The depression near -300 meV, relative to the tip,
denotes the position of Dirac point, indicating a clear shift of the Fermi level as a result of
nitrogen doping.
To understand why acetonitrile uniquely contributes to the formation of nitrogen cluster, the
dissociation of C-C-N and C-N groups on Cu(100) surface was calculated. It is found that the
dissociation energy for C-C-NC+CN is only 0.42 eV, while the dissociation energy for
C-NC+N is as high as 2.2 eV (fig. S10). These results clearly show that the CN group
produced by the dissociation of acetonitrile on the Cu(100) surface is highly stable. This result
explains the uniqueness of acetonitrile in the formation of nitrogen cluster in graphene lattice. In
comparison with acetonitrile, NH3, which was broadly used to grow nitrogen doped graphene,
can be dissociated into single N atom only.
Fig. S8. STM images of the clustered nitrogen dopants in graphene lattice. (A to F),
Representative STM image of nitrogen-cluster doping centers observed in Nc-G film deposited
directly on Cu foil. Inset: fast Fourier transform (FFT) of topography shows atomic peaks (outer
hexagon, denoted by yellow cycles) and intervalley scattering peaks (inner hexagon, denoted by

cyan cycles). (B) is enlarged image of (A). The white arrow denotes the presence of cluster of
nitrogen dopants. For the STM measurements, the NG samples were transferred into the
ultra-high vacuum (UHV) system soon after preparation, followed by annealing in vacuum at
∼600°C to remove adsorbates. Scanning conditions: (A and B) 0.002 V, 30.77 nA; (B) -0.002V,
23.86 nA; (D) -0.002 V, 26.25 nA; (E) -0.002 V, 30.78 nA; (F) -0.021 V, 2.75 nA.
Fig. S9. The STM and STS characterization of single-substitutional nitrogen-doped
graphene. (A) STM image of the single-substitutional nitrogen-doped graphene film on Cu foil,
indicating the configuration of individual N-doping center, denoted by white arrows. (B) dI/dV
curves taken on the bright topographic feature (marked in the inset) near single-point doping
center on Cu. Inset: enlarged STM image of the location where the spectrum was taken.
Scanning conditions: (A) 1.0 V, 200 pA; (B) 0.8 V, 200 pA.

Fig. S10. Calculated dissociation energy of C-C-N. The calculated dissociation energy of
C-C-N (A) and C-N (B).
Fig. S11. STS measurements of Nc-G films. (A) An enlarged STM image from fig. S8A and
the locations where the STS spectra was taken. (B) dI/dV curves taken on the bright topographic
features in the nitrogen-cluster doping center, offset vertically for clarity. The red dashed lines
represent the positions of Dirac points. Scanning conditions: (A) 0.002 V, 30.77 nA; (B) -0.002 V,
16.83 nA.

Fig. S12-16: Density functional theory (DFT) calculation of the formation mechanism of
Nc-G films
In this section of the supporting information, density functional theory (DFT) is used in
calculations of the formation energy of various C-N clusters configurations.
Fig. S12. N atoms prefer to stay on the edge of a C-N cluster. (A) C18N3 clusters, forming four
different structures, and their formation energies. Lowest energy in each group is indicated in red.
The CN clusters with N atoms (blue) at the edges have lower formation energies. (B) C12N9
clusters: three different structures and their formation energies. The CN cluster with all N atoms
at the edge has the lowest formation energy. When three of the N atoms are moved to the center
of the cluster, the formation energy increases. The C12N9 cluster with only 3 N atoms at the edge
has the highest formation energy.

Fig. S13. C-N clusters without N atoms at the center are more stable. (A) The structures and
formation energies of C15N6 and C12N9 clusters. By replacing the three C atoms at the center of
C15N6 with N atoms, the CN cluster becomes unstable, which is indicated by the increase of
formation energy. (B) C18N6 and C15N9 clusters. Replacing the three C atoms at the center of
C18N6 with N atoms increases the formation energy of the CN cluster. (C) C7N6 and C6N7
clusters. Replacing the C atom at the center of C7N6 with N atoms leads to C6N7 and increased
formation energy. (D) C7N7 and C6N8 clusters. Replacing the C atom at the center of C7N7 with N
atoms leads to C6N8 and increased formation energy.

Fig. S14. C-N cluster with flat structure is more stable. The structures and formation energies
of CN clusters with and without pentagons. (A) A CN cluster composed of 3 hexagons is more
stable than 3 pentagons. (B) A CN cluster of 6 hexagons is more stable than one composed of 3
hexagons and 3 pentagons. (C) A CN cluster composed entirely of hexagons is more stable than
those composed of 3 pentagons and 4 hexagons.

Fig. S15. C-N cluster with high ratio of N atoms at the edge is more stable. The structures
and formation energies of triangular (black squares) and hexagonal (red circles) CN clusters with
different ratios of N atoms at the edge. For both triangular and hexagonal CN clusters, the
formation energy of the cluster decreases with an increase of the N ratio at the cluster edge. The
formation energy is lowest when all edge atoms are N atoms. Compared to the hexagonal-shaped
CN clusters, the triangular-shaped CN clusters have lower formation energy.
Fig. S16. A series of triangular shaped C-N clusters with N edges have very low formation
energies.

Fig. S17: The tunability of the Fermi level of Nc-G films
The controllability of dopant concentration in Nc-G films can be realized by tuning the growth
temperature of Nc-G film. As reported in previous works, increasing the growth temperature
usually results in a low doping concentration in graphene as a result of the formation energy
(bonding energy) for the C-C bond being higher than for C-N. Consequently, at elevated
temperature, formation of the more stable C-C bond is favored, resulting in a reduced doping
level. Thus, we tune the Fermi level of the as-formed Nc-G by growing Nc-G at different
temperatures (900 oC, 950
oC, and 1000
oC).
The adjustment range of Fermi level is shown to be around 350 meV relative to intrinsic
graphene, as verified by the UV photoelectron spectroscopy (UPS) (fig. S17A). Furthermore,
transport measurements were performed to evaluate the electrical properties of the Nc-G films
grown at various temperatures, as well as to characterize the potential to adjust the Fermi levels.
The transfer-characteristic curves of samples denoted 900 Nc-G, 950 Nc-G, and 1000 Nc-G (fig.
S17B) all exhibit negative charge neutrality points (Dirac points) with different shifts relative to
the zero value, which is the hallmark of different electron-doping levels. The distance between
the charge neutrality point and the zero value corresponds to the Fermi level shift in Nc-G films
compared to pristine graphene. Using the formula (58)
𝐸F = ℏ𝜈F√𝜋𝑛 (S1)
Where 𝜈F denotes the Fermi velocity.
We extract the Fermi level shift (∆𝐸F) in different samples from these curves, revealing that the

∆𝐸F can be tuned from 180 meV (900 Nc-G) to 90 meV (1000 Nc-G), relative to intrinsic
graphene. XPS measurements were also performed to probe the nitrogen concentration indicating
that the atomic concentration of nitrogen dopants can be tuned from 1.4% to 0.6% (fig. S17C).
Raman spectroscopic characterization is also informative with respect to doping level in
graphene. In particular, the D band intensity reflects the concentration of dopants or defects in
the graphene lattice, and the intensity ratio of the D band to the G band (ID/IG) is usually
presented to reflect the doping concentration (45). The Raman spectra of the Nc-G films, formed
at different temperatures (fig. S17D), exhibit a reduction of the D band intensity with elevated
growth temperature with ID/IG ratios of 0.60, 0.25, and 0.15 for 900 Nc-G, 950 Nc-G, and 1000
Nc-G, respectively. ID/IG ratios for single-substitutional nitrogen doped graphene (Ns-G) and
nitrogen doped graphene containing pyridinic and substitutional nitrogen (Np,s-G) are 0.70 and
0.96 respectively. Without using the oxygen-assisted growth, a higher D band intensity was
observed in Np,s-G than that of Nc-G using the oxygen-selective-etching strategy. Furthermore,
it was found that the intensity ratio of the D and D′ peak is reduced in Nc-G indicating an
elimination of defective pyridinic nitrogen after the oxygen-selective-etching step.
In addition, nitrogen-doping induces a noticeable shift of the G band position, which can be used
to directly measure the carrier concentration and Fermi level of doped graphene. In this regard, a
successive blue shift of G band in the 900 Nc-G, 950 Nc-G, and 1000 Nc-G samples were
observed, confirming the dependence of introduced carrier concentration on the formation
temperature (fig. S17E). Thus, all the observations above confirm the possibility in tuning the
doping levels in Nc-G samples through the control over growth temperature.
Nitrogen doping is a method to inject additional electrons into the graphene system, and to shift
graphene Fermi level. Thus, the conductivity would be enhanced in nitrogen-doped graphene to
broaden its electronic application appeal. However, the doping configuration uniquely
determines the doping efficiency, i.e. how much added nitrogen in a graphene system is required

to achieve a desired shift of the Fermi level. In particular, only substitutional doping can induce
n-doping in graphene, as pyridinic nitrogen would disrupt the graphene lattice and usually induce
a contradictory doping effect (p-doping) (14). The doping efficiency is an important parameter in
graphene doping, because the nitrogen atoms in the graphene lattice function as electronic
scattering centers, reducing the quality (i.e. carrier mobility) of the as-formed doped graphene.
Consequently, a method with lower doping efficiency requires a higher quantity of nitrogen
dopant to induce the same number of electrons than one with a higher doping efficiency. In turn,
this would lead to a significant reduction in carrier mobility and conductivity.
Firstly, we compare the nitrogen doping efficiency of Nc-G sample with single-substitutional
nitrogen doped sample (20,52). The previously reported value of nitrogen concentration in
single-substitutional doped sample is 2.0%, higher than the 1.6% in 900 Nc-G (results obtained
from XPS results). However, as evidenced by the G band shift of Raman results (fig. S17D, E),
the ∆𝐸F in single-substitutional doped graphene (~257 meV) is clearly smaller than that in
Nc-G sample (~363 meV). Thus, a lower carrier concentration was obtained in
single-substitutional doped sample (~3.1×10-12
cm-2
) than that of Nc-G (~8.0×10-12
cm-2
),
consistent with the theoretical calculation results (Fig. 1E in the main text). These results clearly
indicate an improved doping efficiency through the clusterization of dopants in Nc-G. To provide
a comparison of doping efficiency in Nc-G with other previously reported CVD-doped graphene
(8, 47-51), the nitrogen concentrations (gained from XPS in our works and previous works)
versus the ∆𝐸F and carrier concentrations are plotted in fig. S17F., confirming the higher doping
efficiency in Nc-G.

Fig. S17. The doping efficiency of Nc-G film. (A) UPS spectra of the 900, 950, 1000 Nc-G, and
pristine graphene films on Cu foil. The Fermi level is shifted by 300, 150, and 50 meV
(compared to pristine graphene) for 900, 950, and 1000 Nc-G, respectively. (B) Transfer curves
of 900 (red), 950 (green), and 1000 Nc-G (orange) devices. (C) XPS spectra of the samples are
identified as in (A). Note that all the films indicate a single peak, corresponding to graphitic
doping. The dopant concentrations of 900, 950, and 1000 Nc-G are 1.4, 1.0, and 0.6%,
respectively. (D) Raman spectra of the samples (identified as in (A)), Ns-G and Np,s-G, all of
which indicate the prominent doping-related D bands. (E) Enlarged Raman G band spectra. The
G band positions of 900, 950, 1000 Nc-G, and Ns-G are 1593, 1591, 1588, 1589 cm-1
respectively. Note that 900 Nc-G exhibits a higher carrier concentration than that of
single-substitutional doped graphene, and a similar ID/IG ratio, indicating that less defects are
required to achieve the same ∆𝐸F in Nc-G. (F) The ∆𝐸F and carrier concentration function as
the nitrogen dopant concentration in Nc-G of this work (red), single-substitutional doped
graphene (blue) and values reported in the literature (yellow). The data points are labeled with
the reference number from whence they came in brackets.

Fig. S18: The large-scale conductivity and transmittance
(1) The large-scale transmittance of Nc-G film.
Optical transmittance is an important parameter in the increased integration of doped graphene
into optoelectronic components such as transparent conductive films, touch screens, and solar
cells (59). To characterize this, as-synthesized Nc-G films were transferred onto quartz substrates
for UV-Vis measurements. Using the same black quartz substrate as reference, the UV-Vis shows
an optical transmittance of 97.7% at 550 nm for Nc-G film, comparable to its intrinsic
counterpart (fig. S18A). Furthermore, multilayer graphene still exhibits good transmittance
(higher than 90%), consistent with other works on fabricating multilayer graphene using a
layer-by-layer transfer method (fig. S18A) (38, 60).
(2) Large-scale conductivity of monolayer and multilayer Nc-G films
During the transfer of graphene, before the removal of the polymethyl methacrylate (PMMA)
layer, the composite structure was heated at 150 oC for an additional hour to minimize the
transfer-related charged impurities. The sheet resistance of large-scale films was characterized
using a four-probe resistance measuring meter to eliminate contact resistance. Four metal probes
were configured in a straight line at intervals of 1 mm, which is suitable for large-area
characterization of film conductivity (fig. S18B, inset). Note that the conductivity value on a
centimeter-sized scale is usually larger than that obtained from the micrometer-sized devices,
presumably owing to unavoidable transfer-related wrinkles on a larger scale. For a better
understanding of the influence of nitrogen doping in enhancing conductivity, the monolayer
intrinsic graphene film, consisting of the same domain size as the Nc-G film, was also
characterized. In contrast to the sheet resistance of intrinsic graphene (~720 Ω sq-1
), a clearly
reduced value of around 155 Ω sq-1
for the Nc-G film was observed, confirming the significantly

enhanced conductivity caused by the nitrogen-cluster doping (fig. S18B).
To enhance the conductivity, multilayer Nc-G films were fabricated by PMMA-assisted
layer-by-layer transfer (38,60). In particular, CVD-grown doped graphene exhibits no screening
of the dopant, which is typical in doped graphene fabricated by post-treatment doping techniques.
Consequently, the sheet resistances of bilayer graphene and trilayer Nc-G films are in the range
of 102 and 58 Ω sq-1
, respectively (fig. S18B), confirming the capability of further reducing sheet
resistance through forming multilayer structures. No change in the sheet resistance was observed
in transferred monolayer, bilayer and trilayer Nc-G after being kept in the air for at least 7 days.
Fig. S18. The large-scale conductivity and transmittance of Nc-G films. (A) UV-vis
transmittance spectra of the monolayer, bilayer and trilayer Nc-G films and the intrinsic
graphene film. (B) The sheet resistance of monolayer intrinsic graphene (green), also containing
1-mm sized domains and continuous monolayer, bilayer (2L) and trilayer (3L) Nc-G film (red),
fabricated by a layer-by-layer transfer technique. Inset: Photograph of graphene film transferred
onto SiO2/Si substrate and the four-probe station for characterizing the sheet resistance. (Photo
Credits: Li Lin, Peking University)

Fig. S19: Potential application of Nc-G films
The large-scale mapping results were presented in fig. S19A, indicating a good conductivity
uniformity, which is important for further applications, such as touch screen. The detailed
fabrication process of touch screen devices is as follows: First, the as-grown Nc-G film on Cu
foil is transferred onto a polyethylene terephthalate (PET) substrate with the assistance of
thermal release tape (Nanjing XFNANO Materials Tech Co., Ltd) using a roll-to-roll transfer
method (38). In our four-wire touch screen device, the Nc-G on foil serves as the bottom panel,
with commercial ITO-PET as the top panel (fig. S19B). Demonstration of touch screen is
presented in fig. S19C and movie S1. Note that the uniformity of the as-transferred Nc-G films,
for instance, is subject to cracking during the transfer, which would strongly influence the
performance of the touch screen. Furthermore, due to their enhanced conductivity, Nc-G films
can also be used as a conductive channel for powering light emitting diode (LED) indicators (fig.
S19D).

Fig. S19. Potential application of Nc-G films. (A) The spatial distribution of sheet resistance
on a 4 cm × 4 cm Nc-G film transferred onto PET. (B) Schematic of a Nc-G film-based touch
screen panel structure. (C) Demonstration of touch screen based on Nc-G. (D) Photograph of
bilayer Nc-G films transferred onto a SiO2/Si substrate to lighten up a green LED indicator. The
sample size is 3 cm × 3 cm. (Photo Credits: Li Lin, Peking University)

Fig. S20: High electrostatic potential and quasi-bound states induced by nitrogen clusters
Fig. S20. High electrostatic potential and quasi-bound states induced by nitrogen clusters.
(A) Electrostatic potential of 3N-cluster doped Nc-G. (B) Energy bandgap, density of states and
the partial charge distribution of valance band maximum (VBM) and conducting band minimum
(CBM) of 3N-cluster doped Nc-G.

Movie S1. Demonstration of a flexible touch screen device made from Nc-G film.
Supplementary Method: transport measurement of Nc-G
(1) Device fabrication:
The Nc-G samples were transferred onto SiO2/Si substrates with marks for alignment,
heat-cleaned, and imaged by atomic force microscopy (AFM) to check for flatness. Next, the
graphene samples were etched into a Hall bar geometry using a PMMA etch mask (PMMA 950K
A2 @ 4000 rpm) with EBL and O2 (RIE). After the samples were patterned, AFM imaging was
performed again to ensure that the channel regions were free of winkles and residues. Finally,
after using EBL to design a PMMA mask, contacts were fabricated on the samples (5 nm Ti and
90 nm Au) using an electron-beam evaporator, followed by a standard metal lift-off technique.
The carrier mobilities of single substitutional nitrogen doped graphene and nitrogen doped
graphene containing a content of pyridinic nitrogen (Fig. 1E) were also characterized.
(2) Sheet resistance measurements:
Four-probe measurements of the sheet resistance at room temperature in a vacuum probe station
were performed using a Keithly Semiconductor Characterization System (Model 4200-SCS) in
the DC current-bias sweep mode. The four-point configuration applies the sweep current bias to
the outside probes and measures the voltage between the inside probes to eliminate contact
resistance.
(3) Nonlinear fitting method for field-effect (FET) mobility:
The carrier density (electrons or holes) in the graphene channel regions ntot can be approximated

by (61)
𝑛𝑡𝑜𝑡 = √𝑛(𝑉𝑔)2 + 𝑛02 (S2)
where n0 represents the density of carriers at the Dirac point (denoted: residual carrier density).
If using the two-terminal method, the metal/graphene contact resistance should be taken into
account during the fitting
𝑅to t = 𝑅contact + 𝑅channel = 𝑅contact + L
W
1
e u√𝑛(𝑉𝑔)2+𝑛02 (S3)
where Rcontact represents the contact resistance, L and W represent the channel length and width,
and μ represents the FET mobility. Ignoring quantum capacitance, the gate-induced carrier
density can be calculated by
𝑛(𝑉𝑔) =𝐶𝑜𝑥∗(𝑉𝑔−𝑉𝑑𝑖𝑟𝑎𝑐)
𝑒 (S4)

In the four-terminal method, contact resistance is eliminated, and Rtot can be written as
𝑅tot = 𝑅channel = 𝐿′
W
1
e 𝑢√𝑛(𝑉𝑔)2+𝑛02 (S5)
where L′is the distance between the two inner probes.
(4) Low temperature magneto-transport measurements:
Shubnikov-de Haas oscillations of Rxx were observed at magnetic fields below 700 mT (Fig. 3A,
inset), while Rxy revealed well-developed plateau-like structures coinciding with Rxx minima at
higher magnetic fields. Low temperature magneto-transport measurements of the graphene
device fabricated on a SiO2/Si substrate show well-developed plateaus at filling factors 2, 6, 10,
and 14 at a quite low magnetic field of 4 T, indicating a high electronic quality (46, 61, 62) (Fig.
3B).

Table S1. Mobilities and sheet resistance of previously reported intrinsic graphene.

Table S2. Mobilities, conductivity, and stability of previously reported doped graphene.
Notes: the method for calculating conductivities is similar with that in fig. S7. The nμ values,
obtained from literature, is not the experimental result, which is only used for reelecting material
conductivity (13).