School of Medicine Department of Pharmacology and Chemical ... · biology is an area that the...
Transcript of School of Medicine Department of Pharmacology and Chemical ... · biology is an area that the...
2
GENERAL DESCRIPTION ................................................................................................................................... 3-5
RESEARCH AND OTHER SCHOLARLY ACTIVITIES ................................................................................ 6-94
RESEARCH INTERESTS.......................................................................................................................................... 7-38STUDY SECTIONS, ADVISORY COMMITTEE MEMBERSHIPS AND EDITORIAL SERVICE........................................ 38-54RESEARCH GRANTS AND CONTRACT ACTIVITY................................................................................................. 55-66PERCENT OF FACULTY SUPPORT ON RESEARCH GRANTS........................................................................................ 67TRAINING AND PROJECT GRANTS............................................................................................................................ 68PARTICIPANTS IN RESEARCH .............................................................................................................................. 69-72MAJOR COLLABORATIONS................................................................................................................................. 73-81ENTREPRENEURIAL ACTIVITIES .............................................................................................................................. 81AWARDS AND HONORS ...................................................................................................................................... 81-82INVITED TALKS.................................................................................................................................................. 82-94
TEACHING ACTIVITIES ............................................................................................................................... 95-109
EDUCATIONAL CREDIT UNITS SUMMARY ........................................................................................................ 96-107TEACHING AWARDS.............................................................................................................................................. 108POST-DOCTORAL FELLOWS............................................................................................................................ 108-109
FACULTY DATA ............................................................................................................................................ 110-120
CURRENT FACULTY ....................................................................................................................................... 111-112NEW FACULTY...................................................................................................................................................... 113MEMBERSHIP IN PROFESSIONAL SOCIETIES ................................................................................................... 113-120
THREE YEAR BIBLIOGRAPHY ................................................................................................................. 121-165
FINANCIAL PLAN ......................................................................................................................................... 166-172
4
During the 2011-2012 academic year, the Department of Pharmacology & Chemical Biology continued to grow its strengths in discovery and education related to the practice of pharmacology. The discovery component of our departmental missions employs basic chemical principles in developing an understanding of cell signaling events, and then applies these insights in the creation of new therapeutic strategies.
This past year, the department made significant new advances in understanding fundamental mechanisms of cell and tissue communication, how these events impact on cell growth and function, the creation of new drugs to control these processes and, from this, the generation of new intellectual property (patents) that allows the commercialization of these discoveries. In spite of challenging times, the concerted effort of both faculty and staff in making and communicating seminal research advancements has been rewarded by a continued growth in extramural research support that now totals $19,952,114 annually.
The teaching missions of the department are a high priority, thus they both thrive and continue to evolve. Donald DeFranco, Ph.D., has been highly effective in his position as Vice Chair for Education, as has Patrick Pagano, Ph.D., Director of our Molecular Pharmacology Graduate Program. We have continued to restructure and revise the Ph.D. Program in Molecular Pharmacology to be more responsive to student needs and the rapidly evolving training requirements for minting a competitive pharmacologist in the contemporary job market. They are using multiple strategies to inculcate in our students new expertise spanning from synthetic organic chemistry to physiology. In this regard, the Molecular Pharmacology Graduate Program has refocused its areas of specialization to provide additional focus on molecular aspects of signal transduction, cell and organ systems pharmacology, cancer pharmacology and drug discovery. We are especially pleased with student reactions regarding our recent integration of combined clinical simulator- and murine-based “hands-on” training in organ physiology and pharmacology into our graduate curricula. Due to the interest of students outside of the Molecular Pharmacology Graduate Program, we are further expanding these organ physiology and pharmacology teaching missions. Important contributions and leadership have also been lent in graduate education by Guillermo Romero, Ph.D., Associate Director of Graduate Education and Daniel Altschuler, Ph.D., Co-Director of the Molecular Pharmacology course, in addition to our overall departmental faculty.
By new mechanisms that regularly evaluate both mentors and students, Drs. DeFranco, Pagano and Freeman have succeeded in reducing our average time for graduation of Ph.D. students to four, and sometimes less than four, years for exceptionally productive students. Departmental faculty are particularly proud of our current outstanding Molecular Pharmacology Ph.D. and M.D./Ph.D. students, who are contributing important new insight into fundamental cell signaling processes, drug actions and drug discovery.
Medical education has also thrived under Dr. DeFranco’s leadership. He is one of the most highly esteemed educators and researchers in the Department of Pharmacology & Chemical Biology and the School of Medicine. Dr. DeFranco, along with Stephan Tofovic, M.D., Ph.D., have dramatically improved the integration of pharmacology instruction into the organ-based, modular instructional approach currently given to Pitt medical students. Additionally, all of the Pharmacology & Chemical Biology faculty participate in the execution of multiple small-group and team-based workshops, focusing on clinically relevant case studies in pharmacology. With constant attention to designing course content, optimal teaching strategies and their execution, we strive to continuously evolve and fulfill our mission to educate medical students and physicians in the conceptual basis for drug selection and administration.
Pharmacology & Chemical Biology faculty are also dedicated to inspiring and growing the next generation of young scientists and physicians. Michael Palladino, Ph.D. continues to direct our highly acclaimed summer undergraduate research program, which attracts some of the brightest young, aspiring basic and physician-scientists from around the country. This program is in part funded by the American Society of Pharmacology and Experimental Therapeutics, as well as participating School of Medicine centers and departments. Our undergraduate research program supports the summer living and laboratory expenses of students who are interested in a broad range of research themes. These students are matched with a laboratory that fits their
5
general interests and by pursuing a research project become exposed to challenging new laboratory skills, classroom experiences and an opportunity to interact with our internationally renowned departmental investigators. This represents a significant investment of time and resources that pays untold future dividends.
The stellar research environment that exists in the Department of Pharmacology & Chemical Biology is reflected by the high-impact publications and abundant extramural support levels cited in this report. In order to grow and evolve this vibrant environment, new investments are continually being made in the research tools utilized by departmental investigators.
Career development and strong mentorship for junior faculty are critical elements for nurturing a healthy future for the department. A new faculty mentoring process was instigated in recent years, whereby the Chair and senior faculty regularly meet with non-tenured and associate professor-level faculty to discuss and strategize how best to pursue career progression and key research objectives.
In summary, the practice of Pharmacology & Chemical Biology is unique among basic sciences, as it embraces a broad range of expertise in our efforts to understand fundamental cell communication events, drug-target molecule reactions and, finally, the design, synthesis and therapeutic application of new drugs in patients. This latter effort requires strengths or critical collaborations in computational drug design, synthetic organic chemistry, biophysics, structural biology and organ physiology. Thus, Pharmacology & Chemical Biology maintains strong ties with a variety of basic science and clinical disciplines, while maintaining firm roots in fundamental elements of chemistry, cell and molecular biology, drug actions and drug metabolism. These precepts are exemplified by our premier educational and research missions that include the institution of new educational strategies, the creation of new centers of excellence, the recruitment of stellar new faculty, and the scientific and teaching contributions of our dedicated faculty. We thus invite you to explore in more detail our annual report and, if interested, to join us in fulfilling the departmental mission to excel in education, drug discovery and improved patient care.
7
Research Interests
Bruce A. Freeman, Ph.D Professor and Chair Ph.D., University of California, Riverside, 1978
The basic and clinical research activities of the Freeman Laboratory focus on the eukaryotic cell production, reactions and signal transduction properties of oxidizing and free radical inflammatory mediators (e.g., superoxide, hydrogen peroxide, nitric oxide (NO), peroxynitrite, nitrogen dioxide, oxidized/nitrated lipids). In particular, we are interested in the action of these species as both redox signaling mediators under basal conditions and as pathogenic agents in inflammatory diseases. Our observations regarding O2 and NO-derived reactive species have lent new insight into redox-dependent cell signaling and have revealed new therapeutic strategies for treating acute inflammation, metabolic syndrome, respiratory disorders and cardiovascular diseases.
In the late 1980s, his group studied the cellular and subcellular organelle production of superoxide and hydrogen peroxide. Following the landmark description of endothelial-derived relaxing factor (EDRF) as the free radical NO, the Freeman laboratory pioneered the concept that the inflammatory and signal transduction mediator NO displays unique redox signaling actions following reaction with superoxide, oxidizing fatty acids and heme peroxidases. The “oxidative inactivation” of NO is a kinetically fast reaction, yielding “reactive nitrogen species” as products. This array of reactions of O2-derived species with NO can serve to both impair and transduce NO signaling via non-cGMP dependent mechanisms.
There is now a rapidly expanding appreciation that NO-derived reactive species display distinct chemical reactivities and exert cell signaling actions beyond the activation of guanylate cyclase – e.g., via thiol oxidation, electrophilic addition and receptor-dependent reactions. This aspect of redox-related chemical biology is an area that the Freeman laboratory continues to investigate, with the intent of defining the linkages between reactive oxygen species and NO-dependent cell signaling mechanisms. From a translational research perspective, his group is addressing how these interactions impact cell and organ function, with particular directed towards metabolic, cardiovascular and pulmonary diseases.
Dr. Freeman's laboratory observed that NO reacts with superoxide (O2-) to yield the potent biological oxidizing and nitrating species peroxynitrite (ONOO-)and its conjugate acid, peroxynitrous acid (ONOOH). Groundbreaking observations were made in this area by Joe Beckman, PhD and Rafael Radi, MD, PhD. Their work showed that peroxynitrite is both a direct oxidant and, after homolytic scission of peroxynitrous acid, yields the potent oxidant hydroxyl radical (OH) and the oxidizing and nitrating species nitrogen dioxide (NO2)(Fig. 1). Also, they identified thiols and carbon dioxide as the principal biological targets of peroxynitrite. It is now known that peroxynitrite accounts for many of the pathogenic actions previously ascribed to its precursors - superoxide (and its products) and NO. Work from many laboratories continues to affirm that peroxynitrite mediates redox cell signaling actions upon the oxidation or nitration of target molecules such as thiols, aromatic amino acids, nucleotides and unsaturated fatty acids – with downstream cell signaling events and reactions of peroxynitrite now appreciated to be a consequence of its potent and unique reactivities.
Daniel Altschuler, Ph.D Associate Professor Ph.D. (Biology), University of Buenos Aires, Argentina, 1989
Dr. Altschuler's laboratory studies mechanisms of signal transduction by the second messenger cAMP in cell proliferation. cAMP-dependent protein kinase (PKA) and Exchange protein activated by cAMP (Epac) represent the main effectors of cAMP action. Both pathways converge at the level of the small GTPase Rap1b, via its Epac-mediated activation and PKA-mediated phosphorylation. The role of Rap1 activation (Epac) and
8
phosphorylation (PKA) coordinating the early rate-limiting events in cAMP-dependent cell proliferation are studied using a multidisciplinary approach including molecular and cellular biology techniques in vitro, as well as in vivo validation using transgenic/knock in technologies in endocrine tumor models.
Palaniappa Arjunan, Ph.D. Research Instructor Ph.D., Indian Institute of Science, Bangalore, India, 1985
Dr. Arjunan determines the structure of macromolecules of biological interest, and then analyses structure-function relationships. He primarily uses X-ray crystallography to accomplish this.
Dr. Arjunan's current research includes the high resolution three-dimensional structure determination of thiamin diphosphate (ThDP)-dependent enzymes, the yeast pyruvate decarboxylase (PDC) and pyruvate dehydrogenase multienzyme complex (PDHc) from Escherichia coli. The refined structure is then used to address long-standing issues regarding the structure and function of thiamin diphosphate-dependent enzymes. The structure determination also includes the structure of PDHc E1 in complex with a covalently bound reaction intermediate analogue. Other interests are: a) the crystal structural analysis of native and mutant ThDP-dependent enzymes, either alone or in complexes with substrates, inhibitors, activators or with other related enzymes and b) development of techniques for the determination and analysis of macromolecular crystal structure.
Paul R. Baker, Ph.D. Research Assistant Professor Ph.D., Wake Forest University, Winston-Salem, NC, 2002
Dr. Baker’s research interests are focused on understanding how cells communicate with one another during inflammation. Chemical messages called lipid signaling molecules act to initiate, propagate and resolve inflammatory responses. Dr. Baker is particularly interested in a new class of lipid signaling molecules called ‘nitrated lipids;’ these molecules mediate generally anti-inflammatory effects and may play an important role in the resolution of inflammation.
Dr. Baker’s research has centered on a novel class of lipid mediators, nitrated fatty acids, which have distinct bioactivity from their non-nitrated counterparts. We have discovered that these signaling molecules are present clinically in a variety of vascular cells and plasma under basal conditions as well as clinical pathologies. The current hypothesis is that nitrated unsaturated fatty acids are formed by the “interplay” of nitric oxide and lipid oxidation/signaling reactions. In vitro, nitrated linoleic acid has been shown to regulate vessel relaxation by cGMP-dependent mechanisms and to modulate inflammatory responses including inhibiting neutrophil function and platelet activation via a mechanism involving cAMP. His work has involved identifying and quantifying nitrated lipid species in red blood cells and plasma and has found novel signaling actions that, in general, are anti-inflammatory. Specifically, he has found that nitrated linoleic and oleic acids (LNO2 and OA-NO2, respectively): a) up-regulate heme-oxygenase 1 protein expression; b) serve as a potent PPARα, δ and γ ligands that rival or exceed fibrates and thiazolidinediones in their ability to mediate PPAR activation; c) are strong electrophiles that covalently bind biological nucleophiles such as glutathione and protein histidine and cysteine residues, which has been shown to regulate protein functions including inhibition of cytokine release in LPS-stimulated inflammatory cells via adduction to the p65 unit of NF-κB; and d) prevent restenosis in animal models of vessel injury. These observations have broad implications in the clinical pathologies of atherosclerosis and diabetes. Furthermore, he has found that nitrated fatty acids can release nitric oxide during degradation, suggesting that allylic nitro derivatives may be a store of releasable nitric oxide.
9
Recent advances indicate that the diversity of endogenous nitrated lipids extends well beyond LNO2 and OA-NO2 to include nitrated fatty acids of varying chain length, multiple oxidized nitrated fatty acids (nitro-hydroxy and nitro-hydroperoxy adducts), complex lipids (e.g., nitrated cholesterol linoleate) and enzyme Co-A derivatives. His primary interest is to elucidate the endogenous formation of these species to better understand the role(s) they play in inflammation and basal tissue homeostasis. Using mass spectrometry as a tool, He is currently engaged in structural identification of novel nitrated lipid species and studies to determine the mechanism(s) of their formation.
Dr. Alessandro Bisello Assistant Professor Laurea (Chemistry), University of Padova, Italy, 1992
The general scientific theme in the laboratory is to define the role of accessory/scaffolding proteins (such as caveolin and EBP50/NHERF1) in the regulation of cellular and tissue functions. Our efforts focus on two specific areas:
Role of EBP50/NHERF-1 on vascular remodeling. The Ezrin-Radixin-Moesin Binding Phosphoprotein of 50 kDa (EBP50), also known as NHERF-1 is a PDZ domain-containing scaffolding protein. Our studies show that EBP50 is expressed at low levels in healthy vessels but is up-regulated following arterial injury. EBP50 contributes to the proliferation of vascular smooth muscle cells (VSMC). The temporal expression of EBP50 following arterial injury and its ability to regulate specific cell cycle proteins and signaling receptors suggest that this adaptor protein plays a key role in the integrated response of VSMC to injury. Current studies aim at determining the role of EBP50 on vascular remodeling.
Cellular regulation of the glucagon-like peptide 1 (GLP-1R) receptor and its role in regulating beta cell function, proliferation and survival. One of the most promising therapeutic targets for the treatment of type 2 diabetes is the glucagon-like peptide 1 receptor (GLP-1R). The well documented ability of GLP-1R agonists, either GLP-1 itself or exendin-4, to stimulate glucose-dependent insulin secretion and increase beta cell proliferation and survival led to the approval of exendin-4 for the treatment of type 2 diabetes. Our studies show that the GLP-1R interacts with caveolin-1 and this is necessary for the trafficking of the GLP-1R to the cell membrane and directs its localization to lipid rafts. The central hypothesis of this project is that the interaction between GLP-1R and caveolin-1 and its localization in lipid rafts is a fundamental mechanism controlling both the insulinotropic and the proliferative actions of GLP-1 and exendin-4.
Gustavo Bonacci, Ph.D. Research Instructor Ph.D., School of Chemical Sciences, National University of Cordoba, 2004
Dr. Bonacci’s research plan is focused on understanding the mechanisms that lead to the formation of nitro-fatty acid during inflammation. In response to inflammatory process, several bioactive lipids are generated as a result of enzymatic oxygenation or free-radical reactions. These bioactive lipids are involved in modulation of different stages of the inflammatory process, ranging from initiation to its resolution. Lack of regulation can be translated in exacerbated inflammatory responses, leading to detrimental chronic stages. Among these bioactive lipids, the generation of electrophilic nitro-fatty acid derives from radical reactions mediated by pro-oxidative species and reactive nitrogen oxides (RNS), such as nitric oxide (•NO), nitrogen dioxide (•NO2), nitrite (NO2-) and peroxynitrite (ONOO-). The electrophilic nature of nitro-fatty acid facilitates their adduction with cellular nucleophiles such as cysteine and histidine residues in proteins. In terms of cellular signaling, we described several protein targets of posttranslational nitroalkylation modification, resulting in activation of pro-MMP9, PPAR¥ and the antioxidant pathway Keap1/Nrf2 and the inhibition of the pro-inflammatory transcription factor NF-kB.
10
Recent findings indicate that mitochondria may act as a reactor for lipid nitration reactions under inflammatory condition of ischemia/reperfusion, in a process characterized by 1) exacerbation of oxidative stress, 2) low O2 tension, 3) acidic conditions and 4) availability of unsaturated fatty acids as a substrate. Thus, the implication of nitro-fatty acid signaling on mitochondrial respiration and function are closely associated with the cytoprotective effect found on cardiac tissue.
Because of our interest on the biogeneration of lipid signaling mediators in vivo, we have developed different approaches using mass spectrometry to structurally characterize and identify new nitrated lipid compounds. Particular efforts are directed to elucidate the endogenous molecular mechanisms leading to the nitration reactions of unsaturated fatty acids, with particular emphasis on substrate and product characterization. Furthermore, the implications of nitro-fatty acid formation and signaling under inflammatory conditions using cellular and animal models are being studied.
Dinara Bulgari, Ph.D. Research Assistant Professor Ph.D., Kazan State Medical University, Russia, 1993
Dr. Shakiryanova’s interests are focused on signaling mechanisms that regulate neuropeptide release and induce dense-core vesicles mobilization. Because neuropeptides control pain, appetite, mood, and sleep, understanding basic mechanisms underlying neuropeptide release could lead to clinical applications.
The vesicle motion, release and signal transduction in nerve terminals are detected optically by in vivo imaging of fluorescent protein constructs in transgenic Drosophila melanogaster larvae. Specifically, voltage-gated Ca2+
channels, Ca2+-induced Ca2+ release channels and Ca2+/calmodulin-activated kinase II are studied because they are required for sustained mobilization of peptidergic vesicles and post-tetanic potentiation of neuropeptide secretion.
Alicia M. Celotto, Ph.D. Research Assistant Professor Ph.D., University of Connecticut Health Center, CT, 2002
Dr. Celotto is currently involved in three research projects:
1. The link between glycolysis and Mitochondrial energy production and their effect on the Na/K pump and how their dysfunction may lead to seizures.
2. Allotopic ATP6 expression used to rescue the Drosophila MILS model of mitochondrial encephalomyopathy.
3. Drug discovery using Drosophila neurodegenerative models.
Dr. Celotto’s interests are focused on studying the connection between energy production and neurological conditions, such as seizures, migraine and neurodegeneration. She has discovered mutations causing degeneration and reduced longevity that markedly reduce glycolysis or mitochondrial oxidative phosphorylation, neither of which results in a bioenergetic crisis. These results demonstrate that energy derived from different sources cannot fully compensate for such impairments suggesting certain essential processes are dependent upon specific sources of energy. It is believed that ATP produced through glycolysis is specifically generated at sites of high need in the neuromuscular system, for example the Na+/K+ pump, an important regulator of cellular ion homeostasis. Thus changes in the source of ATP production due to mutations affecting glycolysis or mitochondrial function may result in neurological disease by altering Na+/K+ activity. To study this, she uses Drosophila mutants affecting the glycolytic enzyme, triose phosphate isomerase (TPI), a component of the mitochondrial ATP synthase (ATP6) and the Na+/K+ pump (ATPalpha).
11
Eugenia Cifuentes-Pagano, Ph.D. Research Instructor Ph.D., State University of New York at Stony Brook, 1994
Dr. Cifuentes-Pagano’s research interests focus on the understanding of the molecular mechanisms of action of novel NADPH oxidase isoforms and their regulation in the vasculature. The phagocyte NADPH oxidase (or respiratory burst oxidase) is a well-characterized reactive oxygen species (ROS)-generating system that catalyzes the one-electron reduction of oxygen to O2-, the precursor to a variety of other reactive oxygen species. The NADPH oxidase paradigm is a multi-subunit enzyme complex that includes two membrane-spanning subunits, p22-phox and nox2, and three cytoplasmic subunits, p40-phox, p47-phox and p67-phox. Our laboratory was the first to discover a nox2-based oxidase in the vasculature and to develop specific inhibitors targeting this robust source of ROS. Since that initial discovery, various isoforms of NADPH oxidase have been described which differ from the nox2 system in unique modifications of their nox-subunit amino acid sequence as well as the cytoplasmic components that they require. Besides their structural differences, the various isoforms present differential tissue and cellular distribution. The multi-level complexity of this family of proteins provides an opportunity to develop new tools to dissect the role of each of the isoforms in vascular function and pathology.
Marsha Cole, Ph.D. Research Instructor Ph.D., University of Kentucky, 2004
Dr. Cole’s research focuses on the endogenous generation of nitrated fatty acids (NO2-FA) in metabolic diseases, such as diabetes. Hyperglycemic and hyperlipidemic conditions support the presence of a pro-oxidative milieu in type II diabetes which can lead to increased mitochondrial production of reactive oxygen species and nitric oxide synthase activity. In particular, NO2-FA are generated in heart mitochondria through pNO-dependent oxidation and nitration and can mediate reactions that may beneficially modify mitochondrial
function.
NO2-FA derivatives are present endogenously and mediate anti-inflammatory effects through predominately cGMP–independent mechanisms that include electrophilic posttranslational protein modification and potent peroxisome proliferator-activated receptor (PPARR ) ligand activity. As a result of robust thiol reactivity, NO2-FA also activate phase II enzyme expression in vitro and in vivo (e.g., heme oxygenase 1, HO-1), through nuclear factor E2-related factor 2 (Nrf2) activation of antioxidant response element (ARE) dependent gene expression.
Taken together, generation of NO2-FA can impact mitochondrial respiratory function, myocardial efficiency, and cardiac contractility in diabetes. And although NO2-FA are endogenously generated as adaptive cell signaling mediators, therapeutic administration (in higher doses than endogenous concentrations) is of interest as to potentially attenuate hyperglycemia and hyperglycemic-induced myocardial injury.
Donald DeFranco, Ph.D. Professor & Vice Chair, Education Ph.D., Yale University, 1981
Glucocorticoid hormones are widely used as prenatal agents for mothers at risk for preterm delivery and as postnatal agents in premature infants in order to decrease medical complications of prematurity. However, animal and clinical studies suggest that exposure of fetuses to glucocorticoids during development could affect future cognitive function and brain development. Delayed neurological effects of early glucocorticoid exposure may be mediated by reduced neural progenitor cell survival, proliferation or differentiation. We are using a number of in vivo and in vitro models to examine the role of the glucocorticoid receptor protein in survival and
12
function of developing neurons in the cerebral cortex. Both genomic and nongenomic mechanisms of glucocorticoid receptor action are being studied to uncover both
Communication between the epithelial and stromal compartments of the prostate that is mediated by growth factors and cytokines is crucial for the maintenance of prostate growth and function. However, alterations in the expression and response to these factors can occur during prostate cancer progression and alter signaling between these compartments. We are examining various signaling pathways within the tumor microenvironment that mediate cross talk between cells within the stromal compartment and prostate cancer cells. For example, we have identified a prostate stromal cell specific transcriptional coactivator, the Hic-5protein, that functions in both androgen and vitamin D signaling pathways. Hic-5 influences androgen regulation of paracrine factors in stromal cells. In addition, it is an important mediator of vitamin D response acting to regulate vitamin D metabolism in stromal cells and theantiproliferative response of this vitamin in prostate cancer cells.
We are also studying the TGF-beta signaling pathway with a particular interest in understanding the mechanism for its divergent action as both a tumor suppressor and tumor promoter. Recent studies revealed that human prostate cancer associated fibroblasts maintain a secreted activity that limits the migration of prostate cancer cells. However, TGF-beta derived from aggressive prostate cancer cells can block this migration inhibitory activity through activation of an ROS signaling pathway in the cancer-associated fibroblasts.
W. Chet de Groat, Ph.D. Distinguished Professor Ph.D., University of Pennsylvania Medical School, 1965
Dr. de Groat is interested in the autonomic nervous system and the neural regulation of pelvic visceral functions. Current studies focus on the reflex control of the urogenital tract and the mechanisms underlying transmission at central and peripheral autonomic synapses. These experiments are designed to examine (1) the neurotransmitters in the reflex pathways, (2) neuroplasticity during postnatal development or following neural injury, (3) the neural pathways responsible for the detection of visceral pain, and (4) the actions of drugs used to treat urogenital dysfunction. Experiments are conducted on a variety of preparations ranging from intact animals to isolated tissues, like spinal cord slices and dissociated neurons.
Julie Eiseman, Ph.D. Research Associate Professor Ph.D., Cornell University Medical College, 1980
Research in the Eiseman laboratory is directed at the preclinical evaluation of potential anti-cancer agents. Studies include the determination of the maximum tolerated dose, pharmacokinetics, pharmacodynamics and efficacy. The laboratory is also interested in non-invasively measuring compounds with absorbance spectra in the long visible range.
Specific studies include the pharmacokinetics and efficacy of the pyrimidine compounds, fluorodeoxycytidine (FdCyd) and gemcitabine (dFdCyd) in combination with a cytidine deaminase inhibitor, tetrahydrouridine in CD2F1 mice and SCID mice with human pancreatic cancer xenografts.
The pharmacokinetics and efficacy of tubulin interactive agents including docetaxel, paclitaxel and 6-epi-dictyostatin are also under investigation. Studies with docetaxel have examined the interaction with 9-nitrocamptothecin in an ovarian cancer xenograft (SK-OV3) and a physiological based pharmacokinetic model was developed to describe the disposition of docetaxel. This model will be evaluated for its usefulness in predicting patient docetaxel pharmacokinetics.
13
Dr. Eiseman is interested in understanding the mechanisms involved during photodynamic therapy with Pc 4 and other phototherapeutic agents and use elastic scattering spectrometry to measure changes in drug concentrations and hemoglobin saturation during and following photodynamic therapy. For these studies, we measure the concentrations of the drug and hemoglobin non-invasively as well as through destructive methods such as HPLC and LC/MS-MS.
Other agents investigated include a wide range of potential cancer chemotherapeutics including DB-67, CKD-602, 2,2-dimethylbutyrate, DA-3003-1, Zebularine, 17-allyl aminogeldanamycin and 17-dimethylamino-geldanamycin.
Melanie Flint, Ph.D. Research Assistant Professor Ph.D., Imperial College, University of London, England, 1998
Dr. Flint researches hormonal influences on cell cycle regulation, targeted molecular therapies, drug metabolism, drug resistance and cancer. Her primary research project involves the direct interplay betweenstress hormones (cortisol, NE, and E), cancer and chemotherapy. This is accomplished through a mechanistic study of administration of stress hormones to cancerous cells, and observing these effects both in vitro and in rodent models. The goal is to identify predictive characteristics for molecular response, elucidating the mechanism of action of hormones in cancerous cells, and characterizing the genomic/proteomic profiles encoding cell cycle regulation.
Dr. Flint’s primary research project involves the direct interplay between stress hormones (cortisol, NE, and E), cancer and chemotherapy. This is accomplished through a mechanistic study of administration of stress hormones to cancerous cells, and observing these effects both in vitro by proteomics and in rodent models. We are first investigating paclitaxel, a drug used to treat metastatic breast cancer which acts on the cell cycle.
Dr. Flint has conducted a pilot-scale investigation to assess the adaptation of MDA-MB-231 breast cancer cells to the SILAC medium and 10% dialyzed FBS, to evaluate the ability to identify isotopomeric peptides from this cell system by MS and to demonstrate the presence of a measurable proteomic effect underlying the stress hormone influence on drug resistance. She cultured one population of MDA-MB-231 cells in ‘light’ SILAC medium (supplemented with L-lysine and L-arginine with natural abundance isotopes) and one population of cells in SILAC medium supplemented with 13C6-lysine and 13C6
15N4-arginine. The cells were cultured in the ‘light’ and ‘heavy’ isotope-containing SILAC medium for 6 passages. Importantly, she observed no notable differences in morphology or cell proliferation due to either the heavy isotope containing medium or light medium compared to normal media. To test the effect of stress hormones on drug resistance, the light media served as our control population and the cells were exposed to 10-7M paclitaxel for 48 hr.
The cells in heavy isotope containing media served as our experimental population and were incubated with 10-7M paclitaxel, cortisol, NE and E for 48 hours. The cells were then washed, pelleted and counted and mixed together with equal cell numbers. The samples were lysed by boiling in Laemmli buffer prior their resolution by 1D SDS-PAGE. The gel was stained Coomassie blue to provide a general estimation of the protein lysate integrity. 20 protein bands of equivalent size were excised from the gel and digested overnight with sequencing grade trypsin. The extracted tryptic peptides from each of the 20 gel bands were separately analyzed by nanoflow reversed phase (RP) LC coupled online with a hybrid linear ion trap-Orbitrap MS. Please see the 'scientific graphics' page for an illustration of the SILAC workflow for quantifying relative changes in protein abundance from two cell populations in culture.
14
Peter Friedman, Ph.D. Professor Ph.D., SUNY Upstate Medical Center, 1975 Studies in Dr. Friedman’s laboreatory focus on spatiotemporal regulation of protein-protein interactions governing GPCR signaling and function. We are especially interested in the parathyroid hormone receptor (PTHR), which controls extracellular mineral ion homeostasis and bone turnover. Key advances have been made in understanding cell-specific PTHR signaling, trafficking, and post-translational modifications. Recent observations indicate that PTHR activation, desensitization and endocytosis are mediated through distinct structural states that derive from specific interactions between ligand andreceptor. Agonist- or antagonist-occupied receptor states induce discrete conformations with accessibility to intracellular receptor domains. The differential or inducible involvement of these domains in coupling to G proteins may represent a molecular basis for ligand-selective responses notonly for the PTHR, but also for other G protein-coupled receptors, and are novel drug targets. Current work is directed at elucidating the molecular and structural mechanisms of how cytoplasmic PDZ proteins such as NHERF1 legislate cell-, ligand-, and stage-specific receptor trafficking. The resulting information will be valuable in understanding mineral ion homeostasis undernormal conditions, as well as disordered calcium balance in renal failure, hyperparathyroidism, or osteoporosis.
William Furey, Ph.D. ProfessorPh.D., The State University of New Jersey, 1977
Dr. Furey’s research involves the structure determination and analysis of large biological molecules and complexes by x-ray crystallography, and correlating the results with known functions. The work currently focuses on thiamin (vitamin B1) dependent enzymes and cell cycle regulating enzymes, as well as crystallographic methods development. Results of these studies could lead to development of therapeutic agents directed against pathogenic organisms, and anti-cancer drugs.
The pyruvate dehydrogenase multienzyme complex (PDHc, MW 4.7 million Daltons, 60 protein subunits & 60 active sites for the E. coli version) is present in most organisms and is critical for carbohydrate metabolism where it converts pyruvate, the product of glycolysis, to acetyl-CoA via a complicated process of substrate channeling within the confines of the complex. Structural analyses of the complex and its three major enzymatic components E1 (24 copies), E2 (24 copies), & E3 (12 copies) are underway, and Dr. Furey has already determined high resolution crystal structures for some of the components and reaction intermediates from the E. coli version.
The E1 components are rate determining and require thiamin diphosphate as a cofactor, but must interact with a flexible segment [lipoyl domain (LD) and associated lipoamide side chain] on an E2 to transfer the first reaction product, an acetyl group, to the E2 active site. The acetyl group is then transferred to co-enzyme A within the E2 active site, and the product acetyl-CoA is released. The E2 bound lipoamide group (now reduced) then moves to an E3 (FAD dependent) active site, where it is oxidized to restore the initial conditions. Binding of the flexible segment to E1 and E3 subunits is mediated by additional binding to a peripheral subunit-binding domain (PSBD), shown bound to E1.
Mechanistic details regarding the catalytic reactions in each active site are sought, as well as identifying structural aspects critical for assembly of the individual components to form the complete multienzyme complex. Specific mutations in some of the components are associated with hereditary diseases in humans, and detailed analyses of the structure-function relationships may suggest development of plausible therapeutic agents to counter the effects of the mutations. Additionally, given the critical nature of this system in overall energy production for cellular function, development of inhibitors binding at any of the catalytic sites, or at sites disrupting protein-protein assembly, may considerably weaken or kill the organism. Lack of appreciable
15
sequence homology between PDHc’s from humans and pathogenic bacteria therefore suggests that effective, pathogen specific antibacterial agents may be developed.
Early expression or over expression of Cdc25 proteins can cause the cell to prematurely progress leading to oncogenic effects, making these enzymes exciting targets for anti-cancer drug development. As part of a collaborative effort with Dr. John Lazo’s group, several potent inhibitors of Cdc25 proteins have been discovered, and structural analysis of their complexes with the enzymes are underway to reveal both where and how these inhibitors function. Dr. Furey has crystallized the catalytic domain of Cdc25b and determined its high-resolution structure. His group is currently co-crystallizing the catalytic domain with several inhibitors, as a step towards development of effective anti-cancer agents via structure-based drug design procedures.
In collaboration with the Hauptman-Woodward Institute for Medical Research, Dr. Furey's group is developing new computational methods for solving macromolecular crystal structures by automated techniques. This work involves creating and developing a software package BnP, which is a merging of the PHASES package developed in the Furey lab, and the SnB package developed in Buffalo. A simple, graphical user interface is developed to enable automatic creation of an interpretable electron density map starting from observed x-ray diffraction data, with only a few mouse clicks and text field entries required. This will invoke automatic scaling of data, determination of heavy atom/anomalous scatterer sites, refinement and validation of sites, calculation of protein phases, phase refinement, and phase improvement via solvent flattening/negative density truncation. A few more mouse clicks enable automated building of a complete or nearly complete model by interfacing with other externally developed software. The idea is to make it simple for novices to determine good quality crystal structures, while enhancing the productivity of more sophisticated users as well.
Ferruccio Galbiati, Ph.D. Associate Professor Ph.D., University of Milan, 1996
Most cells can not divide indefinitely due to a process termed cellular senescence. Because cancer cells need to escape cellular senescence in order to proliferate and eventually form tumors, it is well accepted that cellular senescence is a powerful tumor suppressive mechanism. In addition, since several molecular changes that are observed in senescent cells occur in somatic cells during the aging process, investigating the molecular mechanisms underlying cellular senescence will also allow us to better understand the more complicated aging process. Thus, molecules that regulate cellular senescence represent potential therapeutic targets for theprevention/treatment of cancer as well as the fight against aging.
Our work is directed at unraveling the role of caveolin-1 as a novel mediator of cellular senescence. Caveolin-1 is the structural protein component of caveolae, invaginations of the plasma membrane involved in signal transduction. Caveolin-1 acts as a scaffolding protein to concentrate, organize, and functionally modulate signaling molecules within caveolar membranes.
Senescent human diploid fibroblasts express higher levels of caveolin-1, as compared to non-senescent cells. We showed that mouse embryonic fibroblasts derived from caveolin-1 overexpressing transgenic mice are arrested in the G0/G1 phase of the cell cycle and display a premature senescent phenotype. In addition, we demonstrated that oxidative stress induces premature senescence by stimulating caveolin-1 gene transcription through p38 MAPK/Sp1-mediated activation of two GC-rich promoter elements in fibroblasts and epithelial cells. Interestingly, oxidative stress-induced premature senescence (SIPS) does not occur in fibroblasts where caveolin-1 expression is reduced using an antisense mRNA-based approach. Moreover, oxidative stress does not induce premature senescence in caveolin-1-negative MCF-7 breast cancer cells and reintroduction of caveolin-1 in these cells restores IPS.
16
Taken together, these data indicate that caveolin-1 plays a central role in the signaling events that lead to cellular senescence. We are currently investigating, at the molecular level, the signaling pathways that link caveolin-1 function to oxidative stress-induced premature senescence. These investigations will contribute to elucidate the molecular mechanisms underlying aging and cancerous cell transformation.
Stacy Gelhaus, Ph.D. Research Assistant Professor Ph.D., University of Maryland Baltimore County, Baltimore, MD, 2005
Nitrated and oxidized metabolites of O-3 and O-6 fatty acids, such as docosahexaenoic acid (DHA), eicosapentaenoic acid (EPA), docosapentaenoic acid (DPA) arachidonic acid (AA) and linoleic acid (LA), are potent signaling mediators involved in inflammation and resolution, amongst a variety of other regulatory pathways. These oxidized metabolites are produced by reactive oxygen and nitrogen species as well as through enzymatic pathways including cyclooxygenase (COX), lipoxygenase (LO), and cytochrome P450s (CYP450). Dr. Gelhaus’ research is specifically focused on understanding the anti-inflammatory mechanisms of a specific subgroup of these metabolites; the electrophilic fatty acids. Examples of electrophilic fatty acids include nitrated fatty acids such as nitro-oleic and nitro-linoleic acid or oxidized lipids that contain an a-,ß-unsaturated ketone or epoxide. Electrophilic fatty acid metabolites can exert their effects through the modulation of transcriptional regulatory proteins. Many transcriptional regulatory proteins contain a nucleophilic amino acid residue, such as a cysteine or histidine, to which the electrophilic moiety of the fatty acid can form a reversible Michael addition. Many of these interactions have been described with the nitrated fatty acids, particularly nitro-oleic acid. In terms of cellular signaling, the Freeman lab has described several protein targets of posttranslational nitroalkylation modification, resulting in activation of pro-MMP9, PPAR? and the antioxidant pathway Keap1/Nrf2 and the inhibition of the pro-inflammatory transcription factor NF-kB.
The current focus of Dr. Gelhaus’ research is on the mechanism of these electrophilic fatty acids in asthma. Asthma is a complicated disease that much like cancer is comprised of numerous disease states and phenotypes. In many ways it is an umbrella of respiratory diseases that share some similar phenotypes such as airway hyper-responsiveness, increased mucus secretion, increased smooth muscle contraction and decreased airflow. In the most severe of cases, airway remodeling and corticosteroid resistance are not uncommon. It affects over 30 million worldwide and is an economic burden with therapeutic costs upwards of $19 billion dollars per year. While the number of asthmatics in Westernized countries seems to be plateauing, the world-wide number of asthmatics is projected to hit over 100 million by 2025, mostly in low/middle economically developing countries.
Dr. Gelhaus is looking at the formation of electrophilic fatty acids in transformed stable cell lines while collaborating with clinicians to study the formation and signaling of electrophilic fatty acids in mild to moderate asthmatic subjects. In this study, subjects undergo a baseline bronchoscopy after which they are placed randomly in one of three groups, control, aspirin, or indomethacin. The thought here is that indomethacin will completely inhibit cyclooxygenase activity; therefore, shunting metabolism to other pathways including lipoxygenase or CYP450. Aspirin will inactivate COX-1, but acetylate COX-2 at S516, thus altering enzymatic activity and the stereochemistry of product formation. Following 5 days of treatment, subjects return for a second bronchoscopy. At each bronchoscopy, blood, urine, bronchoalveolar lavage, and bronchial brushings are taken. The epithelial cells from the brushings can be cultured at the air liquid interface for mechanistic studies and identification of key electrophilic fatty acids. To reach these end goals, the lab utilizes molecular biology and analytical techniques, primarily mass spectrometry, (triple quadrupole and ion trap) to elucidate the structures of novel electrophilic species, accurately detect and quantify key electrophilic fatty acid oxidation products in biological matrices, and establish mechanisms of action in asthma and other lung and airway
17
diseases. Furthermore, the implications of electrophilic fatty acid formation and signaling under inflammatory conditions using animal models are also being determined.
Eun-Ryeong Hahm, Ph.D. Research Instructor Ph.D., Seoul National University, Seoul, Korea, 2003
Pamela Hershberger, Ph.D. Research Assistant Professor Ph.D., Case Western Reserve University, 1991
Hormones such as estrogen and 1,25-dihydroxyvitamin D3 play an important role in controlling cell growth; estrogen tends to promote growth, whereas vitamin D tends to inhibit growth. The goal of Dr. Hershberger’s research is to understand how such hormone responses are generated and controlled, and how they may be exploited to suppress the development or growth of lung cancer (a disease that claims the lives of more than 160,000 individuals in the United States annually). Molecules which are found to be important in regulating hormone responsiveness in our studies represent potential new targets for therapeutic intervention in lung cancer chemoprevention and treatment.
Dr. Hershberger’s research uses molecular and cellular techniques to evaluate the role of nuclear steroid hormone receptors and their signaling pathways as therapeutic targets in solid tumors. Because the hormone vitamin D exerts anti-proliferative activity in a variety of tumor model systems, we initiated studies to explore its potential as a novel lung cancer therapy. She has found that although lung cancer cells express the vitamin D receptor, they were only moderately sensitive to vitamin D-mediated growth inhibition. In exploring the mechanistic basis for this response, we found that primary human lung tumors but not normal lung tissues express CYP24, the enzyme that catabolically inactivates vitamin D. As the only known function for CYP24 is the regulation of vitamin D metabolism, its frequent up-regulation in human lung tumors implies that tumor establishment and/or progression requires escape from the anti-proliferative action of vitamin D. Based on these findings, our lab is now exploring (1) the effect of CYP24 inhibitors on vitamin D pharmacokinetics and anti-tumor activity in lung tumor xenograft models (2) the potential use of CYP24 as a diagnostic or prognostic marker in lung cancer, and (3) the mechanisms contributing to CYP24 over-expression in lung cancer cells. Ultimately, she seeks to suppress CYP24 action in lung cancer cells to restore endogenous growth control by vitamin D.
Jing Hu, Ph.D. Assistant Professor Ph.D., Karolinska Institute, Sweden, 1997
The main focus of our research is to understand how posttranslational modifications—particularly by ubiquitin-related modifiers such as SUMO—of cancer-related factors, regulate cellular process in cancer biology and treatment. Through our research we hope to provide a novel angle of understand why chemotherapy often fails. Our goal is to identify novel molecular targets or events that have potential to guide the clinical development of new means to inhibit tumor progression and chemoresistance.
One of our research aims is to investigate how HDAC2 (Histone deacetylase 2) promotes tumorigenesis through enhancing substrate sumoylation. HDAC2 is a key regulator of oncogenic processes and is elevated in several human cancers, but how HDAC2 functions to promote carcinogenesis remains elusive. A commonly known feature of HDAC is to remove the acetyl group from an acetylated lysine and, consequently, our view of HDAC has for many years been solely from the deacetylase perspective. Intriguingly, we have found that HDAC2 possesses a deacetylase-independent sumoylation-promoting substrate sumoylation.
18
We are also interested in uncovering the HDAC mechanism responsible for the limited efficacy of histone deacetylase inhibitor (HDACi) treatment in solid tumors. HDACi is a new, targeted class of anticancer drugs. Two HDACi, Vorinostat and Romidepsin, are licensed by the United States FDA for the treatment of advanced cutaneous T-cell lymphoma (CTCL). Currently more than 10 HDACi from at least 18 companies are being extensively tested in clinical trials for solid tumors. However, in contrast to their powerful anti-tumor effect in patients with CTCL, HDACi as monotherapy only exhibit limited clinical efficacy against solid tumors. Developing strategies to overcome this challenge is of great interest to the scientific, pharmaceutical and medical community, and requires our clear understanding of underlying HDAC biology that renders a particular type of tumor refractory to HDACi. Using tissue culture and tumor xenograft models, currently we are testing our hypothesis that HDAC2 mediates refractoriness to HDACi therapy through promoting substrate Sumoylation.
Yi Huang, Ph.D. Research Assistant Professor Ph.D., Medical University of South Carolina, Charleston, SC, 2001
Dr. Huang’s research interests focus on the investigation of epigenetic regulation of gene expression in human breast cancer. In breast cancer, DNA hypermethylation frequently acts in collaboration with abnormal histone modifications leading to decreased chromatin activating marks such as H3K4me and AcH3K9 and increased repressive marks such as H3K9me and H3K27me that result collectively in the aberrant silencing of specific genes such as tumor suppressor genes. Over the last decade, significant progress has been made in identification and characterization of aberrantly silenced genes in breast cancer development and progression. Importantly, epigenetic changes, unlike mutations or loss of chromosomes, are reversible. Therefore, it is possible through treatment to restore normal growth control by reversing epigenetically silenced genes. Our main research objective is to define in depth the mechanisms and biological consequences of functional interplay between epigenetic modifiers in chromatin remodeling and gene transcription in breast cancer. The laboratory is also interested in identifying novel agents in targeting epigenetic alterations in human breast cancer. Our recent work demonstrated that activities of histone lysine-specific demethylase 1 (LSD1) and histone deacetylases (HDACs) are intimately linked in breast cancer cells. LSD1 inhibitor in combination with HDAC inhibitor led to enhanced levels of active histone marks such as H3K4me2 and AcH3K9, and displayed synergy in blocking growth of breast cancer cells. Genome wide microarray screening identified a unique subset of genes whose expression was significantly induced by combination treatment with inhibitors of LSD1 and HDAC. We are investigating the genome wide crosstalk between LSD1 and HDACs in breast cancer by using high-throughput approaches. We will also determine if inhibitors of LSD1/HDAC crosstalk are more efficacious in reactivating aberrantly silenced genes and inhibiting tumor growth than current strategies and thus represent a novel targeted therapy for breast cancer.
Recently, a second mammalian FAD dependent histone demethylase, LSD2 (also known as KDM1B or AOF1), has been identified. Amino acid sequence analysis reveals that LSD1 and LSD2 share considerable overall similarity in the amine oxidase domain. Our studies showed that LSD2 possesses the activity to demethylate H3K4 in human breast cancer cells, suggesting the existence of a more sophisticated FAD-dependent histone demethylase family whose members play a role in chromatin remodeling and transcription regulation in breast cancer. Despite structural and catalytic similarities, little is known about the epigenetic activity and biological function of LSD2 in breast cancer. LSD2 is likely to be part of chromatin-remodeling complexes that are different from those involving the LSD1/CoREST/HDAC. We are examining the epigenetic activity of LSD2 as well as the interaction between LSD2 and other histone modifying enzymes in breast cancer cells.
19
Edwin Jackson, Ph.D. Professor Ph.D., University of Texas at Dallas, 1979
Adenosine System: Adenosine is a naturally occurring chemical in the body that serves many functions. We have discovered that adenosine protects the heart from damage induced by a myocardial infarction (heart attack), the brain from damage due to traumatic head injury and (unfortunately) tumors when attacked by the immune system. We are currently investigating how adenosine levels in organ systems are regulated by the cAMP-adenosine pathways, what adenosine does to cardiovascular/ renal/ brain/ immune cells and how we can better modulate the adenosine system with drugs for clinical benefit in diseases of the heart, kidneys, blood vessels and brain and in cancer.
Hypertension (high blood pressure): Our recent suggests that NPY1-36 (a peptide released from sympathetic nerves) and PYY1-36 (a peptide released from the intestines) trigger proliferation of and extracellular matrix production by preglomerular vascular smooth muscle cells (PGVSMCs) and glomerular mesangial cells (GMCs) in kidneys from genetically-hypertensive animals, a phenomenon mediated via Y1 receptors and that involves signaling by RACK1 (receptor for activated C kinase 1). Dipeptidyl peptidase IV (DPPIV) metabolizes NPY1-36 and PYY1-36 (Y1 receptor agonists) to NPY3-36 and PYY3-36 (inactive at Y1 receptors). We are investigating whether a new class of antidiabetic drugs (DPPIV inhibitors) may adversely affect the kidneys of hypertensive subjects by preventing the conversion of PYY1-36 and NPY1-36 to less active metabolites and thereby promoting inappropriate cell proliferation and extracellular matrix production.
Tija Jacob, Ph.D. Assistant Professor Ph.D., University of California, Berkeley, 2002
How does the neurotransmitter GABA produce myriad forms of inhibition in the central nervous system (CNS), restraining and shaping electrical activity to prevent anxiety, agitation, seizures, chronic pain and sleep disturbance? The majority of fast synaptic inhibition in the CNS is mediated by GABA type A neurotransmitter receptors (GABAARs) which are Cl- selective ligand-gated ion channels composed of 5 subunits (from up to 17 different subunits), with differential expression across brain regions, cell types and subcellular localization.
The Jacob lab’s broad goal is to understand the impact of dynamically regulated GABAAR surface levels and distribution in normal development and pathological conditions. The lab uses a combination of molecular, biochemical, cell biological and live-imaging approaches. GABAAR are the sites of action of many clinically important drugs, including the benzodiazepines (BZ), which are front line treatments for anxiety, insomnia, schizophrenia and epilepsy. The Jacob lab is investigating modulation of GABAAR trafficking and synaptic inhibition by BZ and other GABAergic agents.
Another area of research in the lab focuses on the role of GABAergic signaling in CNS development and plasticity. The majority of excitatory synapses in the brain are located at the end of dendritic spines, small protrusions from neuronal processes, with neighboring GABAergic synapses predominantly located on dendritic shafts. We have shown that higher GABAAR surface levels leads to more inhibitory synapses, enhanced inhibitory synaptic transmission and a deficit in mature dendritic spines. Alterations in the excitatory/inhibitory ratio of neuronal signaling, abnormal spine morphology and mutations in GABAARsubunits are associated with many neurological disorders including autism and other neurodevelopmental disorders. The Jacob lab is investigating the contribution of GABAergic inhibition to dendritic spine morphology, movement and plasticity. These studies aim to improve understanding of how GABAergic dysfunction contributes to human neurodevelopmental disorders including autism
20
Yu Jiang, Ph.D. Associate Professor Ph.D., Yale University, 1995
Dr. Jiang’s laboratory is interested in intracellular signaling pathways governing cell growth and metabolism. The laboratory’s current research projects concern the signaling mechanism of the mammalian target of rapamycin (mTOR). mTOR is a protein ser/thr kinase that plays a key role in translation, autophagy and mitochondrial biogenesis. Its activity is regulated by signals of various origins, including nutrient, growth factor, energy and stress. The laboratory has previously identified FKBP38 that acts as an inhibitor of mTOR. Three projects centering on the role of FKBP38 in mTOR regulation are on-going.
The first project concerns the activity of mTOR in mitochondrial function. We have recently found that FKBP38 is involved in recruitment of mTOR to mitochondria. The project investigates the role of the mito-chondrial localized mTOR in mitochondrial functions and cell senescence.
The second project aims at the mechanism of FKBP38 in apoptosis regulation. FKBP38 has been shown to interact with the anti-apoptotic proteins, Bcl-2 and Bcl-xL. The project is to determine whether nutrient, growth factor and oxygen levels control the anti-apoptotic activity of Bcl-2 and Bcl-xL through FKBP38.
The third project focuses on the role of primary cilium in mTOR regulation. Primary cilium is a vital cellular organelle that functions as a signaling hub in many eukaryotic cells. mTOR has been recently found to be a key effector of primary cilium-mediated signaling. This project explores the mechanisms through which primary cilium controls mTOR activity.
Thomas Kensler, Ph.D. Professor Ph.D., Massachusetts Institute of Technology 1976
Research interests in my laboratory focus on the biochemical and molecular mechanisms involved in the induction of cancer by chemicals to serve as a basis for the prevention, interruption or reversal of these processes in man. One of the major mechanisms of chemical protection against carcinogenesis, mutagenesis and other forms of toxicity mediated by carcinogens is the induction of enzymes involved in their metabolism, particularly enzymes such as glutathione S-transferases, UDP-glucuronosyl transferases and NAD(P)H:quinone reductase that facilitate the detoxication and elimination of carcinogens. Furthermore, animal studies indicate that induction of these cytoprotective enzymes is a sufficient condition for obtaining chemoprevention and can be achieved in many target tissues by administering any of a diverse array of naturally-occurring and synthetic chemical agents. Our work utilizes animal and cell culture models to elucidate mechanisms of inhibition of aflatoxin hepatocarcinogenesis by dithiolethiones such as oltipraz, isothiocyanates such as sulforaphane and triterpenoids such as CDDO-Im. While induction of glutathione S-transferases clearly play an important role in chemoprevention of aflatoxin hepatocarcinogenesis, ongoing studies are seeking to identify additional genes induced by these agents. The Keap1-Nrf2 signaling pathway is activated by these classes of chemopreventive agents and leads to increased expression of genes that attenuate oxidative stress and inflammation among other actions. Their contributions to protection against carcinogenesis are under investigation.
A practical goal of our research has been to develop the tools to test the hypothesis that enzyme induction is a useful strategy for chemoprevention in humans. Hepatocellular carcinoma is the leading cause of cancer death in parts of Asia and Africa and may relate to hepatitis B virus infection and aflatoxin ingestion. Longitudinal surveys and prospective case-control studies in Qidong, P.R. China demonstrate consistent exposure of individuals in this region to aflatoxins and indicate a prime role for aflatoxin in the etiology of liver cancer, respectively. As a consequence, we have conducted clinical chemoprevention trials of oltipraz and other agents in Qidong. The initial randomized, placebo-controlled intervention of oltipraz demonstrated an increased
21
excretion of aflatoxin-mercapturic acid, a derivative of the aflatoxin-glutathione conjugate, in the urine of participants receiving oltipraz. This study highlights the general feasibility of inducing Nrf2-regulated enzymes in humans. Follow-up trials are evaluating more effective agents and are assessing whether protective alterations in aflatoxin metabolism can be sustained for extended periods of time and whether diminished incidence of liver cancer can be achieved in this high-risk population.
Nicholas Khoo, Ph.D. Research Assistant Professor Ph.D., University of Iowa, 2003
Dr. Khoo investigates the basic molecular mechanisms underlying the development of metabolic syndrome and the role of electrophilic lipids, particularly nitro-fatty acids (NO2-FAs) in preventing this pathogenesis. His specific research projects include:
1) Determination of the molecular mechanism(s) responsible for the anti-inflammatory cell signaling actions of electrophilic NO2-FAs resulting in insulin senstivity.
Obesity induces chronic inflammatory responses that are characterized by abnormal cytokine production, increased reactive oxygen species (ROS) generation and activation of inflammatory signaling pathways. Preliminary studies demonstrate these inflammatory conditions induce the oxidation and nitration of fatty acidsto electrophilic products, specifically NO2-FA derivatives, that serve as potent anti-inflammatory cell signaling mediators. These electrophilic NO2-FA species potently bind peroxisome proliferator-activated receptors (PPARs), inhibit NF-kB activity and induce heme oxygenase (HO)-1. The treatment of NO2-FAs results in improved glucose homeostasis in mouse models of obesity and diabetes. The electrophilic nature of these NO2-FA signaling molecules and their anti-inflammatory properties are being examined using cultured mammalian cells as well as mouse models of obesity and diabetes. Additionally, a mass spectrometer approach is being used to characterize the formation of NO2-FA derivatives in insulin responsive tissues from murine models of obesity and diabetes (ob/ob, db/db or high fat diet). This is being complemented by similar approaches in cell culture.
· Identify molecular signaling pathways modulated by NO2-FA treatment in mice subjected to a high-fat diet. Currently, three putative pathways for the anti-inflammatory actions of electrophilic NO2-FAs are being examined. The signaling pathways of all three PPAR isotypes, NF-kB and HO-1 are being explored in insulin-responsive tissues (adipose, liver and muscle).
· Define mechanistic roles of all three PPAR isotypes, NF-kB and HO-1 in cultured cells. The knockdown of these putative signaling pathways using si-RNA will be tested in cultured adipocytes, hepatocytes and skeletal muscle cells. Additionally, mouse embryonic fibroblasts isolated from Nrf2 knockout mice will explore the potential mechanism(s) of electrophilic NO2-FA-induced HO-1 expression.
2) Determination of the impact of NO2-FA derivatives on ROS and oxidative stress in insulin-responsive cultured cells and tissues of mouse models of obesity and diabetes. While oxidative stress and ROS are emerging as key culprits in the pathogenesis of obesity-induced insulin resistance, the sources of ROS remain unclear. Emerging studies suggest a link between mitochondrial dysfunction, insulin resistance and diabetic complications, suggesting that mitochondrially derived ROS could play a role in pathogenesis. How does this increase in ROS result in oxidative stress? Is there a decrease in antioxidant enzyme expression and activity in insulin-responsive tissues? These questions are currently being addressed by utilizing cutting edge techniques to detect ROS levels and antioxidant enzyme activity/expression in the cell culture and mouse models of obesity and diabetes described above.
22
3) The PPAR conundrum- Identification of novel PPAR agonists. The activation of PPARs have been shown to regulate glucose and lipid metabolism. These receptors are molecular targets for a number of marketed drugs. The hypolipidemic fibrates activate the isotype PPARa whereas PPAR? is the molecular target of thiazolidinedione (TZD) class of antidiabetic drugs. The activation of PPAR?-dependent downstream signaling has shown to improve insulin sensitivity. Rosiglitazone works as an insulin sensitizer by activating PPAR? andits downstream signaling pathways. Unfortunately, concerns about the severe adverse side effects have drastically limited the use of rosiglitazone despite excellent glycemic control in patients with diabetes. Thus, the development of new therapeutic strategies, such as dual PPARa/? activators or selective PPAR? partial agonists, that retain their antidiabetic efficacy without adverse side effects are appealing, such as NO2-FAs.
In summary, these research interests will generate insights into mechanisms leading to obesity and its associated myriad of health problems and/or diseases such as diabetes, atherosclerosis and other cardiovascular complications, which will hopefully elucidate novel preventative and therapeutic strategies. The potential for electrophilic NO2-FA mediated therapy to prevent obesity-induced type 2 diabetes complications, without the known secondary effects exerted by TZDs, is currently being studied.
Joan M. Lakoski, Ph.D. Professor Ph.D., University of Iowa, 1981
Elucidating the cellular and molecular neuropharmacology of the aging brain is the focus of the Lakoski laboratory. Using multidisciplinary approaches to investigate biogenic amine receptor expression and function, both normal and pathological aging processes are being investigated in young, middle-aged and senescent small animal models. We are investigating the roles of the steroid hormones estrogen and corticosterone on serotonin receptors, their receptor-effector coupling to G-proteins and related signaling transduction cascades, including the 5-HT1A and 5-HT2A receptor subtypes, and the serotonin neurotransporter (SERT) in discrete brain regions including cortical, hippocampal and midbrain regions; radioligand binding techniques, receptor autoradiography and functional neurochemical assays are among the technical approaches used to study the impact of the circulating hormone environment on the aging serotonergic neuronal system.
Related ongoing studies are utilizing in vivo microPET image analysis techniques to elucidate SERTexpression and function with respect to aging and hormone treatment. In addition, the impact of selective neurotoxic insults to the dopamine-containing neuronal system is being investigated using behavioral, neurochemical and molecular approaches to better understand how this neurotransmitter system responds and recovers from neuronal injury across the lifespan.
Our goal is to contribute new information to understand the biology of central nervous system aging, including normal and neurodegenerative processes, in neurotransmitter systems established as key components in cognitive declines, mood disorders, and stress-related disorders common in the elderly. Ultimately, our aim is to improve the quality of life with advancing age by pharmacological interventions to delay the onset of neuronal decline and/or enhance endogenous repair mechanisms of the biogenic amine neurotransmitter systems.
Adrian Lee, Ph.D. Professor Ph.D., University of Surrey, Guildford, Surrey, England, 1993
We are investigating the endocrine regulation of mammary gland development and progression to mammary cancer. Specifically we are interested in interaction between steroid hormones (estrogen and progesterone) with the growth hormone (GH)/insulin-like growth factor-I (IGF-I) axis. These endocrine hormones are all critical
23
for normal mammary development, but have also all been implicated in risk for breast cancer and in breast cancer progression.
We have shown that IGF-IR and IRSs are hormonally regulated in breast cancer, and we have now found that they are also developmentally and hormonally regulated during normal mammary gland development. We have found in breast cancer cell lines that estrogen can sensitize cells to insulin-like growth factor (IGF) stimulation by increasing expression of many of the IGF signaling components such as the IGF-IR and its downstream signaling intermediates IRS-1 and IRS-2.
We have also found that overexpression of IGF-IR, IRS-1, or IRS-2 causes transformation of mammary epithelial cells in culture, combined with epithelial to mesenchymal transition. We have also created transgenic mice that overexpress IGF-IR, IRS-1 or IRS-2 in the mammary gland and all mice develop mammary tumors. Mammary tumors in these mice show multiple cell lineages and expansion of putative mammary stem/progenitor cells. We are currently investigating how these pathways impact upon stem/progenitor cell renewal and cell fate determination.
Edwin Levitan, Ph.D. Professor Ph.D., Brandeis University, 1986
The Levitan lab studies biochemical and electrical signaling that controls neuronal and cardiac function with live cell imaging, electrophysiology and molecular biology. Current projects include in vivo imaging of green fluorescent protein (GFP) constructs in transgenic Drosophila nerve cells and serotonin in mammalian brain slices to determine how patterned electrical activity and synapses control transmitter release. We are also studying remodeling of rhythmic electrical activity in the heart and midbrain dopamine neurons by therapeutically important hormones and drugs.
Zhaohui Liu, M.D., Ph.D. Research Instructor M.D., School of Medicine, Xi’an Jiaotong University, Xi’an, China, 1996Ph.D., School of Medicine, Xi’an Jiaotong University, Xi’an, China, 2006
Neurodegenerative diseases manifest progressive loss of structure or function of neurons in the brain. Mitochondria are critical organelle in the neuron, which produce ATP, buffer calcium and generate reactive oxygen species (ROS). Mitochondrial dysfunction, associated with enhanced reactive oxygen species (ROS) production or loss of calcium homeostasis, is associated with the pathogenesis of degenerative diseases. Dr. Liu uses genetically encoded indicators to monitor redox state and dynamic change in calcium concentration with Drosophila neurodegenerative disease models. According to these redox and calcium state changes, his study is focused on the role of mitochondria dysfunction and its downstream signal transduction pathway in the pathology of neurodegenerative diseases and his final goal is to find new mechanisms in pathogenesis of neurodegenerative diseases and new targets for treatment.
Tatyana Mamonova, Ph.D. Research Instructor Ph.D., Kazakh National Academy of Sciences, Kazakh Scientific Research Institute of Catalysis and Electrochemistry, Almaty, Kazakhstan, 1995
Dr. Mamonova research focuses on molecular modeling of the interactions of the adapter protein EBP50/ NHERF1 with its target ligands, including parathyroid hormone receptor and type II sodium-dependent phosphate co-transporters. The goal of these studies will contribute to our understanding and prediction of
24
conformational reorganization and the structure-function relationships in NHERF1 proteins associated with the ligand binding. The results will provide insights into developing drugs for selective therapeutic applications.
Carola Neumann, M.D. Visiting Associate Professor M.D., Ludwig-Maximilian’s University Medical School, Munich, Germany, 1997
Yuen-Keng Ng, Ph.D. Research Instructor Ph.D., University of Pittsburgh, 2001
Steffi Oesterreich, Ph.D. Professor Ph.D., Humboldt University, Max-Delbrück Center for Molecular Medicine, Berlin, Germany, 1992
The Oesterreich lab includes technicians, graduate students and postdoctoral fellows who are trained in a multi-disciplinary research environment to work in basic, translational, and clinical aspects of breast cancer research. Specifically, our research projects focus on the role of co-regulator proteins in estrogen response in breast cancer. Estrogen mediates its potent mitogenic effects through the estrogen receptor (ER), which has been a successful target for endocrine therapy in breast cancer. Despite the success of such treatment, de novo or acquired resistance remains a major problem. A better understanding of how ER works is critical for the development of more efficient therapies, and better prediction for who should receive which form of endocrine therapy.
Over the last years, many dogmas in hormone response have changed, which has opened many exciting novel research areas. Examples are estrogen-mediated repression of gene transcription, and the role of co-repressors in this process, the close connection between estrogen signaling and epigenetic regulation of gene transcription, and the role of regulatory elements which are located far outside the promoter of the estrogen regulated genes, and which might even be on other chromosomes. We are studying these processes using state-of-the-art molecular and cellular techniques, mouse models, and clinical specimens. The ultimate goal of Dr. Oesterreich's research is to use this knowledge for improved diagnosis and endocrine treatment of breast cancer patients.
Patrick J. Pagano, Ph.D. Professor Ph.D., New York Medical College, 1991
Dr. Pagano’s research focuses on the modulatory role of the adventitia in vascular function and structure under both physiological and pathophysiological conditions. Dr. Pagano’s laboratory was among the first to identify a non-phagocytic NADPH oxidase in the vascular wall, demonstrating a critical role for essential subunit p67phox in its activity. He subsequently cloned vascular p67phox and illustrated its potent activation at the mRNA and protein level in response to the potent pro-hypertensive hormone angiotensin II. Stemming from these early discoveries, Dr. Pagano was the first to develop specific cell- and tissue-permeant peptidic and adenoviral inhibitor of NADPH oxidase, which is widely considered the most specific NADPH oxidase inhibitor available. These and his other more recently developed inhibitors of novel isoforms of NADPH oxidase are expected to provide a platform for the development of new therapies aimed at treating hypertension and other cardiovascular diseases. Moreover, Dr. Pagano is broadly recognized for his pioneering work examining the role of adventitia-derived reactive oxygen species (ROS) and, in particular, superoxide anion and hydrogen peroxide in the modulation of vascular tone, inflammation, and remodeling.
25
Michael Palladino, Ph.D. Associate Professor Ph.D., University of Connecticut, 2000
The Palladino lab uses Drosophila (the fruit fly) as a genetic model system, as well as mice and human cell culture to study progressive neurological and neuromuscular disorders. We are currently focusing on elucidating the mechanism by which mutations affecting Na/K ATPase, triose phosphate isomerase (TPI), and ATP6 function result in RDP (rapid-onset dystonia parkinsonism), glycolytic enzymopthy, and mitochondrial encephalomyopathy, respectively. Our research program is directed toward four main goals 1) discovering and characterizing novel pathways that cause progressive disease, 2) understanding the physiological, cellular and molecular dysfunction that causes dysfunction in vivo, 3) understanding the molecular basis of progressive diseases, and 4) using our animal model system to identify novel therapeutic approaches.
Guillermo Romero, Ph.D. Associate Professor Ph.D., University of Virginia, 1980
G-protein coupled receptors (GPCR) are the largest family of cell surface receptors found in mammalian organisms. These receptors are a major target for drug development. Dr. Romero is interested in the dynamics and traffic of GPCR, with special emphasis on the parathyroid hormone receptor type 1 (PTH1R). His approach is based on the use and development of novel optical techniques to study membrane proteins and their interactions with other cellular components in live cells.
Dr. Romero’s research focuses on two main areas: a) the role of the PDZ proteins sodium-hydrogen exchange regulatory factor (NHERF1) and Disheveled-2 in the regulation of the dynamics and traffic of GPCR; and b) the role of phospholipase D in the regulation of receptor traffic and function.
Dr. Romero’s approach is based primarily on the analysis of the physical properties of molecules of interest in live cells, using advanced optical techniques such as confocal microscopy, fluorescence recovery after photobleaching (FRAP), total internal reflection microscopy (TIRFM), image correlation spectroscopy (ICS), quantum dots, and others. Using these techniques, Dr. Romero has developed novel methods to examine protein-protein interactions in the temporal domain. For example, he has recently demonstrated that the PTH1R is tethered to the cytoskeleton and accumulates in the vicinity of subjacent actin stress fibers, forming bundles that are highly dynamic structures, moving along these bundles much more rapidly than between them.
Because of his interest in traffic, Dr. Romero is actively pursuing new approaches to the study of endocytic processes. To this effect, he recently developed novel methodologies for the purification and preparation of endosomes based on the use of magnetic nanoparticles attached to peptide ligands. In this technique, peptide ligands, such as insulin or epidermal growth factor, are adsorbed to colloidal iron nanoparticles (5-10 nm in diameter) and applied to the external surfaces of cells. These particles are sufficiently small to be internalized via the standard endocytic pathway and, because of the ferromagnetic properties of the colloidal iron, allow for a simple and rapid isolation of the endocytic vesicles containing the particle. He is using this novel technology to study the role of specific proteins in the endocytic pathway in a time-resolved manner. Figure 2 shows electron micrographs of cells treated with insulin-iron complexes at various times, demonstrating endosome maturation.
26
James Roppolo, Ph.D. Research Assistant Professor Ph.D., University of Michigan, 1970
Dr. Roppolo’s research is concerned with the autonomic nervous system’s control of bladder activity in normal animals and those with central nervous system injuries. A variety of techniques are used to examine, at the level of the lumbosacral spinal cord and brainstem, the various neuronal processes that occur in maintaining normal excretory function.
These methods include: (1) anatomical techniques (HRP tracing and immunohisto-chemical techniques) to determine the location of neurons and possible neuropeptides involved in these processes, (2) neurophysiological techniques (evoked potentials, intracellular and extracellular single neuron recordings) to determine the types of neuronal interactions that occur in this system, (3) neuropharmacological techniques (systemic and iontophoretic application of drugs), (4) behavioral techniques and microstimulation of the of the lumbosacral spinal cord.
Francisco Schopfer, Ph.D. Research Associate Professor Ph.D., University of Buenos Aires, Argentina, 2001
Dr. Schopfer’s research is focused on the understanding of the biological effects of electrophilic fatty acids. In particular, he studies the mechanism by which nitrated fatty acid activate and signal through peroxisome proliferator-activated receptor gamma (PPARγ). This receptor is the target of currently used antidiabetic drugs (thiazolidinediones). The activation of the receptor regulates fat and glucose metabolism, resulting in an overall decrease of glucose levels to normal values in patients with type II diabetes. The targeting of this receptor by nitrated fatty acids results in a decrease of the glucose levels to normal values like thiazolidinediones, but without the known secondary effects exerted by thiazolidinediones. In addition to the intrinsic therapeutic value of nitrated fatty acid, they will aid in the understanding of the biological mechanism involved in PPARγ activation, leading to improved designs of anti-diabetic drugs targeting the PPARγ receptor.
The role of the PPARγ receptor in diabetes has been well established. Nonetheless, the role of endogenoussignaling molecules on the activation of PPARγ is still unclear and under debate. Nitrated fatty acids are endogenously formed and bind to PPARγ with high affinity rivaling Rosiglitazone (thiazolidinediones), resulting in receptor activation. In addition, nitrated fatty acids covalently modify a critical cysteine (cys285) in the ligand binding pocket of PPARγ, promoting a particular conformational change that results in partial receptor activation. This partial activation results in the expression of a particular subset of genes under PPARγ regulation and a biological outcome that differs from the one obtained when activating the receptor with Rosiglitazone. Dr. Schopfer’s work focuses on understanding the mechanism of this selective activation and how it avoids the side effect presented upon full activation by agonist like Rosiglitazone.
Electrophilic fatty acids are constantly formed as fatty acid breakdown products during oxidative stress and as signaling messengers by enzymatic or non enzymatic pathways. Dr. Schopfer studies the formation of biologically relevant electrophiles, in particular nitrated fatty acids, and their signaling mechanisms. The study involves the detection and characterization of novel electrophiles formed during inflammation. Once the molecules are characterized, a chemical synthesis approach is used to generate enough quantities for biological experiments.
Electrophiles induce an important cellular response that includes the induction of phase II genes. This will in turn set up a more protective environment against damaging electrophilic molecules. A key player in the initiation of this biological response is the Keap 1/Nrf 2 couple. Keap 1 is usually bound to Nrf 2 in the cytoplasm. Upon formation of electrophiles, Keap 1, which contains several highly reactive cysteine, is
27
targeted, dissociates from Nrf2 and is routed to degradation by the proteosome. These lead to Nrf2 nuclear translocation and activation of phase II genes. In particular, we study the mechanism by which different biologically relevant electrophiles target KEAP 1 and activate Nrf 2 responses. In addition, a more general proteomic approach is use to evaluate and characterize different electrophilic cellular protein targets. Once critical targets are identified using a mass spectrometry approach, a functional study of the modification is performed to determine the relevance and its cellular effects.
Sruti Shiva, Ph.D. Assistant Professor Ph.D., University of Alabama at Birmingham, 2004
Dr. Shiva’s lab focuses on the mechanisms by which reactive nitrogen species (particularly nitrite and nitric oxide) regulate mitochondrial function during hypoxia and ischemia, the factors that influence this regulation and the implications of this regulation on pathology such as ischemia/ reperfusion injury.
Active projects in her lab include: The role of heme proteins in regulating nitrite-dependent modulation of mitochondrial respiration. The anion nitrite (NO2-) is an endocrine storage form of nitric oxide (NO) in blood and tissues that can be reduced to bioavailable NO by heme proteins in conditions of low oxygen. In blood, the reduction of nitrite by hemoglobin mediates hypoxic vasodilation. We are interested in understanding how tissue nitrite reductases regulate mitochondrial function. Specifically, myoglobin, when deoxygenated, can efficiently reduce nitrite to NO and this NO subsequently inhibits mitochondrial respiration by binding to complex IV of the mitochondrial respiratory chain. We are interested in other ways that this interaction between nitrite and myoglobin regulates mitochondrial function as well as characterizing the physiological interplay between mitochondria and myoglobin with nitrite/NO acting as a signaling molecule linking the two.
The regulation of mitochondrial function by nitrite during ischemia/reperfusion. Low concentrations of nitrite have been shown to mediate cytoprotection in a number of models of ischemia/reperfusion of the brain, liver, heart and kidney. However, the mechanism of this cytoprotection is not known. The mitochondria play a central role in the progression of ischemia/reperfusion injury. Hence, we are interested in how nitrite regulates mitochondrial function during ischemia/reperfusion.
We have recently demonstrated that nitrite administered to animals before or during ischemia/reperfusion modulates mitochondrial function by S-nitrosating thiols on mitochondrial complex I, which leads to decreased reactive oxygen species generation, less oxidative damage of mitochondrial proteins, and prevention of cytochrome c release. We think that these modifications of function prevent mitochondrial dysfunction after reperfusion and lead to cytoprotection.
We are currently using isolated mitochondria, the Langendorff isolated and perfused heart, and in vivo ischemia/reperfusion models to further characterize nitrite-dependent cytoprotection, particularly in relation to other cytoprotective programs, such as ischemic preconditioning.
Mechanisms of nitrite generation and metabolism. Another focus of the lab is determining the mechanisms by which nitrite is formed and metabolized physiologically. Conventionally, nitrite is thought to be formed by the oxidation of nitric oxide. However, in vivo, the reaction of nitric oxide with oxygenated hemoglobin (which produces nitrate) is more kinetically favorable than the reaction with oxygen to produce nitrite. We have recently identified a role for the multicopper oxidase, ceruloplasmin, as an “NO oxidase” that can compete with the nitric oxide-hemoglobin reaction to oxidize NO to nitrite. We are currently further characterizing the role of ceruloplasmin in regulating nitrite levels in physiology and pathology, and in plasma and tissue.
28
Jill Siegfried, Ph.D. Professor Ph.D., Yale University, 1981
Dr. Siegfried investigates the role of growth factors and hormones in the development and growth of human lung cancer. The laboratory focuses on the effects of these cytokines on activation of cell signaling pathways and control of tumor growth, as well as their role in risk for cancer. Growth factors and their receptors currently under investigation include estrogen and its receptors and hepatocyte growth factor and its receptor, c-Met. Growth factors and hormones are also being investigated as possible therapeutic targets and diagnostic or prognostic indicators for lung cancer. Hepatocyte growth factor has been found to be a strong negative prognostic indicator for non-small cell lung cancer, and expression of the enzyme aromatase that mediates estrogen production has also been linked to poor outcome in women with lung cancer. Circulating growth factor levels may also correlate with active cancer. These studies are directed toward development of new methods to identify undetected lung cancer and new therapeutic strategies through increased knowledge of growth-regulatory processes in lung cancer cells.
Hepatocyte growth factor (HGF) and its receptor c-Met are a ligand-receptor pair that initiates signaling pathways promoting proliferation, survival, angiogenesis, and invasion. HGF is a mainly paracrine growth factor that is secreted by fibroblasts in the lung and acts upon the c-Met receptor expressed by airway epithelial and endothelial cells. In lung cancer, c-Met is often upregulated and the tumor cells induce elevated HGF production by neighboring stroma. Elevated HGF in lung cancer patients with early stage disease has identified a population of patients who are most likely to recur and die from their disease. As shown in the Kaplan-Meier survival curves, HGF levels above the median of 22 units was associated with a higher proportion of deaths from all causes (A), lung-cancer specific deaths (B), and recurrence events (C). This observation among others has led us to focus on therapeutic targeting of HGF and its receptor c-Met for control of lung cancer.
To test therapeutic strategies, Dr. Siegfried has engineered a transgenic mouse that over-expresses the human HGF gene in the airways by placing the HGF gene under the control of the Clara cell secretory protein (CCSP) promoter. The lungs of these mice produce human HGF mRNA and protein and contain 2-3 times as many clara cells per micron of airway length as wild-type mice. While the mice show some abnormalities in airway branching, they have normal airway function and they do not develop lung tumors spontaneously at a rate above that of wild-type animals. However, the HGF transgenic mouse is more susceptible to the development of lung tumors initiated by the tobacco carcinogen NNK. Lung tumors produced by carcinogen treatment in the HGF transgenic animal are more invasive and contain higher numbers of blood vessels than wild-type tumors. These effects can be inhibited by a neutralizing antibody to HGF that is now in development for clinical use.
The Siegfried laboratory was one of the first to demonstrate the functional significance of estrogen receptors in lung tumors. This figure shows immunohistochemical staining of a non-small cell lung tumor for the estrogen receptor β, which we have found to be expressed to some degree in over 85% of lung tumors. Staining is observed both in the nucleus and cytoplasm, and we have evidence that both nuclear signaling through ERE elements in the promoter regions of estrogen-sensitive genes, as well as non-nuclear signaling through the EGFR and MAPK pathway occur in lung tumor cells.
Dr. Siegfried also has evidence that estrogen is locally produced by the enzyme aromatase within lung tumors, and this suggests an autocrine ligand-receptor exists for estrogen and its receptor in many lung tumors. Clinical trials are currently on-going to test the clinical activity of estrogen antagonists for therapy of lung cancer.
29
Shivendra Singh, Ph.D. Professor Ph.D., Banaras Hindu University, India, 1984
The Singh laboratory is interested in preclinical and clinical development of novel agents, derived from dietary sources and traditional oriental and Indian medicinal plants, for prevention of prostate and breast cancer in humans.
The research interests of the Singh laboratory include preclinical and clinical development of novel cancer chemopreventive agents and rational design of combination chemoprevention regimens. Cellular and transgenic animal models are used to screen potential cancer chemopreventive constituents from dietary sources and traditional oriental and Indian medicinal plants. Cutting edge cellular, molecular biological, Omics, and imaging techniques are used to (a) determine the mechanism of action of promising cancer chemopreventive agents, (b) monitor effects on cancer progression, and (c) identify biomarkers of response potentially useful in future clinical trials.
Cancer chemoprevention is a relatively new but rapidly emerging sub-discipline in oncology and refers to the use of natural or synthetic agents to reverse or delay the process of carcinogenesis. Long latency of most epithelial cancers, including prostate and breast cancers, provides a large window of opportunity for intervention to prevent or slow disease progression. Accordingly, identification of agents that are relatively safe but can be used to prevent cancers is highly desirable to reduce disease-related cost, mortality, and morbidity for a large segment of population.
Epidemiological studies suggest that dietary intake of certain vegetables (e.g., cruciferous vegetables) may be protective against the risk of different types of cancers. The Singh laboratory has determined cancer chemopreventive efficacy of a few chemicals derived from edible plants, including watercress constituent phenethyl isothiocyanate (PEITC). The PEITC selectively inhibits OXPHOS in prostate cancer cells, but not in normal prostate epithelial cells, to trigger ROS-dependent apoptosis. Administration of PEITC in the diet (3 µmol PEITC/g diet) significantly decreases incidence as well as burden (affected area) of poorly differentiated prostate cancer in a transgenic mouse model (TRAMP mice) in association with induction of autophagy and overexpression of E-cadherin. Plasma proteomics reveals distinct changes in the expression of several proteins (eg, suppression of clusterin) in the PEITC-treated mice that could be used as biomarkers to assess PEITC response in future clinical trials.
Other ongoing projects in the Singh laboratory are focused on preclinical development of other natural products including garlic constituent diallyl trisulfide, broccoli constituent sulforaphane to name a few. One common yet unique characteristic of these natural products is that they selectively cause death of cancer cells.
Robert Sobol, Ph.D. Associate Professor Ph.D., Temple University, 1991
DNA damage is implicated as playing a causal role in numerous disease processes. Hence, it is suggested that DNA repair proteins, which maintain the integrity of the nuclear and mitochondrial genomes, play a critical role in reducing the onset of multiple disease phenotypes. Conversely, the requirement for DNA repair and genome maintenance in response to radiation and genotoxic chemotherapeutics implicates DNA repair proteins as prime targets for improving response to currently available anti-cancer regimens. Further, cancer-specific DNA repair defects offer novel approaches for tumor-selective therapy. It is now expected that all cancer cells will be found to be defective in some aspect of DNA repair encoded by one of the 150 different proteins that catalyze DNArepair. To help in our understanding and treatment of cancer, geneticists and molecular biologists must explore the detailed consequences of an alteration in each of these repair pathways. It is our expectation that a detailed
30
genetic and mechanistic understanding of the cellular phenotypes associated with specific DNA repair deficiencies will offer the opportunity to identify novel drug targets, optimize and validate new small molecule inhibitors of DNA repair and provide a mechanistic underpinning for the development of tumor selective therapeutic strategies.
In Dr. Sobol’s lab, they have identified two compensatory pathways that respond to defects in DNA repair mediated by the base excision repair pathway, including proteins involved in the synthesis and degradation of poly-ADP-ribose (PAR) and those involved in the biosynthesis of NAD+. In particular, we study the convergent roles of DNA Repair, PAR metabolism and NAD+ biosynthesis in response to chemotherapy.
Novel approaches to enhance tumor cell cytotoxicity of alkylating agents: Glioblastoma is the most commonly diagnosed brain malignancy and a major cause of cancer related death in the United States. Limited success in the treatment of glioblastoma has been demonstrated with the alkylating agent Temozolomide (TMZ). The overall goals of this project are to discover strategies to circumvent resistance to TMZ and enhance the cytotoxicity and efficacy of this alkylating agent. The base excision repair (BER) pathway provides significant resistance to TMZ by repairing damaged bases but some of the activity of TMZ is due to the accumulation of cytotoxic BER intermediates that result from incomplete or failed repair (termed BER Failure). As the rate-limiting enzyme in BER, DNA polymerase ß (Polß) is important to facilitate repair and to maintain cell survival following DNA damage. Therefore, inhibition of Polß will enhance TMZ response. We posit that BER mediated by Polß is a regulated process that signals BER failure through poly(ADP)ribose (PAR) synthesis and NAD+/ATP depletion by a process that requires activation of PARP1 & PARP2 and is regulated by the enzyme PARG. Specifically, we hypothesize that TMZ efficacy can be increased in glioma cells by increasing post-translational modification and inhibition of Polß. BER failure-induced cell death results from energy (NAD+ &ATP) depletion due to elevated PAR synthesis mediated by the PARP1/PARP2 BER sensor complex, suggesting that the response to TMZ can be enhanced via increased PAR synthesis or further depletion of cellular NAD+ synthesis and/or deregulation of the BER enzyme PARG. Overall, we are testing the hypothesis that the BER pathway is a determinant of resistance to TMZ and therefore selective targeting of the BER pathway will significantly enhance TMZ efficacy.
Development and characterization of isogenic DNA Repair deficient human cell lines: To extend our analysis beyond base excision repair, we have embarked on a large-scale project for the development, characterization and transcriptome analysis of isogenic human cell lines deficient in all known DNA repair genes (>150) in three unique cell backgrounds (glioma, breast cancer and neuronal). These include genes involved in Base Excision Repair, Direct Reversal of Damage, Mismatch Excision Repair, Nucleotide Excision Repair, Homologous Recombination, Non-homologous End-Joining, the modulation of nucleotide pools, DNA polymerases, editing and processing nucleases, the Rad6 pathway, Chromatin Structure, DNA Repair genes defective in diseases and conserved DNA Damage Response genes and Fanconi Anemia/DNA crosslink repair. Further, each will be analyzed for alterations in PAR metabolism and NAD+ biosynthesis. With the expectation that DNA repair capacity impacts basic cellular functions both spontaneously and in response to genotoxic stress, alters the transcriptional and epigenetic landscape and dictates the cellular response to stress, the development of a complete panel of isogenic DNA repair deficient cell lines across multiple backgrounds will be a valuable platform for gene and drug discovery, validation of inhibitor specificity and the identification of response biomarkers and novel targets for gene/drug synthetic-lethality combinations.
Ongoing and future projects also include the identification of novel DNA Repair targets for improved chemotherapy response, testing and evaluation of novel DNA repair inhibitors, tumor tissue analysis for defects in expression of enzymes critical for chemotherapy response and evaluating the potential of NAD+ biosynthesis inhibitors in the development and testing of tumor specific, synthetically lethal, chemotherapy combinations.
31
Gyun Jee Song, Ph.D. Research Assistant Professor Ph.D., Seoul National University, Seoul, Korea, 2001
Dr. Song’s research is focused on the study of G-protein coupled receptor (GPCR) signaling. G protein coupled receptors, a family of membrane signaling proteins, are the targets of half of the drugs on the market today. Her past research shows that some GPCRs function as dimer or oligomers, and that specific binding molecules regulate receptor signaling and trafficking. Dr. Song is currently focusing primarily on two projects: the study of parathyroid hormone (PTH) receptor signaling in vascular cells and the study of the glucagon-like peptide 1 receptor signaling in the pancreatic islets.
Development of therapeutic drug for vascular diseases
During the past few years, Dr. Song has concentrated on determining the molecular mechanism underlying vascular disease, with particular emphasis on the role of PTHrP and the PTH receptor signaling in the development of arterial restenosis. Her research has revealed some of the mechanisms regulating expression and signal transduction of the PTH1R in vascular smooth muscle cells. Indeed, these molecules are endogenously expressed in vascular smooth muscle cells and play a key role in mediating proliferation and migration of vascular smooth muscle cells, events that are critical for the development of restenosis (the process of occlusion of an artery) following angioplasty. Dr. Song is also interested in the function of EBP50 (Ezin binding protein, NHERF1) a scaffolding protein in the vasculature. She showed that EBP50 contributes to cell-specific signaling by the PTH receptor and contributes to vascular smooth muscle cell proliferation and migration. These observations suggest that targeting EBP50 represents a completely novel potential approach for treating this important complication of a commonly used surgical procedure.
GLP1 receptor regulating insulin secretion and ß cell proliferation
Dr. Song is also involved in the study on glucagon-like peptide 1 receptor (one of the GPCRs) function in pancreatic islets. In particular, the role of the adaptor protein Caveolin-1 in the regulation of receptor expression and function is under investigation. Furthermore, her research aims to determine the role of Caveolin-1 in determining the efficacy of GLP-1R agonists in stimulating insulin secretion and ß cell proliferation in vivo.
Laura Stabile, Ph.D. Research Assistant Professor Ph.D., West Virginia University, 1999
Dr. Stabile is interested in the role of growth factors and hormones in the development of human lung cancer. The hepatocyte growth factor (HGF)/c-Met pathway and the estrogen pathway both play key roles in the development and progression of lung cancer and represent attractive targeted pathways for drug development. Lung cancer kills more Americans every year than any other type of cancer, and the 5-year survival rate is only 16%. Lung cancer patients are typically diagnosed at a late stage and have very few effective therapeutic options. Thus, new targeted strategies are essential to make an impact on this disease.
c-Met is a receptor tyrosine kinase whose activation by HGF can lead to transformation (conversion of a normal cell into a malignant cell) and tumorigenicity (growth of tumors) in a variety of human tissues. Since c-Met and HGF are frequently overexpressed in lung cancer and there is a strong correlation between overexpression and decreased patient survival, the HGF/c-Met signaling pathway is a potential target for tumor control. Primary projects in this area of interest include: 1) studying the development and inhibition of lung carcinogenesis in a novel transgenic mouse model that overexpresses HGF in the airways 2) preclinical development of therapeutic drugs that target this pathway using a variety of techniques such as neutralizing antibodies to HGF, c-Met small molecule inhibitors, c-Met guanidinium-peptide nucleic acid antisense
32
technology and 3) understanding the mechanism of signaling interactions between c-Met and the epidermal growth factor receptor (EGFR) pathway.
Dr. Stabile has successfully developed a murine model that mimics the overproduction of HGF found in human lung tumors and has shown that a single human HGF neutralizing antibody, L2G7, has profound inhibitory effects on development of lung tumors in this transgenic mouse model. Furthermore, lung tumors with K-ras mutation are resistant to blockage of the HGF pathway using L2G7. In addition, we have recently demonstrated the importance of induction of the cyclooxygenase 2 (COX-2)/prostaglandin E2 (PGE2) pathway and subsequent activation of EGFR by HGF in lung cancer cells. Figure 2 describes the signaling pathway of HGF that we are studying and areas of therapeutic intervention. Another aspect of research involves the estrogen pathway in lung cancer. Lung cancer is becoming increasingly common in women and in the U.S. accounts for more female deaths annually than breast cancer and all other gynecological cancers combined. Epidemiological studies show that male-female differences exist in the presentation of lung cancer. These observations suggest the role of estrogens in lung carcinogenesis. Primary projects in this area of interest include: 1) understanding both genomic and non-genomic effects of estrogen in the lung 2) elucidating cross-talk pathways between estrogen and the EGFR and VEGF pathways in the lung 3) understanding the differences in estrogen signaling in lung cancer patients who actively smoked versus those who never smoked and 4) preclinical development of therapeutic drugs that target this pathway such as estrogen antagonists and aromatase inhibitors.
Dr. Stabile has demonstrated that estrogen receptors are expressed in both normal lung as well as lung tumor cells and that estrogen promotes the growth of lung tumor cells. The growth stimulation is significantly inhibited in vitro and in vivo with the pure estrogen receptor antagonist, ICI 182,780 (Faslodex, fulvestrant).
In addition, Dr. Stabile has demonstrated that the estrogen receptor pathway can cross-talk with the EGFR pathway and targeting both pathways simultaneously using clinically relevant agents show enhanced anti-tumor effects compared to targeting either pathway alone. This drug combination is currently being tested in clinical trials. Dr. Stabile is currently interested in elucidating the role of the newly discovered estrogen receptor, GPR30, which is thought to be responsible for some of the non-genomic actions of estrogen.
The overall goal for both areas of interest are to test different mechanisms by which these pathways control other growth-promoting proteins in the lung and test both available and novel drugs as single agent or combination therapies using novel animal models of lung cancer to determine how to inhibit these pathways most effectively. Optimal preclinical drugs will ultimately be translated to patient clinical trials.
Ben Van Houten, Ph.D.Professor Ph.D., Oak Ridge Graduate School of Biomedical Sciences, University of Tennessee, 1984
Dr. Van Houten’s laboratory studies the formation and repair of DNA damage in nuclear and mitochondrial genomes. We are particularly interested in the structure and function of proteins that mediate nucleotide excision repair and the role of oxidative stress in human disease.
Faulty DNA repair can promote mutations, aging, cancer and cell death. The process by which protein components of repair detect damaged or modified bases within DNA is an important but poorly understood type of protein-DNA interaction. The cell contains a series of pathways designed to protect its DNA from environmental and endogenous damage. One of the most remarkable aspects of nucleotide excision repair (NER) is that it can remove a wide range of DNA lesions that differ in chemistry and structure. During bacterial NER UvrA, UvrB and UvrC proteins work together to identify and remove DNA damage.
33
The UvrA and UvrB proteins are believed to recognize damage-induced distortion in the DNA helix rather than the lesion per se. However, detailed studies of the kinetics, thermodynamics and structures of the Uvr proteins have been limited due to their instability. To overcome this problem, UvrA, UvrB and UvrC from the thermophilic bacteria Bacillus caldotenax and Thermotoga maritima were recently cloned and overexpressed. The proteins maintain optimal activity at 65ºC and are amenable to both structural and biophysical studies. The group is collaborating with Bob London’s NMR group at NIEHS for an analysis of the dynamics of UvrB upon ligand binding using NMR techniques. The group is also collaborating with Caroline Kisker, Ph.D. and her group, in Wurtzburg, Germany, on solving protein and protein-DNA structures by X-ray crystallography. We have recently solved a co-crystal structure of UvrB bound to DNA.
These complexes are being visualized on DNA using atomic force microscopy, performed Hong Wang, Ph.D., and single-molecule techniques using quantum dot labeling, performed by Neil Kad, Ph.D. at the University of Essex. These tools, combined with site-directed mutagenesis and biochemical analyses, allow for structure-function studies of the UvrA, UvrB and UvrC proteins, and form a basis for understanding the fundamental molecular processes of NER. The long-term goal is to have a complete understanding of how structural perturbations induced by specific DNA lesions are detected and removed by the NER machinery at the atomic level. Most recently we have begun to extend our studies to similar proteins found in humans.
Mitochondria represent an important target of reactive oxygen and mitochondrial DNA (mtDNA) appears to be an early and sensitive marker of this stress. Many human diseases are associated with reactive oxygen including cancer, heart disease and neurodegenerative diseases. Mitochondria are essential organelles for generating ATP during oxidative phosphorylation. The mtDNA encodes 13 polypeptides, 11 involved in electron transport and two serving as subunits of ATP synthase. Damage to mtDNA is repaired, but prolonged oxidant treatment results in persistent mtDNA damage, loss of mitochondrial function and apoptosis. These observations suggest that mtDNA damage is important in the toxicity induced by reactive oxygen species (ROS) such as superoxide, hydrogen peroxide and the hydroxyl radical. Our group is testing the hypothesis that ROS generated in the mitochondria results in mtDNA damage causing a vicious cycle of damage: mtDNA damage causes a decrease in transcription and loss of essential mitochondrial proteins, causing an inhibition of electron transport and subsequent release in more ROS. This process causes further mitochondrial decline and many degenerative diseases associated with aging. We have developed a very sensitive DNA damage assay based on quantitative PCR that allows us to examine damage to nuclear and mitochondrial DNA from as little as 100 microliters of human blood. We are currently examining the role of mtDNA damage and repair in several human diseases including cancer and Friedreich’s ataxia.
Jean-Pierre Vilardaga, Ph.D. Associate Professor Ph.D., Free University of Brussels, Belgium, 1996
The Vilardaga Laboratory is directed at understanding molecular mechanisms of G protein-coupled receptor (GPCR) signaling and trafficking – two key processes in biological signaling in general and, more specifically, in pharmacology and drug research. Adrenergic and peptide receptors, which transmit signals, respectively for small neurotransmitters (such as noradrenaline and dopamine) and larger peptide hormones (vasopressin, parathyroid hormone, parathyroid hormone related peptide), are two well characterized distinct subtypes of GPCRs that serve as useful models for analyzing GPCR mechanisms. The objective of this line of research is to elucidate the general principles of signal transduction from the extracellular ligand binding event to intracellular signaling cascades, which are involved in systems as diverse as neurotransmitter and hormonal signaling.
Optical approaches (eg. FRET, TIRF microscopy) are used to monitor the activation/deactivation steps along the signaling cascades of GPCRs in live cells. This approach revealed fundamental mechanisms of GPCRs signaling and trafficking in live cells for neurotransmitter and peptide hormones such as the PTH, which were
34
published in 2003-2007 in Nature Biotech, Nature Methods, Nature Chemical Biology, the Journal of Biological Chemistry and PNAS.
Recently Vilardaga laboratory also discovered the new concept that persistent cAMP production mediated by parathyroid hormone receptor endocytosis may mediate potent catabolic signaling actions via PTH (PNAS 2008, Nature Chem Biol 2009). This prolonged cAMP production from intracellular compartments further indicate that the traditional concept that cAMP production triggered by GPCRs originates exclusively at the cell membrane must be revised. The main focus of my current research aims at determining the origin of the prolonged signaling by GPCRs and its termination. These events and consequent signaling patterns are quite novel and important for cellular signaling.
Andreas Vogt, Ph.D. Research Assistant Professor Ph.D., University of Hamburg, Germany, 1990
Dr. Vogt’s major research interest is the discovery of new therapeutic agents for diseases related to cell proliferation and intracellular signaling. Specific targets of interest are the mitogen-activated protein kinase phosphatases (MKPs), cellular enzymes involved in cancer and inflammation that have thus far eluded discovery efforts. An important part of his research is the development of analysis tools to increase information content of biological assays and to enable small molecule drug discovery screening in whole organisms. It is his hope that the combination of high information content assays with whole organism models of disease will result in higher quality candidate compounds for drug development.
Dr. Vogt’s target-based discovery efforts center around mitogen-activated protein kinase phosphatases or MKPs. One MKP in particular, termed DUSP-1 or CL100, appears to be a mediator of the malignant phenotype. MKP-1 is overexpressed in many human tumors and can protect cells from apoptosis caused by DNA damaging agents or cellular stress, suggesting that inhibitors of MKP-1 might find applications as novel target-based antineoplastic therapies, either alone or in combination with clinically used antineoplastic agents. The search for small molecule inhibitors of MKP-1 has been challenging due to a lack of structural guidance for inhibitor design, ambiguities associated with in vitro assays for phosphatase activity, and the absence of definitive assays to probe MKP-1 inhibition in the context of the living cell. By developing an image-based, definitive cellular assay for MKPs, which he has termed “chemical complementation,” Dr. Vogt has circumvented many of the challenges associated with MKP inhibitor discovery, screened several thousand small molecules for MKP inhibition in intact cells, and identified several cell-active inhibitors of MKP-1 and MKP-3. Future work will encompass discovery of multiple classes of MKP inhibitors, refinement of their structures and biological activities, evaluation of their mode of inhibition, and their credentialing as potential therapeutic agents. Recently, Dr. Vogt has extended the concept of chemical complementation to collaborations with members of the zebrafish community on the discovery of inhibitors of MKP-3.
Dr. Vogt’s second focus is the continued development of novel drug discovery tools that enhance the information content of small molecule screens. Over the past five years, he has been involved in the establishment of the University of Pittsburgh Drug Discovery Institute (UPDDI) as one of only a handful of academic centers with high-content analysis (HCA) capabilities. HCA is an analysis tool to acquire, analyze, and manage multi-dimensional information about target activity and spatial distribution from individual cells. Our emphasis on HCA has substantially contributed to the UPDDI becoming one of nine national screening centers funded through the NIH roadmap initiative on Molecular Libraries and Imaging. Dr. Vogt’s research has contributed to the center four R03 funded high-content screens (two for MKPs, one for microtubule stabilizing agents, and one for autophagy), which he is currently running on 200,000 small molecules from an NIH compound collection.
35
A natural extension of Dr Vogt's prior HCA work is the expansion of image-based analysis to whole organisms such as zebrafish. This work is based upon the observation that advances in high-throughput screening and laboratory automation have substantially improved the speed of target-based drug discovery but that these efforts have not resulted in increased research productivity. An increasingly popular sentiment is that better models are needed to improve the quality of new drug candidates, and it has been proposed that whole organisms could provide such models. Currently, however, there is no animal model that is compatible with the contemporary paradigm of drug discovery encompassing rapid screening of large compound collections. The zebrafish is an animal model that might fill this void. The NIH is currently supporting acollaboration with Neil Hukriede and Michael Tsang (Department of Molecular Genetics and Biochemistry) aimed at developing novel image-based methods to analyze fluorescent transgenic zebrafish embryos. Of particular interest is an intelligent image analysis method termed Cognition Network Technology (CNT). CNT is different from other image analysis methods in that it processes image information in an object oriented fashion, thereby emulating human cognitive processes.
With this approach, Dr. Vogt is able to detect zebrafish embryos in multiwell plates regardless of orientation and to detect and quantify structures of interest within specific parts of the zebrafish embryo. An example is the automated detection and quantification of intersegmental blood vessels in the dorsal trunk of the zebrafish as measure of in vivo angiogenesis. Dr. Vogt developed a rule set that successively identified the entire outline of the embryo and defined regions of interest such as aorta (magenta), head (brown), yolk and yolk tube (yellow), and dorsal area (dark green). This context-based segmentation permitted the isolation and quantification of intersegmental blood vessels (red) without interference from surrounding areas. Because CNT can identify complex zebrafish embryonic structures regardless of orientation, the embryo can be arrayed randomly into any position and CNT will identify these distinct structures. Currently Dr. Vogt is working to generate a rule set that will identify the GFP expression in specific parts of the brain of a transgenic animal expressing an Fgf reporter (Tg (Mkp3:d2eGFP).
Daniela Volonte, Ph.D. Research Assistant Professor Ph.D., University of Milan, Italy, 1996
Tumor development is initiated by a multiplicity of genetic abnormalities. Tumor cells need to escape barriers that limit uncontrolled cell proliferation. One of these barriers is represented by cellular senescence. Cancer cells need to overcome this obstacle to produce a clinically relevant tumor mass. For these reasons, cellular senescence represents a natural tumor suppressor mechanism. Thus, molecules that regulate cellular senescence are potential therapeutic targets for the treatment of cancer and the fight against aging.
Caveolae are invaginations of the plasma membrane enriched in cholesterol. Caveolin-1, the structural protein component of caveolar membranes, acts as a scaffolding protein to concentrate and functionally regulate signaling molecules.
In recent years, several independent lines of evidence have emerged suggesting that caveolin-1 may function as a "tumor suppressor protein" in mammalian cells. For example, caveolin-1 protein expression has been shown to be absent in several transformed cell lines derived from human mammary carcinomas, including MCF-7. In addition, caveolin-1 mRNA and protein expression are lost or reduced during cell transformation by activated oncogenes, such as v-Abl and H-ras (G12V); caveolae are absent from these cell lines. In addition, the human caveolin-1 gene is localized to a suspected tumor suppressor locus (D7S522; 7q31.1), a known fragile site (FRA7G) that is deleted in many types of cancer.
Oxidative stress is a known inducer of cellular senescence. We have shown that up-regulation of caveolin-1 is required for oxidative stress–induced cellular senescence in fibroblasts. To unravel the molecular mechanisms underlying oxidative stress-induced up-regulation of caveolin-1 in senescent cells, Dr. Volonte has shown that
36
oxidants stimulate the activity of the caveolin-1 promoter reporter gene construct in fibroblasts. She has identified Sp1 binding to two GC-boxes as the core mechanism of oxidative stress–triggered caveolin-1 transactivation. In addition, through signaling studies she has shown p38 mitogen-activated protein kinase (MAPK) as the upstream regulator of Sp1-mediated activation of the caveolin-1 promoter following oxidative stress. For the first time Dr. Volonte has delineated the molecular mechanisms that modulate caveolin-1 gene transcription upon oxidative stress bringing new insights into the redox control of cellular senescence in both normal and cancer cells.
Thus, cellular senescence may represent one of the molecular mechanisms through which caveolin-1 acts as a tumor suppressor protein. Current efforts are aimed at identifying the signaling molecules which link caveolin-1’s function to cellular senescence.
Nobunao Wakabayashi, Ph.D. Research Assistant Professor Ph.D., Tohoku University Graduate School, 1959
Loss of cellular homeostasis through exposures to endogenous (e.g., inflammation) and exogenous (e.g., carcinogens) stresses contributes to many diseases including carcinogenesis. The Keap1-Nrf2-ARE signaling pathway evokes an adaptive response to these stresses that serves to enhance cell survival. Through gene expression analyses of the signaling cascade linked to Nrf2, using both Keap1- and Nrf2-disrupted mice, it appears that multiple signaling pathways intersect with Nrf2 signaling. Our research goals are to elucidate novel signaling crosstalk based on genes bearing functional ARE (Nrf2-sMaf recognition enhancer element) in the promoter of target genes and the underlying mechanistic roles of the Keap1-Nrf2 system in protecting against chronic degenerative diseases in vivo. Currently, we are focusing on the role of Nrf2 signaling in tissue repair and regeneration.
Bin Wang, Ph.D. Research Assistant Professior Ph.D., Anhui Medical University, Hefei, China, 1999
Dr. Wang researches parathyroid hormone receptor trafficking, interaction with the adapter protein EBP50/ NHERF1 and other proteins, and their effects on the signaling in kidney and bone cells. The goal of these studies will contribute to our understanding of mineral ion homeostasis under normal conditions, as well as the pathogenesis of diseases that are related to disordered calcium and phosphorus balance in renal failure, hyperparathyroidism, or osteoporosis. The results will provide insights into developing drugs for selective therapeutic applications.
Q. Jane Wang, Ph.D. Associate Professor Ph.D., Creighton University, 1995
Dr. Wang’s lab conducts basic and translational research on oncogenic protein kinases in the area of cancer biology. The main focus is on diacylglycerol (DAG)-targeted protein kinases, particularly protein kinase D (PKD) and protein kinase C (PKC) family kinases. DAG is a key second messenger generated in cells in response to the activation of receptor tyrosine kinases and G protein-coupled receptors. It acts to disseminate vital signals from the cell surface to the cell interior. PKD and PKC are primary targets of DAG that regulate many fundamental cellular processes such as cell growth, survival, apoptosis, motility, and protein trafficking. In particular, PKD is at the center of the DAG signaling network, integrating signals from both DAG and PKC. In recent years, PKD has emerged as a promising therapeutic target for several diseases including cancer and heart disease. Deregulated PKD expression and activity have been demonstrated in a variety of pathological conditions, suggesting an active contribution to disease initiation and progression.
37
Dr. Wang’s laboratory seeks to understand both the basic structure of PKD and how different structural domains in PKD interact to regulate its activity and function. We are particularly interested in the C1 domain, the high affinity DAG binding domain shared among all DAG receptors. Our previous studies identified PKD as a high affinity receptor for DAG and defined the structure-activity requirements for the binding of the PKD C1 domain to DAG and phorbol esters. This work has provided the basis for ligand-specific regulation of PKD isoforms. Our long-term goal is to investigate the intra- and intermolecular regulatory mechanisms controlling PKD activity and assess their impact on the function of PKD in intact cells. Ultimately, we seek to solve the 3D structure of this protein.
Growing evidence has implicated PKD in the pathogenesis of cancer. Using prostate cancer as a model, we will determine the functional relevance and signaling mechanisms of PKD in cancer. We will elucidate the signaling pathways both upstream and downstream of PKD and identify novel PKD substrates relevant to cancer promotion. In previous studies, we have identified PKD3 as a downstream target of the oncogenic kinase PKCe. We found that PKD3 acts through a hyperactive PKCe/PKD3 pathway to modulate the activity of ERK1/2 and Akt signaling, contributing to the growth and survival of prostate cancer cells. Another active area of research in our lab is to determine the in vivo relevance of PKD signaling to cancer. We use a variety of animal models including mouse xenograft models and transgenic/knock-out mice to determine the role of PKD in prostate cancer initiation, progression, and metastasis. In addition, we also study the cross-regulation between PKD and androgen receptor signaling and its implication in prostate cancer. Our studies have now expanded to other types of solid tumors such as head and neck cancer and pancreatic cancer. In the long run, we seek to validate PKD as a therapeutic target for cancer therapy.
In this major research endeavor, we seek to develop potent and selective small molecule inhibitors of PKD as pharmacological tools for dissecting PKD-mediated biological processes and as potential drugs for clinical application. This is an important study in the translation of PKD-targeted therapeutics into the clinic. In collaboration with our colleagues at the University of Pittsburgh Drug Discovery Institute and the Department of Chemistry, we have identified the first potent and selective small molecule PKD inhibitor, CID755673, and subsequently developed a class of unique ATP-noncompetitive inhibitors based on this parental compound. These inhibitors are cell-active and exhibit nanomolar potencies, with high specificity for PKD. Future studies are focused on further developing this series of inhibitors for in vivo and clinical application, and continuing our efforts in new drug discovery.
Dong Xiao, Ph.D. Research Instructor Ph.D., Soochou University, China, 1997
Natural products play a relevant role in cancer therapy today with substantial numbers of antitumor agents used in the clinic being either natural or derived from natural products. Dr. Xiao’s laboratory focuses on the study of some dietary natural products on the effects and biomarker(s) of their prevention/therapy of prostate and breast cancers.
Our field of expertise is chemoprevention and the inhibition of cancer cell growth through the modulation of genes that are associated with cell cycle, autophagy and apoptosis. The long-term goal of Dr. Xiao’s research is to develop a safe and effective strategy for chemoprevention/chemotherapy of the human prostate and breast cancer.
38
Lin Zhang, Ph.D.Associate Professor Ph.D., University of Southern California, 1995
The immediate goal of Dr. Zhang’s research is to understand how anticancer drugs kill cancer cells, and more importantly, why they fail so often. In the long term, we will attempt to use this knowledge to identify novel molecular targets and treatment strategies to improve cancer chemotherapy and chemoprevention.
Cell death in anticancer therapies Our research program has centered on several molecules that control discrete steps of programmed cell death. The first one, PUMA, is a downstream target of the tumor suppressor p53 and a BH3-only Bcl-2 family protein. PUMA is required for DNA damage-induced and p53-dependent apoptosis, and also plays a key role in apoptosis induced by several targeted anticancer drugs. The second one, SMAC, is a mitochondrial apoptogenic protein and a caspase activator. SMAC helps to execute apoptosis induced by anticancer drugs via a mitochondrial feedback loop. Regulators of non-apoptotic cell death, such as the autophagy inducer Beclin 1 and the necrosis regulator RIPK3, have also been studied. Through analyses of these molecules and their associated protein networks, we try to gain deep understanding on how cell death is initiated and executed in human cancer cells, why some cancer cells are not sensitive to anticancer drugs, and what can be done to restore their sensitivity.
Oncogenic stem cells as the target of cancer chemoprevention Prevention of human cancer through the use of chemical agents such as non-steroidal anti-inflammatory drugs (NSAIDs) has emerged as a promising strategy to reduce morbidity and mortality of cancer. Our recent studies showed that intestinal stem cells that have acquired oncogenic alterations are targeted by NSAIDs in chemoprevention of colon cancer. We are investigating how NSAIDs trigger apoptosis in such oncogenic stem cells, and if induction of apoptosis is critical for the chemopreventive effects of NSAIDs. We will also determine if apoptosis regulators can be used as markers to predict outcomes of chemoprevention of cancer patients, and if manipulation of apoptosis regulators can be used to improve the chemopreventive effects of NSAIDs.
Manipulation of cell death regulators To target PUMA, we have developed a high-throughput screening system for identifying small molecules that can activate PUMA in p53-deficient cancer cells. In collaboration with the Pittsburgh Drug Discovery Institute, we will screen compound libraries to identify novel PUMA inducers. We have also identified and characterized small molecules that mimic the functional domains of PUMA and SMAC. Efforts are undertaken to apply these small molecules to chemotherapy and chemoprevention.
Study Sections, Advisory Committee Memberships and Editorial Service
Bruce Freeman, Ph.D. Professor and Chair Associate Editor:
Environmental and Nutritional Interactions Editorial Board:
Journal of Biological Chemistry General Pharmacology: The Vascular System Nitric Oxide: Biology and Chemistry Critical Care Medicine
Grant Reviewer: NCINIEHS
39
Study Section: Lung Injury, Repair and Remodeling Study Section, Center for Scientific Review
Daniel Altschuler, Ph.D. Associate Professor External Reviewer Argentine Society for Science and Technology Ad Hoc Reviewer: Nature Cell Biology Journal of Biological chemistry Proceedings of the National Academy of Sciences USA
Molecular and Cellular Biology Endocrinology Molecular Endocrinology Molecular Pharmacology Journal of Cell Biology Molecular Biology of the CellThe Journal of Pharmacology and Experimental Therapeutics Journal of Cell Science American Journal of Pathology
Dr. Alessandro Bisello Associate Professor Ad Hoc Reviewer: National Heart, Lung and Blood Institute National Institutes of Health
Marsha Cole, Ph.D. Research Instructor Editorial Board: Medicinal Chemistry: Current Research Ad Hoc Reviewer: The Journal of Immunology Journal of Biological Chemistry Environmental Health Perspectives International Journal of Cardiology
Donald DeFranco, Ph.D.Professor Study Section: Biomedical Research and Training (BRT-A) Study Section, NIGMS Editor-in-Chief:
Molecular Endocrinology Ad Hoc Grant Reviewer: American Cancer Society March of Dimes Birth Defects Foundation National Science Foundation
Ad Hoc Reviewer: American Journal of Physiology Biochemical Pharmacology Biochemistry
40
Biotechniques Cancer Research Cell Biology and Toxicology Cytogenetics and Genome Research Endocrinology European Journal of Biochemistry Experimental Neurology Immuno Modulation Journal of American Physiology Journal of Biological Chemistry Journal of Cell Biology Journal of Cell Science Journal of Cerebral Blood Flow and Metabolism Journal of Neurochemistry Journal of Neuroscience Journal of Steroid Biochemistry and Molecular Biology Molecular Cell Molecular Endocrinology Molecular Biology of the Cell Molecular and Cellular Biology Molecular and Cellular Endocrinology Molecular Pharmacology Nature Biotechnology Neurobiology of Disease Neuroscience Oncogene Proceedings of the National Academy of Sciences USA Trends in Cell Biology Trends in Endocrinology and Metabolism Science Steroids Urology
W. Chet de Groat, Ph.D. Distinguished Professor Editorial Board:
Journal of Pharmacology and Experimental Therapeutics Journal of the Autonomic Nervous System Life Sciences
Editorial Consultant: Brain Research Journal of Neurophysiology Science Journal of Urology Journal Comparative Neurology European Journal of Pharmacology Experimental Neurology
Executive Committee, International Society of Autonomic Neuroscience External Advisory Board, UCLA Center for Neurovisceral Sciences and Women’s HealthExternal Advisory Committee, UCSF SCOR Grant of the Women’s Health Research Center External Advisory Board, K-12 Institutional Mentored Urological Research Career Development Program, UCLA
41
Faculty, 1000 Biology, Head of Section for the Genito-Urinary and Reproductive Pharmacology Interdisciplinary Functional Pain Consortium Steering Committee of the International Foundation for Gastrointestinal Disorders
Julie Eiseman, Ph.D. Research Associate Professor Grant Reviewer:
Veteran’s Administration Merit Review (central and local) NCI, Program Project Grants, STIR/SBIR Developmental Therapeutics Reviewer:
Journal of Biological Optics Cancer Chemotherapy and Pharmacology Oncology Research Communications in Molecular Pathology and Pharmacology Clinical Cancer Research Journal of Pharmacology and Experimental Therapeutics Molecular Pharmacology Journal of AOAC International Cancer Research Journal of Photochemistry and Photobiology Molecular Cancer Therapeutics Antimicrobial Agents and Chemotherapy
Melanie Flint, Ph.D. Research Assistant Professor Reviewer:
Journal of Neuroimmunology Toxicology and Applied Pharmacology Carcinogenesis Experimental Dermatology Medical Science Monitor Basic Mechanisms in Cancer
Grant Reviewer: Austrian Science Fund Executive Board
Peter Friedman, Ph.D. Professor Reviewer:
National Science Foundation, Panel on Cellular and Molecular Biology National Science Foundation, Panel on Regulatory Biology
Awards Committee: American Physiological Society, Chair, 2005-2007 Study Section: National Institutes of Health, Molecular and Cellular Endocrinology Study Section Consultant: FDA, novel parathyroid hormone compounds Ad Hoc Reviewer: Journal of Biological Chemistry Nature Chemical Biology PLoS One Tumor Biology
42
Bone Biochemistry Journal of Bone and Mineral Research American Journal of Physiology
William Furey, Ph.D. Professor Ad Hoc Reviewer:
Journal of Molecular Biology Biochemistry Nature Structure Biology Acta Crystallographica Proceedings of the National Academy of Sciences USA Proteins Journal of Biological Chemistry Protein Science
Ferruccio Galbiati, Ph.D. Associate Professor Editorial Board: h Bioscience Ad Hoc Reviewer:
PLos ONE Cancer Research Molecular Cancer Research Molecular Pharmacology Molecular Endocrinology Journal of Neurochemistry Aging Cell Human Genetics European Journal of Cancer Journal of Cell Science Journal of Applied Physiology Experimental Cell Research Journal of Molecular Medicine Proteomics Journal of Cellular and Molecular Medicine Carcinogenesis Cell Research
Stacy Gelhaus, Ph.D. Research Assistant Professor Ad Hoc Reviewer: Chemical Research in Toxicology Analytical Chemistry Prostaglandins, Leukotrienes and Essential Fatty Acids
Pamela Hershberger, Ph.D. Research Assistant Professor Ad Hoc Reviewer:
Cancer Chemotherapy and Pharmacology
43
Leukemia Gene Therapy Molecular Pharmacology Cancer Research
Jing Hu, Ph.D. Assistant Professor Ad Hoc Reviewer:
Molecular Carcinogenesis British Journal of Cancer Biochemical Pharmacology Experimental Cell ResearchCurrent Drug Metabolism Journal of Cancer Research and Clinical Oncology Cellular and Molecular Life Science Molecular Nutrition and Food Research Journal of Biological Chemistry Neoplasia
Editorial Board: American Journal of Cancer Biology Ad Hoc Grant Reviewer: Florida Department of Health
James & Esther King Biomedical Research Program Bankhead-Coley Cancer Research Program
Yi Huang, M.D. Research Assistant Professor Ad Hoc Reviewer: Cancer Biology & Therapy Cancer Research Clinical Cancer Research Clinical & Experimental Metastasis
Life Science Carcinogenesis
Breast Cancer Research and Treatment Molecular Carcinogenesis Neoplasia Cell Biochemistry and Biophysics Molecular and Cellular Endocrinology Editorial Board: Frontiers in Epigenomics
Edwin Jackson, Ph.D. Professor NIH Study Section, Permanent Member:
Hypertension and Microcirculation Editorial Board:
Hypertension Journal of Pharmacology & Experimental Therapeutics Clinical and Experimental Hypertension American Journal of Physiology - Renal Physiology
44
American Journal of Physiology - Regulatory, Integrative and Comparative Physiology American Journal of Physiology – Heart and Circulatory Physiology Cardiovascular Therapeutics
Journal Refereeing: American Journal of Hypertension American Journal of Physiology Biochemical Pharmacology Cardiovascular Research Circulation Circulation Research Clinical and Experimental Hypertension Clinical Pharmacology and Therapeutics Hypertension Journal of Cardiovascular Pharmacology Journal of Clinical Investigation Journal of Laboratory and Clinical Medicine Journal of Pharmacology and Experimental Therapeutics Kidney International Life Sciences Molecular Pharmacology Nephrology Dialysis Transplantation
Yu Jiang, Ph.D. Associate Professor Editorial Board: World Journal of Biological Chemistry Ad Hoc Reviewer:
Breast Cancer Research and Treatment Cell Report Science Journal of Biological Chemistry
Thomas Kensler, Ph.D. Professor Study Section: NCCAM/NIH Special Emphasis Panel ZAT1 SM (25) – Mechanistic Research on Natural Products Editorial Board: Reviews in Mutation Research Carcinogenesis Senior Editor: Cancer Prevention Research Committee Memberships: Society of Toxicology, Disease Prevention Task Force; Chair [2010-2012] Ad Hoc Reviewer: Analytical Biochemistry In Vitro Toxicology Applied Occupational & Environmental Hygiene Journal of Agricultural & Food Chemistry Archives of Biochemsitry and Biophysics Journal of Biological Chemistry Biochemical Journal Journal of Cellular Biochemistry Biochemical Pharmacology J. Chromatography B: Biomed. Sci Appl. Biochemistry Journal of Clinical Investigation Cancer Biochemistry Biophysics Journal of Inorganic Biochemistry
45
Cancer Detection and Prevention Journal of Investigative Dermatology Cancer Epidemiology, Biomarkers & Prevention Journal of Mass Spectrometry Cancer Prevention Research Journal of Medicinal Chemistry Cancer Research Journal of the National Cancer Institute Carcinogenesis Journal of Pharmacy and Pharmacology ChemBioChem Journal of Toxicol. and Environ. Health Chemico-Biological Interactions Laboratory Animal Sciences Chemical Biology & Drug Design Molecular Carcinogenesis Chemical Research in Toxicology Molecular Cellular Biology Drug Metabolism and Disposition Molecular Pharmacology Environmental Health Perspectives Mutation Research Environmental & Molecular Mutagenesis Nutrition and Cancer Environmental Research Oncogene Experimental Cell Research Proc. National Academy of Science USA FASEB Journal Proc. Soc. Experimental Biology Free Radical Biology & Medicine and Medicine Free Radical Research Risk Analysis Gene Science Gut Toxicology Hepatology Toxicology Letters International Journal of Cancer Trends in Molecular Medicine
Nicholas Khoo, Ph.D. Research Assistant Professor Ad Hoc Reviewer: Antioxidants and Redox Signaling Biochemical Journal Brain Research Bulletin British Journal of Pharmacology Dissease Models & Mechanisms Free Radical Biology and Medicine Journal of Biological Chemistry Molecular Nutrition and Food
Joan M. Lakoski, Ph.D. Professor Study Section:
Neuroendocrinology, Neuroimmunology and Behavior Editorial Board: American Journal of Translational Research Committee Memberships:
Travel Awards Selection Committee, National Postdoctoral Association Nomination Awards Committee, National Postdoctoral Association Program Committee, Annual Meeting, National Postdoctoral Association President’s Council, Society for Executive Leadership in Academic Medicine (SELAM) International Board Development Committee, Annual Meeting, National Postdoctoral Association Member, Nominations and Awards Committee, Women Executives in Science and Healthcare (WESH; formerly SELAM)
Ad Hoc Reviewer: Neuroscience Letters Journal of Pharmacology and Experimental Therapeutics
46
Endocrinology Journal of Neuroscience Research International Journal of Developmental Neuroscience Molecular Endocrinology Synapse Psychopharmacology Brain Research BulletinNeuroscience Life Sciences Journal Neuropharmacology Gastroenterology Environmental Research Pharmacology, Biochemistry and Behavior Journal of Neuroscience Journal of Gerontology: Biological Sciences Academic Medicine Society for Clinical and Translational Science Cell Biology Education – Life Sciences Education
Adrian Lee, Ph.D. Professor Peer Review: NIH/NCI Intramural Program Review (MTBC) Chair, Susam G. Komen for the Cure, Tumor Progression Permanent Member, NIH/NCI, Molecular Oncogenesis (MONC)
Ad-Hoc Member, Susan G. Komen for the Cure, Postdoctoral Fellow Awards Editorships: Section Editor, Viewpoints, Breast Cancer Research Corresponding Editor, Hormone Molecular Biology and Clinical Investigation Member, Editorial Board, Hormones and Cancer Member, Frontiers in Endocrinology Editorial Board Associate Editor, Endocrinology Ad Hoc Reviewer: Breast Cancer Research and Treatment Cancer Research Cancer Chemotherapy and Pharmacology European Journal of Cancer Cancer Investigation Molecular Endocrinology Cell Growth and Differentiation International Journal of Cancer Cancer Immunology and Immunotherapy Oncogene Journal of Neurobiology Cancer Breast Cancer Research Journal of Cellular Biochemistry Cancer Epidemiology Biomarkers & Prevention Biotechniques Molecular Cancer Therapeutics Endocrinology
47
Trends in Biochemical Sciences Molecular and Cellular Biology Biochemistry Growth Hormone & IGF Research Journal of the National Cancer Institute Endocrine-Related Cancer Biomedical Microdevices Nature Clinical Practice Oncology Nature Reviews Cancer
Edwin Levitan, Ph.D. Professor Editorial Board:
Molecular EndocrinologyStudy Section:
National Institutes of Health Special Emphasis Panel Chair
Carola Neumann, M.D. Visiting Associate Professor Ad Hoc Reviewer: Frontiers in Genetics Plos One Cancer Research Current Opinion in Pharmacology Journal of Cellular Biology Journal of Cellular Biochemistry Biochemistry
Steffi Oesterreich, Ph.D. Professor Editorial Boards: Endocrinology BMC Cancer Endocrine-Related Cancer Frontiers in Epigenomics Peer Review: Susan G. Komen for the Cure, Career Catalyst Research Study Section: Permanent Member, NIH TBC Study Section Ad Hoc Reviewer: Breast Cancer Research and Treatment Cancer Research Molecular Endocrinology Oncogene Cancer Breast Cancer Research Molecular Cancer Therapeutics Molecular and Cellular Biology Cancer Letters Carcinogenesis Clinical Chemistry
48
Experimental Cell Research FEBS Letters Genomics Journal of Biological Chemistry Journal of Clinical Investigation Journal of Molecular Endocrinology Journal of Molecular Medicine Journal of the National Cancer Institute Molecular Carcinogenesis Molecular Medicine New England Journal of Medicine Nucleic Acid Research NURSA The Journal of Clinical Endocrinology & Metabolism Nature Medicine
Patrick Pagano, Ph.D.Professor Editor: Clinical ScienceEditorial Board: American Journal of Physiology Cardiovascular Research Free Radical Biology & Medicine Study Section: Permanent Member, NIH/NHLBI Study Section HM RFA Special Emphasis Panel, Cellular and Molecular Mechanisms of Arterial Stiffening and its Relationship to Development of Hypertension Ad Hoc Reviewer: American Heart Association Ad Hoc Reviewer:
American Journal of Physiology Cardiovascular Research Circulation Research Hypertension Free Radical Biology & Medicine Journal of Biological Chemistry Journal of Molecular and Cellular Cardiology Journal of Hypertension American Journal of Respiratory Cell & Molecular Biology Antioxidants & Redox Signaling Arteriosclerosis, Thrombosis & Vascular Biology Circulation Journal of Clinical Investigation Journal of Physiology Kidney International Toxicological Sciences
49
Michael Palladino, Ph.D. Associate Professor Editorial Board: Neurobiology of Diseasea ISRN Biotechnology Ad Hoc Reviewer: Genetics FASEB IUBMB Life Journal of Neuroscience Human Molecular Genetics Cell Metabolism Molecular Cell Proceedings of the National Academy of Sciences USA Annals of the New York Academy of Sciences Cell Death and Differentiation Grant Reviews: United Mitochondrial Disease Foundation Study Section: NIH Neurogenetics (NMG) Study Section Ad Hoc Grant Reviewer: Wellcome Trust PEIR Grants
Guillermo Romero, Ph.D. Associate Professor Editorial Board: Frontiers in Endocrinology Ad Hoc Reviewer: BMC Cell Biology Molecular Endocrinology Journal of Cell Biology Endocrinology
Journal of Cell Science Ad Hoc Grant Reviewer: National Science Foundation of the Czech Republic
James Roppolo, Ph.D. Research Assistant Professor Ad Hoc Reviewer: Journal of Urology, Neurourology and Urodynamics Brain Research
Francisco Schopfer, Ph.D. Research Associate Professor Grant Reviewer: American Heart Association Ad Hoc Reviewer: Free Radical Biology and Medicine Journal British Journal of Pharmacology
50
Sruti Shiva, Ph.D. Assistant Professor Ad Hoc Reviewer: Cardiovascular Research Free Radical Biology and Medicine Mitochondria Circulation Reserach Journal of Biological Chemistry Antioxidant and Redox Signaling Proceedings of the National Academy of Sciences British Journal of Pharmacology Nitric Oxide Grant Reviewer: American Heart Association Review Committee Membranes and Subcellular Organelles
Jill Siegfried, Ph.D. Professor Committee Member:
Subcommittee A - Cancer Centers of the National Cancer Institute Initial Review Group University of Colorado Cancer Center, External Advisory Board SPORE Investigators’ Workshop Planning CommitteeNational Lung Cancer Partnership Board of Directors Eastern Cooperative Oncology Group, Lung Cancer Biology Subcommittee
Grants Reviewer: NCI Special Emphasis Panels for Tumor Microenvironment and Metastasis Study SEction Smith Charitable Trust State of Kentucky Tobacco Research Fund
Editorial Board: Molecular Pharmacology Clinical Lung CancerRespiratory Research
Ad Hoc Reviewer: Cancer Research American Journal of Respiratory Cell and Molecular Biology Experimental Lung Research Radiation Research Molecular Carcinogenesis American Journal of Physiology British Journal of Cancer Lung Cancer Carcinogenesis American Journal of Respiratory and Critical Care Medicine
Scientist Reviewer: Department of Defense, U.S. Army Medical Research and Materiel Command, Congressionally Directed Medical Research Programs – Lung Cancer Research Program
Shivendra Singh, Ph.D. Professor Study Section Member:
Chemo/Dietary Prevention Study Section Special Emphasis Panel, ZRG1 OTC-B 03M
51
Editorial Board: Molecular Pharmacology Molecular Cancer Therapeutics Open Access Pharmacology Journal Journal of Cell Death-Open Access Cancer Prevention Research Clinical Cancer Research
Associate Editor: Molecular Carcinogenesis Ad Hoc Reviewer:
Cancer Research Carcinogenesis Prostate Molecular Cancer Therapeutics Urology Clinical Cancer Research
Committee: Data Safety Monitoring Board, PEITC Trial, University of Minnesota, Minneapolis, MN External Advisory Board, University of South Dakota Translation Research Center
Robert Sobol, Ph.D. Associate Professor Editorial Board:
DNA Repair Journal of Carcinogenesis and Mutagenesis
Editorial Advisory Board: The Open Toxicology Journal
Ad Hoc Reviewer: American Journal of Biotechnology Glia Analytical Biochemistry Head and Neck BBA Journal of Biological Chemistry Biochemistry Journal of Cerebral Blood Flow and Metabolism Biology of Reproduction Journal of Medicinal Chemistry Brain Pathology Journal of Neuro-Oncology Cancer Journal of Neurochemistry Cancer Cell Journal of Surgical Oncology Cancer Chemotherapy & Pharmacology Leukemia Research Cancer Research Mechanisms of Aging and Development Carcinogenesis Molecular & Cellular Biochemistry Cell Biology & Toxicology Molecular & Cellular Biology Cell Cycle Molecular Cancer Chemical Research in Toxicology Molecular Cell Chemistry & Biology Molecular Pharmacology Clinical Cancer Research Mutation Research Digestive Diseases and Sciences Nature Structural & Molecular Biology DNA & Cell Biology Nucleic Acids Research DNA Repair Oncogene EMBO J PLoS Genetics EMBO Reports PLoS ONE Environmental and Molecular Mutagenesis PNAS Environmental and Toxicology & Pharmacology Science Translational Medicine
52
Expert Opnion on Investigational Drugs The Open Toxicology Journal FASEB J The Protein Journal FEBS Letters Toxicological Sciences Gene Therapy TIBS Journal of Bacteriology Tumor Biology Ad Hoc Grant Reviewer: NIH Study Section, MGC NIH Study Section, ZRG1 NIH Study Section, Somatosensory and Chemosensory Systems
Gyun Jee Song, Ph.D. Research Assistant Professor Ad Hoc Reviewer: Human Reproduction Journal of Andrology Asian Journal of Andrology Fertliity and Sterility
Laura Stabile, Ph.D. Research Assistant Professor Ad Hoc Reviewer:
American Journal of Physiology- Lung Cellular and Molecular Physiology Steroids Molecular Carcinogenesis Cancer Chemotherapy and Pharmacology Cancer Biomarkers Lung Cancer Cancer Research Clinical Cancer Research Head and Neck Evidence-Based Complimentary and Alternative Medicine Molecular Endocrinology
Grant Reviewer: Study Section Reviewer, Susan G. Komen for the Cure Research Program
Study Section Reviewer, Susan G. Komen for the Cure Research Program, Postdoctoral Fellow Grants-Basic-Tumor Progression Panel
Ben Van Houten, Ph.D. Professor Ad Hoc Reviewer:
Cell Metabolism Chem. Res. Tox. Chemical Biology Chemical Biology Current Biology Free Radical Biology and Medicine Molecular and Cell Biology Molecular Cell Molecular Pharmacology Nucleic Acids Research Oncogene PLoS One PLos Genetics
53
Proceedings National Academy of Sciences Nature Reviews Science Signaling Science Tox. Sci. Editorial Board: DNA Repair Mutation Research Reviews Journal of Biological Chemistry Molecular Carcinogenesis Committees: NIEHS Technology Transfer Committee NIEHS Microarray User Committee NINDS, Scientific Advisor for Specialized Neuroscience Research Program
Jean-Pierre Vilardaga, Ph.D. Associate Professor Ad Hoc Reviewer: Nature Chemical Biology Proceedings of the National Academy of Sciences American Journal of Physiology Molecular Endocrinology EMBO Report Biochem Biophys Acta Science Signaling Nature
Andreas Vogt, Ph.D. Research Assistant Professor Ad Hoc Reviewer: Molecular Pharmacology Cancer Chemotherapy and Pharmacology Cancer Research Molecular Cancer Therapeutics FASEB Journal Chemistry & Biology Biochemical Pharmacology Ad Hoc Grant Reviewer: North Carolina Biotechnology Center
Daniela Volonte, Ph.D. Research Assistant Professor Editorial Board: Landes Bioscience Journal
Q. Jane Wang, Ph.D. Associate Professor Editorial Board: PLoS ONE International Journal of Clinical and Experimental Medicine
54
Reviewer: Carcinogenesis Chinese Journal of Cancer Biotechnology Journal Mol Biol Cell PLoS One Grant Reviewer: Ad Hoc Member, NIH/CSR, Drug Discovery and Molecular Pharmacology (DMP) Study SEction
US Army Department of Defense Study Section, Prostate Cancer Research Program
Dong Xiao, Ph.D. Research Assistant ProfessorAssociate Editor: Frontiers in Pharmacology of Natural Products Ad Ho Reviewer:
Molecular Cancer Therapeutics Journal of Nutritional Biochemistry BMC Cancer Nutrition and Cancer Acta Pharmacol Sin
Lin Zhang, Ph.D. Associate Professor Editorial Board: World Journal of Gastroenterology Associate Editor: Molecular Carcinogenesis Ad Hoc Reviewer: Oncogene PLoS One Autophagy Neoplasia Journal of Medicinal Chemistry Cellular & Molecular Biology Letters Cell Reports Cancer Microenvironment World Journal of Gastroenterology Study Section Review Panel Membership: Standing Member, NIH ONC Drug Discovery and Molecular Pharmacology (DMP) Study Section
National Cancer Institute, Division of Cancer Prevention PREVENT Cancer Program’s Special Emphasis PanelAmerican Lung Association Research Grant Review Committee
Ad Hoc Review Panel: ZCA1-SLRG-1(J1) SEP: Cancer Biology 1, NCI Omnibus R21/R03 Review Panel DoDCDMRP Lung Cancer Concept Award – Cell Biology Review Panel VA Merit Review Panel for Gastroenterology
FY12�Extramural�Spo
nsored
�Project�Fun
ding�
Arm
y�Gr
ants
�$6
65,2
49Fo
unda
tions
�and
�Ass
ocia
tions
�Fun
ding
$1,5
66,2
81In
dust
ry�G
rant
s/Co
ntra
ct$6
91,9
13N
IH�C
ente
r�Gra
nts
$968
,404
NIH
�Con
trac
ts$7
42,6
04N
IH�D
evel
opm
enta
l�Gra
nts
$469
,713
NIH
�Pro
gram
�Pro
ject
�Aw
ards
$261
,792
NIH
�Res
earc
h�Aw
ards
$12,
910,
028
NIH
�Tra
inin
g�Gr
ants
$206
,452
Fello
wsh
ips
$52,
431
Vete
rans
�Adm
inist
ratio
n�an
d�O
ther
�Gov
ernm
ent�A
war
ds$1
,417
,248
Total�Extramural��Fun
ding�
$19,952,114
FY12�Nationa
l�Institutes�of�H
ealth
�Fun
ding�
Nat
iona
l�Can
cer�I
nstit
ute
$6,5
52,8
37N
atio
nal�C
ente
r�for
�Com
plim
enta
ry�&
�Alte
rnat
ive�
Med
icin
e$3
54,2
00N
atio
nal�H
eart
,�Lun
g,�a
nd�B
lood
�Inst
itute
$2,3
23,6
76N
atio
nal�I
nstit
ute�
on�A
ging
$422
,855
Nat
iona
l�Ins
titut
e�of
�Dia
bete
s�&�D
iges
tive�
&�K
idne
y�Di
seas
es$3
,134
,305
Nat
iona
l�Ins
titut
e�of
�Gen
eral
�Med
ical
�Sci
ence
s$1
,665
,990
Nat
iona
l�Ins
titut
e�of
�Env
ironm
enta
l�Hea
lth�S
cien
ces
$380
,186
Nat
iona
l�Ins
titut
e�of
�Neu
rolo
gica
l�Diso
rder
s�and
�Str
oke
$631
,406
Oth
er�N
IH�In
stitu
tes�
$145
,968
Total�N
ationa
l�Institutes�of�H
ealth
�Fun
ding�
$15,611,424
#REF
!
Research�Grant�&�Con
tract�A
ctivity
Depa
rtmen
t�of�P
harm
acology�&�Che
mical�Biology
55
Dep
artm
ent
of P
har
mac
olog
y &
Ch
emic
al B
iolo
gyF
Y12
Ext
ram
ura
l Sp
onso
red
Pro
ject
Fu
nd
ing
Arm
y G
rant
s3.
3%
Foun
dati
ons
and
Ass
ocia
tion
s Fu
ndin
g7.
9%
Indu
stry
Gra
nts/
Con
trac
t3.
5%
NIH
Cen
ter
Gra
nts
4.9%
NIH
Con
trac
ts3.
7%
NIH
Dev
elop
men
tal G
rant
s2.
4%
NIH
Pro
gram
Pro
ject
Aw
ards
1.3%
NIH
Res
earc
h A
war
ds64
.7%
NIH
Tra
inin
g G
rant
s1.
0%
NIH
Fel
low
ship
s0.
02%
Vet
eran
s A
dmin
istr
atio
n an
dO
ther
Gov
ernm
ent A
war
ds7.
1%
56
Dep
artm
ent
of P
har
mac
olog
y &
Ch
emic
al B
iolo
gyF
un
din
g by
In
stit
uti
on F
Y12
Nat
iona
l Can
cer
Inst
itut
e42
.0%
Nat
iona
l Cen
ter
for
Com
plim
enta
ry &
Alt
erna
tive
Med
icin
e2.
3%
Nat
iona
l Hea
rt, L
ung,
and
Blo
od I
nsti
tute
14.9
%
Nat
iona
l Ins
titu
te o
n A
ging
2.7%
Nat
iona
l Ins
titu
te o
f Dia
bete
s&
Dig
esti
ve &
Kid
ney
Dis
ease
s20
.1%
Nat
iona
l Ins
titu
te o
f Gen
eral
Med
ical
Sci
ence
s10
.7%
Nat
iona
l Ins
titu
te o
f E
nvir
onm
enta
l Hea
lth
Scie
nces
2.4%
Nat
iona
l Ins
titu
te o
f N
euro
logi
cal D
isor
ders
and
Stro
ke4.
0%
Oth
er N
IH I
nsti
tute
s0.
9%
57
Last
Nam
eG
rant
Num
Age
ncy
Nam
eTi
tleB
egin
Dat
eEn
d D
ate
Ann
ual D
irect
C
osts
Ann
ual F
&A
C
osts
Ann
ual T
otal
C
osts
ALT
SCH
ULE
R5R
01 D
K06
3069
Nat
iona
l Ins
titut
es o
f H
ealth
Rap
1B
a M
itoge
nic
Sign
al in
Thy
roid
12/0
1/02
06/3
0/13
$213
,172
$109
,784
$322
,956
ALT
SCH
ULE
R1R
03TW
0090
01N
atio
nal I
nstit
utes
of
Hea
lthEx
ploi
ting
the
cAM
P Pa
thw
ay in
Cha
gas D
isea
se
Ther
apy
08/1
9/11
07/3
1/14
$47,
625
$12,
746
$60,
372
BIS
ELLO
5R01
DK
0510
81N
atio
nal I
nstit
utes
of
Hea
lthSt
ewar
t/Pat
hoph
ysio
logy
of P
TH-R
elat
ed P
rote
in (1
-36)
on
Hum
ans
04/0
5/10
03/3
1/13
$7,2
11$3
,714
$10,
924
CO
LE1K
99H
L095
769
Nat
iona
l Ins
titut
es o
f H
ealth
Met
abol
ic S
tress
Indu
ced
Fatty
Aci
d &
Cad
iova
scul
ar
Func
tion
07/0
1/10
09/3
0/11
$20,
833
$1,6
67$2
2,50
0
DEF
RA
NC
O5R
01 D
K07
8394
Nat
iona
l Ins
titut
es o
f H
ealth
Intra
cellu
lar m
echa
nism
s of g
luco
corti
coid
act
ion
07/0
1/86
06/3
0/13
$178
,196
$86,
425
$264
,621
DEF
RA
NC
O5T
32 G
M00
8424
Nat
iona
l Ins
titut
es o
f H
ealth
Pred
octo
ral T
rain
ing
in P
harm
acol
ogic
al S
cien
ces
09/0
1/94
06/3
0/15
$168
,400
$8,3
52$1
76,7
52
DEF
RA
NC
OW
81X
WH
-09-
1-04
97D
epar
tmen
t of D
efen
seH
IC-5
S R
egul
ator
y R
ole
in T
GFB
Sig
nalin
g in
the
Pros
tate
Stro
ma
(Gru
bish
a Pr
eDoc
Fel
low
ship
)07
/01/
0910
/31/
11$1
3,84
0$1
,107
$14,
947
DEG
RO
AT
1R01
DK
0777
83N
atio
nal I
nstit
utes
of
Hea
lthN
euro
plas
ticity
of U
rinar
y Tr
act D
isor
ders
afte
r SC
I05
/01/
0704
/30/
12$7
4,86
3$3
8,55
4$1
13,4
17
DEG
RO
AT
1R01
DK
0777
83N
atio
nal I
nstit
utes
of
Hea
lthA
RR
A A
dmin
Sup
port/
Neu
ropl
astic
ity o
f Urin
ary
Trac
t D
isor
ders
afte
r SC
I09
/29/
0908
/31/
11$5
,043
$2,5
97$7
,639
DEG
RO
AT
2R01
DK
0685
66N
atio
nal I
nstit
utes
of
Hea
lthB
ladd
er &
Sph
inct
er C
ontro
l Afte
rSpi
nal C
ord
Inju
ry07
/01/
0906
/30/
13$4
1,52
8$2
1,38
7$6
2,91
5
DEG
RO
AT
W81
XW
H-1
1-1-
0829
Dep
artm
ent o
f Def
ense
Kan
ai/U
se o
f Opt
ical
Map
ping
to E
valu
ate
Mec
hani
ms
and
New
The
rapi
es fo
r Bla
dder
Dys
func
tion
due
to
09/3
0/11
10/2
9/14
$7,4
41$3
,832
$11,
273
DEG
RO
AT
W81
XW
H-1
1-1-
0819
Dep
artm
ent o
f Def
ense
Tai/A
n Im
plan
tabl
e N
euro
pros
thet
ic D
evic
e to
N
orm
aliz
e B
ladd
er F
unct
ion
afte
r SC
I09
/22/
1110
/21/
14$1
2,79
6$6
,590
$19,
386
DEG
RO
AT
Eli L
illy
Eli L
illy
Eli L
illy
Cor
pora
te R
esea
rch
Agr
eem
ent
02/2
6/10
02/2
6/12
$19,
800
$11,
187
$30,
987
DEG
RO
AT
Cra
ig N
eils
enC
raig
Nei
lsen
Fo
unda
tion
Arti
m/C
yclic
nuc
leot
ide
sign
alin
g in
urin
ary
blad
der
follo
win
g sp
inal
cor
d in
jury
07/0
1/10
06/3
0/12
$60,
000
$0$6
0,00
0
DEG
RO
AT
1R01
DK
0900
06N
atio
nal I
nstit
utes
of
Hea
lthTa
i/New
Stra
tegi
es to
Tre
at O
vera
ctiv
e B
ladd
er09
/30/
1008
/31/
14$1
2,30
5$6
,337
$18,
641
DEG
RO
AT
1R01
DK
0912
53N
atio
nal I
nstit
utes
of
Hea
lthFu
nctio
ns &
Cen
tral P
roje
ctio
ns o
f the
Sym
path
etic
A
ffer
ent N
erve
s08
/01/
1107
/31/
16$1
31,9
75$6
7,96
7$1
99,9
43
DEG
RO
AT
Eli L
illy
Eli L
illy
Eli L
illy
Res
earc
h A
gree
men
t12
/10/
1012
/09/
11$4
,735
$2,6
75$7
,410
DEG
RO
AT
Abb
ott
Abb
vie
Ana
lysi
s of t
he a
ctio
ns o
f a T
RPV
1 an
tago
nist
on
refle
x bl
adde
r act
ivity
05/0
1/12
04/3
0/13
$1,5
16$8
57$2
,372
EISE
MA
NR
01 C
A12
1105
-05
Nat
iona
l Ins
titut
es o
f H
ealth
SMA
C in
Che
mop
reve
ntio
n of
Col
on C
ance
r09
/01/
0707
/31/
12$2
,618
$1,2
70$3
,888
EISE
MA
NR
01 C
A14
0624
-03
Nat
iona
l Ins
titut
es o
f H
ealth
Stru
ctur
e ba
sed
desi
gn o
f nov
el lo
w m
olec
ular
wei
ght
cMyc
Inhi
bito
rs07
/01/
0905
/31/
14$6
4,09
7$3
3,01
0$9
7,10
7
EISE
MA
N5R
01 C
A14
2580
-03
Nat
iona
l Ins
titut
es o
f H
ealth
Dev
elop
men
t of N
ovel
Sm
all M
olec
ule
Inhi
bito
rs o
f Pr
otei
n K
inas
e D
01/0
1/10
12/3
1/14
$14,
235
$7,3
31$2
1,56
6
EISE
MA
NU
01 C
A09
9168
-10
Nat
iona
l Ins
titut
es o
f H
ealth
Early
Clin
ical
Tria
ls o
f New
Ant
i-Can
cer A
gent
s with
Ph
ase
1 Em
phas
is03
/01/
1102
/28/
13$3
,374
$1,7
37$5
,111
EISE
MA
NR
01 C
A14
0624
-04
Nat
iona
l Ins
titut
es o
f H
ealth
Stru
ctur
e ba
sed
desi
gn o
f nov
el lo
w m
olec
ular
wei
ght
cMyc
Inhi
bito
rs06
/01/
1205
/31/
14$3
,236
$1,6
66$4
,902
EISE
MA
NN
01-C
M-5
2202
Nat
iona
l Ins
titut
es o
f H
ealth
Prec
linic
al P
harm
acol
ogic
al S
tudi
es o
f Ant
itum
or a
nd
Ant
i-HIV
age
nts
12/1
5/04
12/1
4/12
$94,
491
$39,
037
$133
,528
58
Last
Nam
eG
rant
Num
Age
ncy
Nam
eTi
tleB
egin
Dat
eEn
d D
ate
Ann
ual D
irect
C
osts
Ann
ual F
&A
C
osts
Ann
ual T
otal
C
osts
EISE
MA
NV
A51
2-D
2501
1V
ETS
MED
CTR
Mec
hani
sms o
f Dru
g R
esis
tanc
e in
Hum
an C
ance
rs10
/01/
1109
/30/
12$2
7,42
6$0
$27,
426
EISE
MA
NH
HSN
2612
0110
0015
CN
atio
nal I
nstit
utes
of
Hea
lthPr
eclin
ical
Pha
rmac
okin
etic
and
Pha
rmal
ogic
al S
tudi
es
with
Ant
ican
cer a
nd O
ther
The
rape
utic
Age
nts
07/0
1/11
06/3
0/16
$87,
483
$45,
053
$132
,536
EISE
MA
NST
AT3
John
son
& Jo
hnso
nC
yclic
Sta
t3 d
ecoy
/mut
ant c
yclic
stat
3 de
coy
12/0
1/11
11/3
0/12
$3,3
64$1
,901
$5,2
64
EISE
MA
NPY
1-3
2In
dian
a U
nive
rsity
Mul
tiple
Dos
e M
TD S
tudy
in T
umor
Bea
ring
Mic
e04
/01/
1209
/30/
12$2
3,13
2$1
3,07
0$3
6,20
2
FLIN
T20
11 R
esea
rch
PA B
reas
t Can
cer
Coa
litio
nD
rug
Res
ista
nce
in B
reas
t Can
cer
07/0
1/11
06/3
0/12
$50,
000
$0$5
0,00
0
FLIN
TD
rug
Res
ista
nce
Wen
dy W
ill C
ase
Can
cer F
und
Dru
g R
esis
tanc
e in
Bre
ast C
ance
r01
/01/
1212
/31/
12$1
5,00
0$0
$15,
000
FREE
MA
N5R
37H
L058
115
Nat
iona
l Ins
titut
es o
f H
ealth
Red
ox T
rans
duct
ion
of N
itric
Oxi
de S
igna
ling
04/0
1/04
05/3
1/13
$259
,372
$133
,576
$392
,948
FREE
MA
N5R
01 H
L064
937
Nat
iona
l Ins
titut
es o
f H
ealth
Red
ox D
eriv
ed P
ulm
onar
y A
nti-I
nfla
mm
ator
y M
edia
tors
06/2
1/06
03/3
1/16
$236
,021
$120
,648
$356
,669
FREE
MA
N1R
01D
K07
9162
Nat
iona
l Ins
titut
es o
f H
ealth
Uta
h/Li
pid
Med
iato
rs in
Ren
al c
ontro
l of B
lood
Pre
ssur
e du
ring
Met
abol
ic S
tress
es08
/01/
0912
/31/
11$1
6,80
8$8
,656
$25,
464
FREE
MA
N1R
C1D
K08
5852
Nat
iona
l Ins
titut
es o
f H
ealth
Gla
dwin
/Die
tary
nitr
ate
activ
atio
n of
PPA
Rga
mm
a im
prov
es in
sulin
sens
itivi
ty09
/30/
0908
/31/
11$1
6,04
6$8
,264
$24,
309
FREE
MA
N1R
01H
L105
114
Nat
iona
l Ins
titut
es o
f H
ealth
Mic
higa
n/N
itro
Fatty
Aci
ds a
nd N
rf2
in V
ascu
lar
Rem
odel
ing
07/0
1/10
06/3
0/15
$100
,000
$51,
500
$151
,500
FREE
MA
NR
imed
Fou
ndat
ion
Rim
ed F
ound
atio
nR
iMed
Fel
low
ship
-Faz
zari
05/1
9/11
04/0
3/13
$50,
000
$24,
250
$74,
250
FREE
MA
NP0
1HL1
0345
5N
atio
nal I
nstit
utes
of
Hea
lthG
ladw
in/V
ascu
lar S
ubph
enot
ypes
of L
ung
Dis
ease
06/0
1/11
04/3
0/16
$122
,632
$63,
155
$185
,788
FRIE
DM
AN
5R01
DK
0541
71N
atio
nal I
nstit
utes
of
Hea
lthPT
H R
ecep
tor:
Post
trans
latio
nal M
odifi
caito
n an
d R
ecyc
ling
in B
one
09/0
1/98
03/3
1/16
$245
,909
$125
,496
$371
,405
FRIE
DM
AN
5R01
DK
0699
98N
atio
nal I
nstit
utes
of
Hea
lthEB
P50
Reg
ulat
ion
of P
TH R
ecep
tor S
igna
ling
and
Traf
ficki
ng02
/01/
0601
/31/
15$2
17,5
00$1
12,0
13$3
29,5
13
FRIE
DM
AN
1R01
DK
0876
88N
atio
nal I
nstit
utes
of
Hea
lthSu
stai
ned
cAM
P si
gnal
s trig
gere
d by
inte
rnal
ized
PTH
re
cept
or: n
ew c
onse
quen
ces f
or c
ell s
igna
ling
05/0
1/10
03/3
1/15
$23,
004
$11,
846
$34,
850
FUR
EY2R
01G
M06
179
Nat
iona
l Ins
titut
es o
f H
ealth
Pyru
vate
Deh
ydro
gena
se E
1: S
truct
ure-
Func
tion
Stud
ies
06/0
1/09
05/3
1/13
$192
,028
$95,
290
$287
,318
GA
LBIA
TI12
SCG
8800
012
Am
eric
an H
eart
Ass
ocia
tion
PTR
F/C
avin
-1, A
Nov
el R
egul
ator
of S
tress
-Ind
uced
Pr
emat
ure
Sene
scen
ce o
f end
othe
lial c
ell s
01/0
1/12
12/3
1/15
$35,
000
$3,5
00$3
8,50
0
GA
LBIA
TI1R
01A
G03
0636
Nat
iona
l Ins
titut
es o
f H
ealth
Cav
eolin
1: a
nov
el m
odul
ator
of t
he P
P2A
/ATM
/p53
pa
htw
ay08
/01/
0907
/31/
14$1
85,4
32$9
5,49
8$2
80,9
30
HER
SHB
ERG
ERP5
0 C
A09
0440
Nat
iona
l Ins
titut
es o
f H
ealth
SPO
RE
in L
ung
Can
cer
06/0
1/01
08/3
0/11
$1,4
13$6
86$2
,099
HER
SHB
ERG
ERP5
0 C
A09
0440
Nat
iona
l Ins
titut
es o
f H
ealth
Labo
rato
ry C
orre
lativ
e St
udie
s to
Supp
ort E
arly
Pha
ce
Clin
ical
Tria
ls o
f Tar
gete
d A
gent
s in
Lung
Can
cer
06/0
1/01
08/3
0/11
$2,5
19$1
,221
$3,7
40
HER
SHB
ERG
ERP5
0 C
A09
0440
Nat
iona
l Ins
titut
es o
f H
ealth
SPO
RE
in L
ung
Can
cer
09/0
1/11
11/3
0/11
$4,2
41$2
,184
$6,4
25
HER
SHB
ERG
ERR
01 C
A13
2844
Nat
iona
l Ins
titut
es o
f H
ealth
Rol
e of
CY
P24
in N
on-S
mal
l Cel
l Lun
g C
ente
r05
/01/
1104
/30/
14$5
6,34
7$2
9,01
9$8
5,36
6
HU
AN
GJH
U P
O#2
0009
1208
7Jo
hns H
opki
ns
Uni
vers
it yB
reas
t Can
cer S
POR
E - P
roje
ct 2
-202
/01/
0909
/29/
12$1
7,06
6$8
,789
$25,
855
59
Last
Nam
eG
rant
Num
Age
ncy
Nam
eTi
tleB
egin
Dat
eEn
d D
ate
Ann
ual D
irect
C
osts
Ann
ual F
&A
C
osts
Ann
ual T
otal
C
osts
HU
AN
GB
CR
F 20
11-2
012
Bre
ast C
ance
r Res
earc
h Fo
unda
tion
BC
RF
- Hua
ng 2
010-
2011
10/0
1/10
09/3
0/11
$29,
034
$5,8
07$3
4,84
1
HU
AN
GH
uang
His
tone
Sam
uel &
Em
ma
Win
ters
Fou
ndat
ion
Cro
ssta
lk b
etw
een
His
tone
Dem
ethy
lase
and
His
tone
D
eace
tyla
se: a
Nov
el E
pige
netic
07/0
1/11
06/3
0/12
$9,0
00$0
$9,0
00
JAC
KSO
N1
P30
DK
0793
07N
atio
nal I
nstit
utes
of
Hea
lthPi
ttsbu
rgh
Cen
ter f
or K
idne
y R
esea
rch
9/1/
087/
31/1
3$1
51,5
00$2
5,75
0$1
77,2
50
JAC
KSO
N2
R01
DK
0685
75N
atio
nal I
nstit
utes
of
Hea
lthR
egul
atio
n of
Ren
al C
ortic
al A
deno
sine
Lev
els
9/1/
098/
31/1
1$4
1,00
9$2
1,12
0$6
2,12
8
JAC
KSO
N2
R01
DK
0685
75-0
8A1
Nat
iona
l Ins
titut
es o
f H
ealth
The
Ren
al 2
', 3'
-cA
MP
Ade
nosi
ne P
athw
ay6/
15/1
25/
31/1
7$1
6,43
8$8
,465
$24,
903
JAC
KSO
N5
R01
DK
0777
77N
atio
nal I
nstit
utes
of
Hea
lthA
deno
sine
Rec
epto
r Fun
ctio
n in
Bla
dder
Uro
epith
eliu
m2/
15/0
811
/30/
12$1
1,54
9$5
,948
$17,
497
JAC
KSO
N2
R01
HL0
6984
6N
atio
nal I
nstit
utes
of
Hea
lthR
ole
of R
enal
Dip
eptid
yl P
eptid
ase
IV4/
1/02
2/28
/17
$245
,563
$120
,840
$366
,403
JAC
KSO
N1R
21N
S700
03N
atio
nal I
nstit
utes
of
Hea
lthR
ole
of C
NPa
se in
TB
I7/
1/10
6/30
/12
$14,
008
$7,2
14$2
1,22
2
JAC
KSO
NR
01D
K09
1190
Nat
iona
l Ins
titut
es o
f H
ealth
Ade
nosi
ne in
Ren
al S
ympa
thet
ic N
euro
trans
mis
sion
9/1/
118/
31/1
6$2
08,3
33$1
07,2
92$3
15,6
25
JAC
KSO
N1F
32D
K09
3210
Nat
iona
l Ins
titut
es o
f H
ealth
Ver
rier/R
egul
atio
n of
Ren
al H
emod
ynam
ics b
y A
deno
sin e
9/1/
118/
31/1
4$4
0,33
2$0
$40,
332
JAC
OB
R03
MH
0902
53N
atio
nal I
nstit
utes
of
Hea
lthB
enzo
diaz
epin
es M
odul
ate
GB
AA
Rec
epto
r Sur
face
2/1/
111/
31/1
3$2
8,87
5$1
4,87
1$4
3,74
6
JIA
NG
1R01
CA
1298
21N
atio
nal I
nstit
utes
of
Hea
lthTh
e R
ole
of F
KB
P38
in tu
mor
igen
esis
ass
ocia
ted
with
Ts
c de
ficie
ncy
04/0
1/08
01/3
1/13
$165
,208
$85,
083
$250
,291
KEN
SLER
5R01
CA
0947
6N
atio
nal I
nstit
utes
of
Hea
lthR
ole
of N
rf2
Cro
ss-T
alk
in C
ance
r Che
mop
reve
ntio
n12
/21/
0103
/31/
17$2
05,1
28$1
00,9
00$3
06,0
28
KEN
SLER
2R01
CA
0394
16N
atio
nal I
nstit
utes
of
Hea
lthM
olec
ular
Mec
hani
sms o
f Che
mop
reve
ntio
n: N
RF2
Si
gnal
ing
06/1
2/10
02/2
8/14
$460
,772
$46,
133
$506
,905
KEN
SLER
W81
XW
H-1
1-1-
0731
Dep
artm
ent o
f Def
ense
Yan
g/M
odul
atio
n of
Est
roge
n D
epur
inat
ion
DN
A
addu
cts b
y su
lfora
phan
e fo
r bre
ast c
ance
r09
/15/
1110
/14/
14$8
2,57
3$4
2,52
5$1
25,0
98
KH
OO
Gile
adG
ilead
Nitr
o-fa
tty A
cids
Lim
it Pu
lmon
ary
Arte
rial
Hyp
erte
nsio
n03
/05/
1203
/04/
14$2
1,66
7$0
$21,
667
LAK
OSK
IR
13R
R02
5929
Nat
iona
l Ins
titut
es o
f H
ealth
Clin
ical
Res
earc
h Sc
hola
rs &
Edu
cato
rs T
rain
ing
03/0
1/09
02/2
8/12
$29,
700
$0$2
9,70
0
LAK
OSK
IU
54G
M08
8491
Nat
iona
l Ins
titut
es o
f H
ealth
Bur
ke/C
ompu
tatio
nal M
odel
s of I
nfec
tious
Dis
ease
07/2
4/09
06/3
0/14
$191
,973
$109
,166
$301
,139
LAK
OSK
IU
54G
M08
8491
Nat
iona
l Ins
titut
es o
f H
ealth
sub
for P
ilot P
roje
cts
07/0
1/10
06/3
0/14
$83,
257
$32,
577
$115
,834
LEE
R01
CA
0941
18N
atio
nal I
nstit
utes
of
Hea
lthIR
S-1
and
-2 si
gnal
ing
in m
amm
ary
deve
lopm
ent a
nd
canc
er02
/01/
1101
/31/
15$1
90,3
70$9
8,04
1$2
88,4
11
LEE
W81
XW
H-1
0-1-
0985
Dep
artm
ent o
f Def
ense
BC
0955
37: S
truct
ural
Rea
rran
gem
ents
in D
NA
Rep
air
Gen
es in
BC
09/2
0/10
10/1
9/12
$187
,788
$96,
691
$284
,479
LEE
PO#5
6005
9619
3B
aylo
r Col
lege
of
Med
icin
eTr
ansl
atio
nal R
esea
rch
in B
reas
t Can
cer
08/0
1/10
11/3
0/12
$33,
199
$17,
097
$50,
296
LEE
MU
SC/B
aylo
r Brid
ge
Pro g
ram
MU
SCM
USC
/Bay
lor B
ridge
Pro
gram
09/2
9/11
09/2
8/15
$9,7
45$4
,995
$14,
740
LEE
SAP#
410
0047
655
Com
m o
f PA
Estro
gen
Rec
epto
r Gen
etic
s in
Bre
ast C
ance
r03
/30/
1112
/31/
12$3
42,4
75$1
76,3
74$5
18,8
49
60
Last
Nam
eG
rant
Num
Age
ncy
Nam
eTi
tleB
egin
Dat
eEn
d D
ate
Ann
ual D
irect
C
osts
Ann
ual F
&A
C
osts
Ann
ual T
otal
C
osts
LEE
SAC
1100
21Su
san
Kom
en C
ance
r Fu
ndR
atio
nale
Com
bina
tion
of A
nti-I
GF-
IR a
nd
Che
mot
hera
py in
Bre
ast C
ance
r07
/01/
1006
/30/
12$2
00,0
00$5
0,00
0$2
50,0
00
LEE
Can
cer R
esea
rch
BC
RF
BC
RF
Car
ryov
er -
Suba
ccou
nt to
Lee
07/0
1/11
09/3
0/12
$190
,400
$38,
080
$228
,480
LEE
Gra
nt#
CA
191-
012
Bris
tol M
yers
Squ
ibb
Det
erm
inin
g th
e O
ptim
al U
se fo
r IG
F-IR
Inhi
bito
rs in
B
reas
t Can
cer
04/2
7/11
07/3
1/12
$102
,236
$57,
764
$160
,000
LEV
ITA
N5R
01 N
S032
385
Nat
iona
l Ins
titut
es o
f H
ealth
Cha
nnel
s, C
alci
um a
nd P
eptid
e Se
cret
ion
08/0
4/95
05/3
1/17
$263
,306
$128
,522
$391
,827
LEV
ITA
N5R
01 H
L080
632
Nat
iona
l Ins
titut
es o
f H
ealth
Reg
ulat
ion
of C
ardi
ac K
v C
hann
el E
xpre
ssio
n12
/20/
0503
/31/
14$2
84,5
00$1
10,0
00$3
94,5
00
LEV
ITA
N2R
01 D
K05
4824
Nat
iona
l Ins
titut
es o
f H
ealth
Nitr
ic O
xide
in B
ladd
er N
eura
l-Epi
thel
ial S
igna
ling
04/0
1/07
01/3
1/12
$2,1
78$1
,056
$3,2
34
LEV
ITA
N1R
21D
A02
5739
Nat
iona
l Ins
titut
es o
f H
ealth
Mul
tipho
ton
Mon
oam
ine
Imag
ing
of G
erot
onin
Neu
ron
Func
tion
09/0
1/08
08/3
1/11
$8,3
33$3
,817
$12,
151
LEV
ITA
N1R
01N
S061
097
Nat
iona
l Ins
titut
es o
f H
ealth
Intri
nsic
cur
rent
s mod
ulat
e sy
napt
ic in
tegr
atio
n in
do
pam
ine
neur
ons
01/0
1/09
12/3
1/13
$40,
166
$20,
686
$60,
852
LEV
ITA
N1F
32N
S078
994
Nat
iona
l Ins
titut
es o
f H
ealth
Tuck
er/D
ynam
ic C
lam
p St
udy
of C
alci
um C
hann
els i
n N
igra
l Dop
amin
e N
euro
ns04
/01/
1203
/31/
15$1
2,10
0$0
$12,
100
OES
TER
REI
CH
R21
DK
0828
25N
atio
nal I
nstit
utes
of
Hea
lthA
Fun
ctio
nal S
RC
1 SN
P in
Bon
e B
iolo
gy05
/01/
1104
/30/
12$1
91,6
39$9
8,69
4$2
90,3
33
OES
TER
REI
CH
xxx4
0696
7xxx
Uni
v of
Mic
higa
n - N
IHG
enet
ic P
redi
ctor
s of A
nti-E
stro
gen
Clin
ical
Act
ivity
in
Bre
ast C
ance
r Pat
ient
s06
/15/
1103
/31/
12$5
1,60
2$2
6,57
5$7
8,17
7
OES
TER
REI
CH
xxx4
0790
7xxx
Uni
v of
Mic
higa
n - N
IHG
enet
ic P
redi
ctor
s of A
nti-E
stro
gen
Clin
ical
Act
ivity
in
Bre
ast C
ance
r Pat
ient
s04
/01/
1203
/31/
13$3
3,49
7$1
7,25
1$5
0,74
7
OES
TER
REI
CH
SAP#
410
0047
655
Com
m o
f PA
Estro
gen
Rec
epto
r Gen
etic
s in
Bre
ast C
ance
r03
/30/
1112
/31/
12$3
30,9
47$1
70,4
38$5
01,3
85
OES
TER
REI
CH
Can
cer R
esea
rch
BC
RF
BC
RF
Car
ryov
er -
Suba
ccou
nt to
Oes
terr
eich
07/0
1/11
09/3
0/12
$168
,360
$33,
672
$202
,032
OES
TER
REI
CH
Res
earc
h In
itiat
ive
PA B
reas
t Can
cer
Coa
litio
nEs
troge
n/IG
F-I C
orep
ress
ion
of G
enes
in B
reas
t Can
cer
Pro g
ress
ion
and
Res
ista
nce
07/0
1/11
06/3
0/12
$50,
000
$0$5
0,00
0
PAG
AN
O7R
01 H
L079
207
Nat
iona
l Ins
titut
es o
f H
ealth
Rea
ctiv
e O
xyge
n Sp
ecie
s in
Vas
cula
r Dis
ease
04/0
1/07
03/3
1/13
$184
,766
$95,
154
$279
,920
PAG
AN
OR
imed
Fou
ndat
ion
Rim
ed F
ound
atio
nR
iMed
Fel
low
ship
-Fra
zzia
no03
/08/
1112
/23/
11$2
5,08
6$1
2,16
6$3
7,25
2
PAG
AN
OA
mer
ican
Hea
rtA
mer
ican
Hea
rt A
ssoc
iatio
nC
syan
i/Rol
e of
Thr
ombo
spon
ddid
-1 in
exi
dativ
e st
ress
07/0
1/10
06/3
0/12
$45,
000
$0$4
5,00
0
PAG
AN
OP0
1HL1
0345
5N
atio
nal I
nstit
utes
of
Hea
lthG
ladw
in/V
ascu
lar S
ubph
enot
ypes
of L
ung
Dis
ease
06/0
1/11
04/3
0/16
$42,
205
$21,
735
$63,
940
PALL
AD
INO
ASP
ETA
mer
Soc
for P
harm
and
Ex
perim
enta
lSu
mm
er U
nder
grad
uate
Res
earc
h Fe
llow
ship
s (SU
RF)
06/0
1/12
05/3
1/15
$9,0
00$0
$9,0
00
PALL
AD
INO
5R01
AG
0250
46N
atio
nal I
nstit
utes
of
Hea
lthU
nder
stan
ding
Mec
hani
sms o
f Neu
ropa
thog
enes
is05
/01/
0604
/30/
12$4
7,46
0$2
4,44
2$7
1,90
2
PALL
AD
INO
1R01
AG
0274
53N
atio
nal I
nstit
utes
of
Hea
lthM
itoch
ondr
ial D
ysfu
nctio
n &
Pro
gres
sive
En
ceph
alom
uopa
thie
s09
/15/
0908
/31/
12$4
6,22
0$2
3,80
3$7
0,02
3
PALL
AD
INO
1R21
NS0
7875
8N
atio
nal I
nstit
utes
of
Hea
lthM
itoch
ondr
ial R
NA
tran
spor
t as a
nov
el th
erap
y01
/15/
1212
/31/
13$7
5,00
0$3
8,62
5$1
13,6
25
RO
MER
OM
agee
Wom
ens R
esM
agee
Wom
ens
Res
earc
h Fo
unda
tion
Rol
e of
NH
ERF
1 in
Est
roge
n-In
depe
nden
t Bre
ast
Can
cer
10/0
1/11
09/3
0/12
$15,
000
$0$1
5,00
0
61
Last
Nam
eG
rant
Num
Age
ncy
Nam
eTi
tleB
egin
Dat
eEn
d D
ate
Ann
ual D
irect
C
osts
Ann
ual F
&A
C
osts
Ann
ual T
otal
C
osts
RO
MER
O2R
01D
K05
4171
Nat
iona
l Ins
titut
es o
f H
ealth
Frie
dman
/PTH
Rec
epto
r: Po
sttra
nsla
tiona
l Mod
ifica
iton
and
Rec
yclin
g in
Bon
e05
/01/
1203
/31/
16$5
,451
$2,8
07$8
,258
RO
PPO
LO99
1764
754
Ethi
con,
Inc.
Ethi
con
Cor
pora
te R
esea
rch
Agr
eem
ent
12/1
5/07
12/3
1/15
$5,0
48$7
89$5
,837
RO
PPO
LO5P
50D
K06
4539
Nat
iona
l Ins
titut
es o
f H
ealth
Wom
en's
Hea
lth &
Fun
ctio
nal V
isce
ral D
isor
ders
Cen
ter
09/0
1/07
08/3
1/12
$9,4
91$4
,873
$14,
364
RO
PPO
LOR
01D
K09
0006
Nat
iona
l Ins
titut
es o
f H
ealth
Tai/N
ew S
trate
gies
to T
reat
Ove
ract
ive
Bla
dder
09/3
0/10
08/3
1/14
$27,
047
$13,
929
$40,
975
RO
PPO
LO2R
01D
K05
8284
Nat
iona
l Ins
titut
es o
f H
ealth
Bird
er/R
ole
of N
itric
Oxi
de in
Inte
rstit
ial C
ystit
is09
/01/
1007
/31/
15$5
,268
$2,7
13$7
,981
RO
PPO
LOW
81X
WH
-11-
1-08
19D
epar
tmen
t of D
efen
seTa
i/An
Impl
anta
ble
Neu
ropr
osth
etic
Dev
ice
to
Nor
mal
ize
Bla
dder
Fun
ctio
n af
ter S
CI
09/2
2/11
09/2
1/14
$15,
794
$8,1
34$2
3,92
7
RO
PPO
LOM
edtro
nic
Med
troni
cTa
i/The
rape
utic
Act
ion
of S
NS
for S
upre
ssio
n of
Ref
lex
Bla
dder
Con
tract
ions
10/0
1/10
09/3
0/11
$7,7
60$4
,384
$12,
144
SCH
OPF
ER1R
01A
T006
822
Nat
iona
l Ins
titut
es o
f H
ealth
Form
atio
n of
Om
ega
3-D
eriv
ed E
lect
roph
iles D
urin
g In
flam
mat
ion
09/0
1/11
07/3
1/16
$227
,273
$117
,045
$344
,318
SHIV
A5R
01G
M08
2830
Nat
iona
l Ins
titut
es o
f H
ealth
Hem
e O
xyge
nase
Enz
ymes
/Car
bon
Mon
oxid
e in
Hep
ati c
Dys
func
tion
from
Hem
mor
hage
07/0
1/10
07/3
1/15
$15,
762
$8,1
17$2
3,87
9
SHIV
AA
HA
Am
eric
an H
eart
Ass
ocia
tion
Myo
glob
in is
a N
itrite
Red
ucta
se th
at R
egul
ates
M
itoch
ondr
ial F
unct
ion
Dur
ing
Isch
emia
/Rep
erfu
sion
07/0
1/09
06/3
0/13
$70,
000
$7,0
00$7
7,00
0
SHIV
A5R
01H
L103
927
Nat
iona
l Ins
titut
es o
f H
ealth
Uni
vers
ity o
f Min
neso
ta/P
hase
II C
linic
al T
rial t
o Ev
alua
te th
e B
enef
its o
f a P
ostc
ondi
tioni
ng in
STE
MI
02/0
1/11
11/3
0/13
$28,
713
$14,
787
$43,
500
SHIV
AD
OD
Dep
artm
ent o
f Def
ense
Smal
l Mol
ecul
e, G
as B
ased
The
rapi
es to
Pre
vent
Org
an
Inju
ry f
rom
Tra
uma/
Hem
orrh
age
12/2
8/10
01/2
7/14
$47,
947
$24,
453
$72,
400
SHIV
A5P
01H
L103
455
Nat
iona
l Ins
titut
es o
f H
ealth
Vas
cula
r Sub
phen
otyp
es o
f Lun
g D
isea
se-P
roje
ct 2
06/0
1/11
04/3
0/16
$7,9
63$4
,101
$12,
064
SHIV
APh
arm
aceu
tical
sPh
arm
aceu
tical
sA
Tra
nsla
tiona
l Ass
essm
ent o
f Nitr
ite a
nd G
SNO
R
e gul
atio
n th
roug
h G
SNO
R In
hibi
tion
02/0
1/11
01/3
1/12
$61,
055
$15,
264
$76,
319
SHIV
AA
ires P
harm
aceu
tical
sA
ires P
harm
aceu
tical
s, In
c.M
easu
rem
ent o
f Nitr
ite P
harm
acok
inet
ics
06/0
1/11
05/3
1/12
$133
,908
$14,
879
$148
,787
SIEG
FRIE
DP5
0 C
A09
0440
Nat
iona
l Ins
titut
es o
f H
ealth
SPO
RE
in L
ung
Can
cer-
Cor
e A
06/0
1/06
08/3
0/11
$29,
376
$12,
699
$42,
075
SIEG
FRIE
DP5
0 C
A09
0440
Nat
iona
l Ins
titut
es o
f H
ealth
SPO
RE
in L
ung
Can
cer-
Proj
106
/01/
0608
/30/
11$2
3,94
7$4
,028
$27,
975
SIEG
FRIE
DP5
0 C
A09
0440
Nat
iona
l Ins
titut
es o
f H
ealth
SPO
RE
in L
ung
Can
cer A
RR
A S
uppl
emen
t08
/01/
0908
/30/
11$4
4,36
5$0
$44,
365
SIEG
FRIE
DP5
0 C
A09
0440
Nat
iona
l Ins
titut
es o
f H
ealth
SPO
RE
in L
ung
Can
cer
09/0
1/11
08/3
0/16
$80,
760
$41,
592
$122
,352
SIEG
FRIE
DP5
0 C
A09
0440
Nat
iona
l Ins
titut
es o
f H
ealth
SPO
RE
in L
ung
Can
cer
09/0
1/11
08/3
0/16
$103
,132
$53,
113
$156
,244
SIEG
FRIE
DP5
0 C
A09
0440
Nat
iona
l Ins
titut
es o
f H
ealth
SPO
RE
in L
ung
Can
cer
09/0
1/11
08/3
0/16
$5,1
38$2
,646
$7,7
84
SIEG
FRIE
D1R
01 H
L107
883
01N
atio
nal I
nstit
utes
of
Hea
lthTh
e Em
phys
emat
ous M
icro
envi
rom
ent P
rom
otes
Lun
g Tu
mor
i gen
esis
and
Pro
gres
sion
07/0
1/11
04/3
0/15
$30,
742
$15,
832
$46,
574
SIEG
FRIE
D5R
01 H
L107
883
02N
atio
nal I
nstit
utes
of
Hea
lthTh
e Em
phys
emat
ous M
icro
envi
rom
ent P
rom
otes
Lun
g Tu
mor
igen
esis
and
Pro
gres
sion
07/0
1/11
04/3
0/15
$4,8
64$2
,505
$7,3
70
SIEG
FRIE
DR
01 C
A09
8372
Nat
iona
l Ins
titut
es o
f H
ealth
GPC
R S
igna
ling
in S
CC
HN
: Int
ergr
atio
n w
ith E
GFR
12/0
1/02
01/3
1/12
$1,1
45$5
86$1
,731
62
Last
Nam
eG
rant
Num
Age
ncy
Nam
eTi
tleB
egin
Dat
eEn
d D
ate
Ann
ual D
irect
C
osts
Ann
ual F
&A
C
osts
Ann
ual T
otal
C
osts
SIEG
FRIE
DR
01 C
A09
8372
Nat
iona
l Ins
titut
es o
f H
ealth
GPC
R S
igna
ling
in S
CC
HN
: Int
ergr
atio
n w
ith E
GFR
02/0
1/12
01/3
1/14
$12,
331
$6,3
50$1
8,68
1
SIEG
FRIE
DR
01 C
A07
9882
Nat
iona
l Ins
titut
es o
f H
ealth
Targ
etin
g th
e H
GF
C M
et P
athw
ay in
Lun
g C
ance
r02
/01/
0901
/31/
12$1
31,4
00$6
7,67
1$1
99,0
71
SIEG
FRIE
DR
01 C
A07
9882
Nat
iona
l Ins
titut
es o
f H
ealth
Targ
etin
g th
e H
GF
C M
et P
athw
ay in
Lun
g C
ance
r02
/01/
1201
/31/
14$9
3,85
7$4
8,33
6$1
42,1
94
SIEG
FRIE
DP5
0 C
A09
7190
Nat
iona
l Ins
titut
es o
f H
ealth
SPO
RE
in H
ead
and
Nec
k C
ance
r_N
ew07
/01/
1006
/30/
15$9
9,50
1$5
1,24
3$1
50,7
44
SIEG
FRIE
D3P
50 C
A09
7190
07S
1N
atio
nal I
nstit
utes
of
Hea
lthSp
ecia
lized
Pro
gram
of R
esea
rch
Exce
llenc
e07
/01/
0206
/30/
15$9
3,70
6$4
8,25
9$1
41,9
65
SIEG
FRIE
DFY
10.0
46.0
09N
atio
nal I
nstit
utes
of
Hea
lthLu
ng C
ance
r Mut
atio
n C
onso
rtium
Tria
l (U
CC
C)
09/2
9/09
08/3
1/12
$7,5
00$3
,863
$11,
363
SIEG
FRIE
DR
01 C
A09
7356
06A
1N
atio
nal I
nstit
utes
of
Hea
lthFa
ctor
s for
Epi
gene
tic S
ilenc
ing
of L
ung
Can
cer G
enes
07/1
2/10
05/3
1/15
$14,
449
$7,4
42$2
1,89
1
SIEG
FRIE
DFY
12.5
96.0
01U
nive
rsity
of C
olor
ado
Can
cer C
ente
rLU
NG
evity
Lun
g C
ance
r Ear
ly D
etec
tion
Proj
ect
07/0
1/11
06/3
0/13
$10,
870
$1,6
30$1
2,50
0
SIEG
FRIE
D1R
01 C
A16
4782
01
Nat
iona
l Ins
titut
es o
f H
ealth
Epig
enet
ic C
hang
es L
ink
CO
PD a
nd L
ung
Can
cer
08/0
4/11
05/3
1/12
$16,
871
$8,6
89$2
5,56
0
SIEG
FRIE
D5R
01 C
A09
7356
08
Nat
iona
l Ins
titut
es o
f H
ealth
Fact
ors f
or E
pige
netic
Sile
ncin
g of
Lun
g C
ance
r Gen
es07
/12/
1005
/31/
15$5
,906
$3,0
42$8
,948
SIEG
FRIE
D1R
01 C
A16
4782
02
Nat
iona
l Ins
titut
es o
f H
ealth
Epig
enet
ic C
hang
es L
ink
CO
PD a
nd L
ung
Can
cer
08/0
4/13
05/3
1/12
$16,
889
$8,6
97$2
5,58
6
SIEG
FRIE
DR
esea
rch
Agr
eem
ent
Som
alog
ic In
cSl
apta
mer
-Bas
ed A
rray
Tec
hnol
ogy
for D
isco
very
of
Lun g
Can
cer S
erum
Bio
mar
kers
07/0
1/09
12/3
1/11
$36,
127
$20,
412
$56,
538
SIEG
FRIE
DLu
ng C
ance
r Tria
lsV
Fou
ndat
ion
Tran
slat
iona
l Lun
g C
ance
r Clin
ical
Tria
ls09
/01/
0908
/31/
13$2
25,0
04$2
2,50
0$2
47,5
05
SIN
GH
R01
CA
1293
47N
atio
nal I
nstit
utes
of
Hea
lthB
reas
t Can
cer P
reve
ntio
n by
Die
tary
Phy
toch
emic
als
09/0
7/07
07/3
1/12
$164
,554
$79,
809
$244
,362
SIN
GH
R01
CA
1017
53N
atio
nal I
nstit
utes
of
Hea
lthA
ntic
arci
noge
nic
Effe
ct o
f ITC
S A
gagi
nst p
rost
ate
Can
cer
11/1
4/03
11/3
0/13
$206
,779
$106
,112
$312
,891
SIN
GH
R01
CA
1291
27N
atio
nal I
nstit
utes
of
Hea
lthTh
e R
ole
of P
KD
305
/01/
0902
/28/
12$5
,930
$3,0
54$8
,985
SIN
GH
5R01
CA
1291
27 0
4N
atio
nal I
nstit
utes
of
Hea
lthTh
e R
ole
of P
KD
303
/01/
1202
/28/
14$5
,872
$3,0
24$8
,896
SIN
GH
R01
CA
1426
04N
atio
nal I
nstit
utes
of
Hea
lthB
reas
t Can
cer P
reve
ntio
n by
Ayu
rved
ic M
edic
ine
Con
stitu
ents
12/0
4/09
11/3
0/14
$203
,379
$104
,696
$308
,076
SIN
GH
2R01
CA
1133
63N
atio
nal I
nstit
utes
of
Hea
lthPr
osta
te C
ance
r Pre
vent
ion
by D
ially
l Tris
ulfid
e04
/01/
0501
/31/
12$2
27,7
16$7
7,39
8$3
05,1
14
SIN
GH
5R01
CA
1133
63 0
8N
atio
nal I
nstit
utes
of
Hea
lthPr
osta
te C
ance
r Pre
vent
ion
by D
ially
l Tris
ulfid
e02
/01/
1201
/31/
15$2
27,7
16$7
7,39
8$3
05,1
14
SIN
GH
R01
CA
1154
98N
atio
nal I
nstit
utes
of
Hea
lthPr
even
tion
of P
rost
ate
Can
cer b
y Su
lfora
phan
e07
/01/
0503
/31/
12$1
40,4
50$7
2,33
2$2
12,7
81
SIN
GH
5R01
CA
1154
98 0
7N
atio
nal I
nstit
utes
of
Hea
lthPr
even
tion
of P
rost
ate
Can
cer b
y Su
lfora
phan
e04
/01/
1203
/31/
16$1
87,2
66$9
6,44
2$2
83,7
08
SIN
GH
1R21
AT0
0523
1 01
A1
Nat
iona
l Ins
titut
es o
f H
ealth
Dire
ct T
arge
ting
of C
ance
r Inv
asio
n an
d M
etas
tasi
s U
sin g
With
ania
Roo
t Ext
ract
s05
/01/
1004
/30/
12$6
,523
$3,3
59$9
,882
SOB
OL
R01
CA
1486
29-0
2N
atio
nal I
nstit
utes
of
Hea
lthN
ovel
App
roac
hes t
o En
hanc
e Tu
mor
Cel
l cyt
otox
icity
of
Alk
ylat
ing
Age
nts
07/0
2/10
05/3
1/15
$177
,466
$90,
215
$267
,680
63
Last
Nam
eG
rant
Num
Age
ncy
Nam
eTi
tleB
egin
Dat
eEn
d D
ate
Ann
ual D
irect
C
osts
Ann
ual F
&A
C
osts
Ann
ual T
otal
C
osts
SOB
OL
U01
DK
0895
38-0
2N
atio
nal I
nstit
utes
of
Hea
lthM
ulti-
Dis
cipl
inar
y A
ppro
ache
s to
Driv
ing
Ther
apeu
tic
Hum
an B
eta
Cel
l Rep
licat
ion
09/1
5/10
06/3
0/15
$28,
370
$14,
611
$42,
981
SOB
OL
2P30
CA
0479
04 2
3N
atio
nal I
nstit
utes
of
Hea
lthC
ance
r Cen
ter S
uppo
rt G
rant
09/1
0/10
07/3
1/15
$31,
286
$16,
112
$47,
398
SOB
OL
2R01
NS0
3770
4 12
N
atio
nal I
nstit
utes
of
Hea
lthM
olec
ular
Mar
kers
as P
redi
ctor
s of O
utco
me
in G
liom
as02
/15/
1101
/31/
16$1
4,23
7$7
,332
$21,
568
SOB
OL
R01
CA
1486
29-0
3N
atio
nal I
nstit
utes
of
Hea
lthN
ovel
App
roac
hes t
o En
hanc
e Tu
mor
Cel
l Cyt
otox
icity
of
Alk
latin
g A
gent
s06
/01/
1205
/31/
15$1
6,11
2$8
,190
$24,
302
SOB
OL
R01
NS0
3770
4-13
Nat
iona
l Ins
titut
es o
f H
ealth
Mol
ecul
ar M
arke
rs a
s Pre
dict
ors o
f Out
com
es in
G
liom
as02
/01/
1201
/31/
16$6
,741
$3,4
72$1
0,21
3
SOB
OL
SUB
AW
AR
D N
-201
0-59
Uni
v of
Tol
edo/
NIH
Nov
el ro
le o
f Bas
e Ex
cisi
on R
epai
r and
Mis
mat
ch
Re p
air i
n C
ipla
tin S
ensi
tivity
03/1
5/10
02/2
8/12
$3,3
33$1
,717
$5,0
50
SOB
OL
2R44
GM
0877
98 0
2N
atio
nal I
nstit
utes
of
Hea
lthD
NA
Rep
air D
efic
ient
Hum
an C
ells
for G
enom
ic
Var
iatio
n A
naly
sis
09/1
3/10
08/3
1/13
$463
,203
$212
,801
$676
,003
SOB
OL
2R44
GM
0877
98 0
2N
atio
nal I
nstit
utes
of
Hea
lthD
NA
Rep
air D
efic
ient
Hum
an C
ells
for G
enom
ic
Var
iatio
n A
nal y
sis
09/1
3/10
08/3
1/13
$52,
815
$27,
200
$80,
015
SOB
OL
Suba
war
d 57
1000
2942
MIT
/NIH
Com
etch
ip E
nabl
ing
Tran
slat
ion
of D
NA
Dam
age
04/0
1/11
03/3
1/12
$46,
664
$24,
032
$70,
697
SOB
OL
GM
0992
13Tr
evig
en, I
ncD
isco
very
Too
ls fo
r Che
mot
hera
py R
esis
tanc
e to
Cel
l D
eat h
02/0
1/12
01/3
1/13
$33,
578
$17,
293
$50,
871
SOB
OL
GM
0992
13Tr
evig
en, I
ncD
isco
very
Too
ls fo
r Che
mot
hera
py R
esis
tanc
e to
Cel
l D
eath
02/0
1/12
01/3
1/13
$12,
399
$6,3
85$1
8,78
4
SOB
OL
Suba
war
d 57
1000
2942
MIT
Com
etch
ip E
nabl
ing
Tran
slat
ion
of D
NA
Dam
age
04/0
1/12
03/3
1/13
$17,
162
$8,8
38$2
6,00
0
SOB
OL
M20
10-0
028
Pitts
burg
h Fo
unda
tion
Tum
or S
elec
tive
Che
mot
hera
py fo
r Glio
blas
tom
a:
Expl
oitin
g Tu
mor
-Spe
cific
Def
ects
12/0
1/10
11/3
0/11
$41,
667
$0$4
1,66
7
SOB
OL
PED
MA
ILG
GLI
OM
AS
CB
TFG
enom
ic A
ltera
tions
in P
edia
tric
Mal
igna
nt G
liom
as12
/01/
1011
/30/
12$3
,625
$362
$3,9
87
SOB
OL
ES02
1116
Trev
igen
, Inc
DN
A R
epai
r on
a C
hip
Spat
ially
Enc
oded
Mic
row
ell
Ass
a ys
09/2
0/11
08/3
1/12
$62,
663
$32,
271
$94,
933
SON
G10
BG
IA35
0000
5A
mer
ican
Hea
rt A
ssoc
iatio
nR
oel o
f EB
P50
in V
ascu
lar S
moo
th M
uscl
e C
ell
Prol
ifera
tion
07/0
1/10
06/3
0/12
$60,
000
$6,0
00$6
6,00
0
VA
N H
OU
TEN
R01
ES0
1956
6N
atio
nal I
nstit
utes
of
Hea
lthD
NA
Dam
age
Rec
ogni
tion
by N
ucle
otid
e Ex
cisi
on
Re p
air P
rote
ins
12/0
9/10
11/3
0/15
$192
,391
$91,
098
$283
,489
VA
N H
OU
TEN
P50
CA
1219
73-0
4N
atio
nal I
nstit
utes
of
Hea
lthSP
OR
E in
Ski
n C
ance
r_D
RP
08/0
1/11
07/3
1/13
$14,
333
$7,3
82$2
1,71
5
VA
N H
OU
TEN
12.8
291_
UPM
CC
hild
ren'
s Hos
pita
l R
esea
rch
Foun
datio
nm
tDN
A H
eter
opla
smy:
A S
ensi
tive
Func
tiona
l B
iom
arke
r of O
xida
tive
Stre
ss09
/01/
0908
/31/
11$5
,622
$2,8
95$8
,517
VA
N H
OU
TEN
Early
Aw
ard-
Toba
cco
Yr1
0PA
CO
MM
OF
Toba
cco
Yea
r 10
Form
ula
Fund
s01
/01/
1112
/31/
13$2
43,9
52$1
25,6
36$3
69,5
88
VA
N H
OU
TEN
2012
HH
MI
How
ard
Hug
hes
Med
ical
Inst
itute
2012
HH
MI R
esea
rch
Trai
ning
Fel
low
ship
Pro
gram
06/0
1/12
05/3
1/13
$3,2
50$0
$3,2
50
VIL
AR
DA
GA
1R01
DK
0876
88N
atio
nal I
nstit
utes
of
Hea
lthSu
stai
ned
cAM
P si
gnal
s trig
gere
d by
inte
rnal
ized
PTH
re
cept
or: n
ew c
onse
quen
ces f
or c
ell s
igna
ling
05/0
1/10
03/3
1/15
$187
,958
$96,
798
$284
,756
VIL
AR
DA
GA
2R01
DK
0541
71N
atio
nal I
nstit
utes
of
Hea
lthFr
iedm
an/P
TH R
ecep
tor:
Post
trans
latio
nal M
odifi
caito
n an
d R
ecyc
ling
in B
one
05/0
1/12
03/3
1/16
$3,7
07$1
,909
$5,6
16
WA
NG
R01
CA
1291
27N
atio
nal I
nstit
utes
of
Hea
lthR
ole
of P
KD
3 in
pro
stat
e ca
rcin
ogen
esis
05/0
1/09
02/2
8/14
$191
,017
$98,
372
$289
,390
64
Last
Nam
eG
rant
Num
Age
ncy
Nam
eTi
tleB
egin
Dat
eEn
d D
ate
Ann
ual D
irect
C
osts
Ann
ual F
&A
C
osts
Ann
ual T
otal
C
osts
WA
NG
1R01
CA
1458
0N
atio
nal I
nstit
utes
of
Hea
lthD
evel
opm
ent o
f sm
all m
olec
ule
inhi
bito
rs o
f pro
tein
ki
nase
D01
/01/
1012
/31/
14$1
62,1
44$8
7,57
2$2
49,7
16
ZHA
NG
R01
CA
1211
05N
atio
nal I
nstit
utes
of
Hea
lthSM
AC
in C
hem
opre
vent
ion
of C
olon
Can
cer
09/0
1/07
07/3
1/13
$181
,443
$77,
942
$259
,385
ZHA
NG
R01
CA
1211
05N
atio
nal I
nstit
utes
of
Hea
lthSM
AC
in C
hem
opre
vent
ion
of C
olon
Can
cer (
AR
RA
)10
/01/
0909
/30/
11$1
6,31
3$8
,401
$24,
714
ZHA
NG
U01
DK
0855
70N
atio
nal I
nstit
utes
of
Hea
lthIn
test
inal
Ste
m C
ell S
urvi
val a
nd R
enew
al C
oord
inat
ely
Re g
ulat
ed b
y PU
MA
and
p21
09/0
1/09
08/3
1/14
$7,5
74$3
,901
$11,
475
ZHA
NG
2R01
CA
1063
48 0
6A2
Nat
iona
l Ins
titut
es o
f H
ealth
PUM
A-M
edia
ted
Apo
ptos
is in
Hum
an C
ance
r Cel
ls05
/01/
1004
/30/
15$1
50,2
49$7
5,24
2$2
25,4
91
ZHA
NG
P50C
A09
0440
-11
Nat
iona
l Ins
titut
es o
f H
ealth
SPO
RE
in L
ung
Can
cer_
Proj
ect 2
_309
/01/
1108
/31/
16$1
,260
$649
$1,9
09
65
Depa
rtmen
tal�Spo
nsored
�Project�Fun
ding�FY0
3�FY12
$10,026,000
$11,413,000
$14,572,000
$17,378,000
$19,131,112
$20,517,922
$20,297,879
$20,369,869
$19,699,291
$19,952,114
$0
$3,0
00,0
00
$6,0
00,0
00
$9,0
00,0
00
$12,
000,
000
$15,
000,
000
$18,
000,
000
$21,
000,
000
2002
�03
2003
�04
2004
�05
2005
�06
2006
�07
2007
�08
2008
�09
2009
�10
2010
�11
2011
�12
66
Percent of Faculty Support on Research Grants – FY12
Altschuler, Daniel 25% Lakoski, Joan 10%
Arjunan, Palaniappa 100% Lee, Adrian 100%
Bisello, Alessandro 5% Levitan, Edwin 65%
Bonacci, Gustavo 100% Ng, Yuen-Keng 50%
Cifuentes-Pagano, Maria 35% Oesterreich, Steffi 100%
Cole, Marsha 100% Pagano, Patrick 62%
Defranco, Donald 25% Palladino, Alicia 100%
Degroat, William 48% Palladino, Michael 50%
Eiseman, Julie 75% Romero, Guillermo 5%
Flint, Melanie 75% Roppolo, James 100%
Freeman, Bruce 80% Schopfer, Francisco 90%
Friedman, Peter 65% Shakiryanova, Dinara 100%
Furey, William 25% Shiva, Sruti 100%
Galbiati, Daniela 95% Siegfried, Jill 87%
Galbiati, Ferruccio 25% Singh, Shivendra 82%
Gelhaus, Stacey 100% Sobol Jr., Robert 85%
Hahm, Eun-Ryeong 100% Song, Gyun Jee 42%
Hershberger, Pamela 85% Stabile, Laura 90%
Hu, Jing 20% Van Houten, Bennett 65%
Huang, Hi 80% Vilardaga, Jean-Pierre 55%
Jackson, Edwin 95% Wakabayashi, Nobunao 50%
Jacob, Tija 5% Wang, Bin 100%
Jiang, Yu 25% Wang, Peng 100%
Kensler, Thomas 50% Wang, Qiming 80%
Khoo, Nicholas 100% Zhang, Lin 85%
Pharmacology Average 68%
67
Last
Nam
eG
rant
Num
Age
ncy
Nam
eTi
tleB
egin
Dat
eEn
d D
ate
Ann
ual D
C
Ann
ual I
DC
A
nnua
l TC
CO
NR
AD
S5
P50
CA
0904
40 1
0N
atio
nal I
nstit
utes
of H
ealth
SPO
RE
in L
ung
Can
cer
09/1
3/06
04/3
0/11
$
6
24
$
32
1 $
945
CO
NR
AD
S5
U01
CA
0849
68 1
0S1
Nat
iona
l Ins
titut
es o
f Hea
lthSe
rum
Pro
teom
ic B
iom
arke
rs fo
r Col
on a
nd P
ancr
eatic
C
ance
r09
/22/
0406
/30/
10 $
937
$
482
$
1,4
19
DEF
RA
NC
O5T
32 G
M00
8424
Nat
iona
l Ins
titut
es o
f Hea
lthPr
edoc
tora
l Tra
inin
g in
Pha
rmac
olog
ical
Sci
ence
s09
/01/
9406
/30/
15 $
156,
820
$
7,51
2 $
164,
332
EISE
MA
N5
P01
CA
0780
39 1
0N
atio
nal I
nstit
utes
of H
ealth
Com
bina
toria
l App
roac
hes
for N
ovel
Ant
ican
cer A
gent
s07
/01/
0506
/30/
11 $
27,
492
$
9,96
5 $
37,
457
EISE
MA
N5
R01
CA
1211
05 0
3N
atio
nal I
nstit
utes
of H
ealth
SMA
C in
Che
mop
reve
ntio
n of
Col
on C
ance
r09
/01/
0707
/31/
12 $
146,
092
$
2
9,21
9 $
175,
311
FREE
MA
N3P
30D
K07
2506
Nat
iona
l Ins
titut
es o
f Hea
lth
Friz
zell/
Bas
ic &
Clin
ical
Stu
dies
of C
ystic
Fib
rosi
s 08
/18/
0907
/31/
10 $
65,
625
$
3
3,79
7 $
99,
422
FUR
EY5P
01 C
A07
8039
Nat
iona
l Ins
titut
es o
f Hea
lthC
ombi
nato
rial A
ppro
ache
s fo
r Nov
el A
ntic
ance
r Age
nts
07/0
1/06
06/3
0/10
$
7,6
79
$
3,72
4 $
11,
403
HER
SHB
ERG
ER3
P30
CA
0479
04 2
1S2
Nat
iona
l Ins
titut
es o
f Hea
lthC
ance
r Cen
ter S
uppo
rt09
/22/
0407
/31/
10 $
215,
519
$
9
2,81
5 $
308,
335
HER
SHB
ERG
ER5
P50
CA
0904
40 1
0N
atio
nal I
nstit
utes
of H
ealth
SPO
RE
in L
ung
Can
cer
09/1
3/06
04/3
0/11
$
10
4,32
3 $
37,
001
$
14
1,32
4
HER
SHB
ERG
ER5
U01
CA
0991
68 0
8N
atio
nal I
nstit
utes
of H
ealth
Early
Clin
ical
Tria
ls o
f New
Ant
i-Can
cer A
gent
s w
ith P
hase
I E
mph
asis
05/1
2/08
02/2
8/13
$
3
1,97
5 $
15,
508
$
4
7,48
3
JAC
KSO
N1
P30
DK
0793
07N
atio
nal I
nstit
utes
of H
ealth
Pitts
burg
h C
ente
r for
Kid
ney
Res
earc
h9/
1/08
7/31
/13
$
23
7,70
6 $
25,
750
$
26
3,45
6
LAZO
2P01
CA
0780
39N
atio
nal I
nstit
utes
of H
ealth
Com
bina
toria
l App
roch
es fo
r Nov
el A
ntic
ance
r Age
nts-
Proj
305
/15/
9806
/30/
10 $
159,
572
$
7
7,39
3 $
236,
965
LAZO
5U19
CA
0529
Nat
iona
l Ins
titut
es o
f Hea
lthC
ance
r str
ess
rele
vant
pro
tein
pho
spho
tase
targ
ets
05/0
1/08
04/3
0/10
$
8
4,18
7 $
43,
356
$
12
7,54
3
LAZO
P01C
A07
8039
Nat
iona
l Ins
titut
es o
f Hea
lthC
ombi
nato
rial A
ppro
ches
for N
ovel
Ant
ican
cer A
gent
s-C
ore
A05
/15/
9806
/30/
10 $
46,
160
$
2
2,38
8 $
68,
548
LAZO
2P01
NS0
4092
Nat
iona
l Ins
titut
es o
f Hea
lthN
ovel
Str
ateg
ies
for B
rain
Tum
or T
hera
py-P
roje
ct 1
Mas
ter
03/0
1/09
02/2
8/13
$
7,7
99
$
4,01
6 $
11,
815
LAZO
5U19
AI0
6802
1N
atio
nal I
nstit
utes
of H
ealth
Expl
oitin
g Sm
all I
nter
ferin
g R
NA
SIR
NA
Lib
rarie
s to
Id
entif
y M
olec
ular
Pro
tein
(Cry
stal
Zel
lefr
ow)
09/0
1/09
08/3
1/10
$
5
2,50
0 $
25,
463
$
7
7,96
3
LAZO
5P01
NS4
0923
Nat
iona
l Ins
titut
es o
f Hea
lthN
ovel
Str
ateg
ies
for B
rain
Tum
or T
hera
py03
/01/
0902
/28/
13 $
3
,964
$
2,
041
$
6,0
05
PAG
AN
OR
imed
Fou
ndat
ion
Rim
ed F
ound
atio
nR
iMed
Fel
low
ship
-Fra
zzia
no06
/01/
1003
/07/
11 $
5
,556
$
2,
694
$
8,2
50
RO
PPO
LO5P
50D
K06
4539
Nat
iona
l Ins
titut
es o
f Hea
lthW
omen
's H
ealth
& F
unct
iona
l Vis
cera
l Dis
orde
rs C
ente
r09
/01/
0708
/31/
12 $
10,
780
$
5,55
2 $
16,
332
SHA
RLO
W2P
50A
G00
5133
Nat
iona
l Ins
titut
es o
f Hea
lthM
olec
ule
Inhi
bito
rs a
s C
hem
ical
Pro
bes
and
Pote
ntia
l Th
erap
y05
/01/
1003
/31/
11 $
3
,729
$
2,
881
$
6,6
10
SIEG
FRIE
D
5 P5
0 C
A09
0440
10
Nat
iona
l Ins
titut
es o
f Hea
lthSP
OR
E in
Lun
g C
ance
r09
/13/
0604
/30/
11 $
399,
962
$
134
,598
$
534,
560
SIEG
FRIE
D
3 P5
0 C
A09
7190
05S
5N
atio
nal I
nstit
utes
of H
ealth
SPO
RE
in H
ead
and
Nec
k C
ance
r07
/01/
0406
/30/
10 $
10,
205
$
5,25
6 $
15,
461
SOB
OL
5 P2
0 C
A13
2385
03
Nat
iona
l Ins
titut
es o
f Hea
lthEn
viro
nmen
tal O
ncol
ogy
Part
ners
hip
Bet
wee
n H
ampt
on
Uni
vers
ity a
nd U
PCI (
2 of
2)
09/0
1/09
08/3
1/10
$
27
4,07
6 $
1
39,8
45
$
41
3,92
1
VAN
HO
UTE
N
3 P3
0 C
A04
7904
21S
2N
atio
nal C
ance
r Ins
titut
eC
ance
r Cen
ter S
uppo
rt09
/22/
0407
/31/
10 $
225
$
109
$
3
35
YALO
WIC
H5U
19 A
I068
021
Nat
iona
l Ins
titut
es o
f Hea
lthM
itoch
ondr
ial T
arge
ting
Aga
inst
Rad
iatio
n D
amag
e09
/30/
0508
/31/
10 $
9
,112
$
4,
419
$
1
3,53
1
ZHA
NG
1 U
01 D
K08
5570
01
Nat
iona
l Ins
titut
es o
f Hea
lthIn
test
inal
Ste
m C
ell S
urvi
val a
nd R
enew
al C
oord
inat
ely
Reg
ulat
ed b
y PU
MA
and
p21
09/3
0/09
08/3
1/14
$
3,0
91
$
1,59
2 $
4
,683
2,
065,
709
$72
7,69
7$
2,79
3,40
7$
68
69
Participants in research
Graduate Students Title LabCourtney Andersen Other Trainee OesterreichWai Chiu Pre Doctoral Fellow PalladinoAndrey Finegersh Grad Student Researcher HomanicsHarshad Ghodke Grad Student Researcher Van HoutenEva Goellner Pre Doctoral Fellow Sobol Jr.Melanie Grubisha Pre Doctoral Fellow DefrancoRekha Gyanchandani Grad Student Researcher GrandisCassandra Henry Pre Doctoral Fellow SiegfriedJeffrey Koenitzer Grad Student Researcher FreemanKristen Leslie Pre Doctoral Fellow BiselloAnne Lipton Grad Student Researcher WangJamie Moroco Other Trainee SmithgallErica Nakajima Other Trainee Van HoutenCallie Norris Other Trainee AizemanDushani Palliyaguru Graduate Student Assistant KenslerShoghag Panjarian Grad Student Researcher KimKelly Quesnelle Other Trainee GrandisDaniel Ranayhossaini Pre Doctoral Fellow PaganoBartholomew Roland Graduate Student Researcher PalladinoRanmal Samarasinghe Other Trainee DefrancoJohn Skoko, Iii Basic Science Fellows KenslerStephen Slocum Basic Science Fellows KenslerMitchell Springer Grad Student Researcher HornHilary Stevenson Grad Student Researcher CaleroDavid Svilar Grad Student Researcher Sobol Jr.Mohammad Towheed Grad Student Researcher PalladinoMelanie Warnes Other Trainee DefrancoMan Wong Postdoctoral Associate LevitanMark Zimmerman Pre Doctoral Fellow Homanics
Undergraduate Students Title LabEric Amoroso Student Worker JacobKristin Arena Work Study Student PalladinoAlison Bank Work Study Student PalladinoMatthew Baumgartner Work Study Student PalladinoAllison Bennett Work Study Student FreemanSarah Boarts Work Study Student PalladinoUsma Chatha Work Study Student PalladinoZachary Clapp Work Study Student DegroatVanessa Cole Work Study Student PalladinoDaniel Contract Work Study Student DegroatEmily Cunningham Work Study Student PalladinoCharmaine Dogans Student Worker PalladinoAlexandra Doyle Student Worker PalladinoKatherine Durgin Work Study Student PalladinoBroderick Engelhard Work Study Student Palladino
70
Undergraduate Students Title LabLorin Grieve Work Study Student PalladinoKatherine Gubish Student Worker AdministrationAmelia Hohenadel Student Worker DegroatBenjamin Kendall Work Study Student PalladinoRobert Kiernan Work Study Student PalladinoNak Kim Work Study Student DegroatAndrew King Work Study Student DegroatKevin Koronowski Student Worker DegroatDesiree Kosmisky Work Study Student PalladinoKevin Lenzen Work Study Student PalladinoDouglas Leung Student Worker PalladinoMaria Macpherson Other Trainee DefrancoLaurentiu Maerean Student Worker PalladinoZane Mclain Work Study Student PalladinoCharles Moon Iii Work Study Student JacobAnh Nguyen Work Study Student PalladinoAlicia Olson Work Study Student PalladinoMatthew Orlowski Other Trainee SiegfriedKellianne Piell Student Worker FreemanJohn Ries Work Study Student PalladinoJennifer Schaber Student Worker AdministrationBryan Seelnacht Work Study Student PalladinoSarah Spaventa Student Worker DefrancoDavid Tofovic Student Worker JacksonJacob Volpe Student Worker DegroatHannah Wills Work Study Student PalladinoMegan Wolfe Work Study Student PalladinoKeith Yatsko Work Study Student PalladinoChelsea Yelk Work Study Student FreemanWendy Yun Work Study Student PalladinoAlison Zeccola Work Study Student PalladinoMatthew Zurenski Work Study Student Palladino
University Staff Title LabGuillermo Barila Research IV AltschulerStacey Barrick Research IV BiselloJoshua Benson Research II PalladinoJennifer Butterfield Administrator I DefrancoSamantha Cavolo Research III LevitanJohn Chaves Research II PalladinoTeresa Cox Research IV AltschulerStephanie Daugherty Research III DegroatHolly Gergely Administrator III AdministrationDelbert Gillespie Jr. Research Specialist V JacksonFranca Golin Bisello Research IV FreemanSonia Gor Research III FreemanRebecca Hohmann Financial II AdministrationJames Kaczynski Administrator IV AdministrationJudith Krigman Research II Romero
71
University Staff Title LabYumei Lai Research III JiangMarcia Lewis Research IV DefrancoBarbara Martin Administrator II AdministrationMelanie Mcclain Administrator I AdministrationJeanette Mcdew Administrator III AdministrationLisa Mcgreal Financial I AdministrationCharlotte Mckinnon Administrator I AdministrationZaichuan Mi Research Specialist V JacksonPhillip Palmer Administrator III LakoskiVirginia Reiner Administrator I AdministrationSonia Salvatore Research Specialist III SchopferRichard Serventi Research I AdministrationRichard Smith Systems / Programmer IV AdministrationPatricia Smith Administrator I AdministrationLynda Sorch Financial III AdministrationBrian Taylor Systems / Programmer III AdministrationChenshan Woodcock Research III FreemanShuping Xu Research III WangYanmei Yang Research III Friedman
Researchers Title LabImad Al Ghouleh Postdoctoral Associate PaganoVeronica Alonso Postdoctoral Associate FriedmanMarie Lue Antony Postdoctoral Associate SinghJuan Ardura Postdoctoral Associate FriedmanDebra Artim Research Associate DegroatMichelle Barbi De Moura Postdoctoral Associate Van HoutenChandra Sekha Bathula Postdoctoral Associate LeePatricia Boley Postdoctoral Associate KenslerDavid Boone Postdoctoral Associate LeeSylvain Bougoin Postdoctoral Associate VilardagaMegan Brady Postdoctoral Associate JacobMonica Buchanan Postdoctoral Associate ZhangBonita Chan Visiting Research Associate LeeKumar Chandra Kuntal Visiting Research Associate SinghKrishnamoorth Chandrasekhar Research Associate FureyDionysios Chartoumpekis Postdoctoral Associate KenslerDongshi Chen Postdoctoral Associate ZhangDongmei Cheng Research Associate JacksonLesley Colgan Postdoctoral Associate LevitanGabor Csanyi Visiting Research Associate PaganoDavide Del Prete Postdoctoral Associate AltschulerLoreto Egana Basic Science Fellows PaganoQingming Fang Visiting Research Associate Sobol Jr.Susan Farabaugh Postdoctoral Associate LeeMarco Fazzari Postdoctoral Scholar FreemanTimothy Feinstein Postdoctoral Associate VilardagaElise Fouquerel Postdoctoral Associate Sobol Jr.
72
Researchers Title LabAmy Furda Postdoctoral Associate Van HoutenChenxi Gao Postdoctoral Associate HuJianxia Guo Research Associate EisemanRyan Hartmaier Postdoctoral Associate LeeDaniel Hochbaum Visiting Research Associate LeeSu Hyeong Kim Research Associate SinghCarolyn Kitchens Postdoctoral Associate Sobol Jr.Joomin Lee Postdoctoral Associate SinghHua Li Postdoctoral Associate ZhangJianfeng Li Research Associate Sobol Jr.Zhongmin Liu Visiting Research Associate GalbiatiYe Peng Postdoctoral Associate Van HoutenRui Peng Basic Science Fellows ZhangWei Qian Postdoctoral Associate Van HoutenAndres Rodriguez Visiting Research Associate PaganoSanghamitra Sahoo Visiting Research Associate PaganoKozue Sakao Postdoctoral Associate SinghAnuradha Sehrawat Visiting Research Associate SinghMatthew Sikora Postdoctoral Associate OesterreichJing Sun Postdoctoral Scholar ZhangManuj Tandon Research Associate WangJingshan Tong Postdoctoral Associate ZhangKristal Tucker Postdoctoral Scholar LevitanJonathan Verrier Postdoctoral Scholar JacksonDario Vitturi Iglesias Postdoctoral Associate FreemanAvani Vyas Postdoctoral Associate SinghPeng Wang Research Instructor ZhangHong Wang Postdoctoral Associate Van HoutenRebecca Watters Postdoctoral Associate OesterreichVanessa Wehbi Postdoctoral Associate VilardagaSteven Woodcock Research Associate FreemanGonghong Yan Research Associate JiangLi Yang Postdoctoral Associate KenslerYongbei Yu Research Associate DegroatXiulin Zhang Research Associate DegroatXuefeng Zhang Postdoctoral Associate AltschulerQiuhong Zhang Research Associate HershbergerLiyong Zhang Research Associate WangChao-Ming Zhou Research Associate LevitanHuafei Zou Research Associate Galbiati
73
Major Collaborations
Bruce Freeman, Ph.D. Professor and Chair
Fadi Lakkis and Timothy Billiar (University of Pittsburgh): Organ preservation for transplantation Mitchell Fink and Derek Angus (University of Pittsburgh): Anti-inflammatory strategies for treating
sepsis/ARDS Robert Squires and David Hackam (University of Pittsburgh):Anti-inflammatory strategies for treating GI
surgical patients Kaikobad Irani (University of Pittsburgh): Prevention of cardiac ischemic injury Mark Gladwin (University of Pittsburgh): Treatment of pulmonary hypertension
Daniel Altschuler, Ph.D. Associate Professor
Mikael Nilsson (Sweden) Sookja Chung (South Korea) Matthias Buck (Cleveland, OH) Joanne Ye (University of Pittsburgh)
Dr. Alessandro Bisello Associate Professor
Peter Friedman (Department of Pharmacology & Chemical Biology, University of Pittsburgh): functional studies on EBP50/NHERF1
Patrick Pagano (Department of Pharmaocology & Chemical Biology, University of Pittsburgh): EBP50 and Nox
Mark Gladwin (Department of Medicine, University of Pittsburgh): nitrate and peroxide in VSMC migration Rupangi Vasavada (Department of Medicine, University of Pittsburgh): pancreatic beta cells Sam Gellman (University of Wisconsin): novel GLP-1 analogs
Donald DeFranco, Ph.D. Professor
Dr. Gordon Rintoul (Simon Fraser University Vancouver, Canada) Dr. Selma Witchel (Division of Pediatric Endocrinology, Children’s Hospital of Pittsburgh)Dr. Elias Aizenman (Department of Neurobiology, University of Pittsburgh) Dr. Robert Bowser (Department of Pathology, University of Pittsburgh) Dr. Charlene Chu (Department of Pathology, University of Pittsburgh) Dr. Wen Xie (Department of Pharmaceutical Sciences, University of Pittsburgh) Dr. Zhou Wang (Department of Urology, University of Pittsburgh) Dr. Dean Bacich (Department of Urology, University of Pittsburgh)
W. Chet de Groat, Ph.D. Distinguished Professor
Dr. Naoki Yoshimura (Department of Urology, University of Pittsburgh): Collaborative urologic research in spinal cord injury
74
Dr. Lori Birder (Department of Medicine, University of Pittsburgh): Collaborative urologic research in spinal cord injury
Dr. Tony Kanai (Department of Medicine, University of Pittsburgh): New treatment for spinal cord injury Dr. Changfeng Tai (Department of Urology, University of Pittsburgh): Mechanisms underlying neurogenic
bladder disorders Dr. Seong-Gi Kim (Radiology-Imaging Center, University of Pittsburgh): Neuroplasticity in the spinal cord Dr. C. L. Cheng (Department of Urology, Taichung Veterans Hospital): Pharmacology of the lower urinary
tract Dr. Michael Chancellor (Department of Urology, Beaumont Hospital, Michigan): Use of liposomes in drug
delivery Dr. Pradeep Tyagi (Department of Urology, Beaumont Hospital, Michigan): Use of liposomes in drug delivery Dr. Hamid Chahine (Department of Physiology, University of Laval, Quebec City, Canada): Sodium channel
blockade Dr. Cemil Gocmen (Department of Pharmacology, Cukurova University, Adana, Turkey): Bladder
pharmacology Dr. James Roppolo (Department of Pharmacology & Chemical Biology, University of Pittsburgh): Bladder
pain
Julie Eiseman, Ph.D. Research Associate Professor
Robert S. Parker, Ph.D. (Department of Chemical and Petroleum Engineering, University of Pittsburgh): Optical pharmacokinetics, modeling and calculations of elastic scattering spectrometer data of Pc4 and motexafin gadolinium; docetaxel pk
Peter Wipf, Ph.D. (Department of Chemistry, University of Pittsburgh): Collaborations on preclinical studies of phosphatase inhibitors and tubulin or motor protein interactive small molecules, Protein kinase D small molecule inhibitors
Dennis Curran, Ph.D. (Department of Chemistry, University of Pittsburgh): Collaborations on preclinical studies tubulin or motor protein interactive small molecules: 6-epi dictyostatin
Billy Day, Ph.D. (School of Pharmacy University of Pittsburgh): Collaborations on preclinical studies of phosphatase inhibitors and tubulin or motor protein interactive small molecules.6-epi dictyostatin
Ed Prochownik, M.D. (Pediatrics, Children’s Hospital): Preclinical studies of myc inhibitors Alex Doemling, Ph.D. (School of Pharmacy, University of Pittsburgh): Tubulycin analogues as anti-cancer
agents, MDM2 and MDM4 antagonists Gabrialla Mustata, Ph.D. (Department of Computation Biology, University of Pittsburgh): Computational
biology of c-myc inhibitors Jerry Collins, Ph.D. (Developmental Therapeutics, NCI): Studies of pyrimidine nucleosides in xenografts
animal pharmacology studies Doug Ross, M.D. (University of Maryland Greenebaum Cancer Center, Baltimore, MD): Mechanisms of drug
resistance due to BCRP Nancy Olienik, Ph.D. (Case Western Reserve University/Ireland Cancer Center, Cleveland, OH): Studies on Pc
4 and other silicon phthalocyanines as photodynamic therapeutics and molecular alterations in xenografts following photodynamic therapy
Ivana Vucenik, Ph.D. (University of Maryland, Baltimore): IP6 pharmacokinetics and inositol Irving Bigio, Ph.D. (Boston University, Boston, MA): Use of optical pharmacokinetics system (ESS) to
measure drugs with absorbance spectra in the long wavelength visible spectra including motexafin gadolineum, motexafin lutetium, PC4, mitoxanthrone
Steve Musser, Ph.D. (FDA, Washington, DC): Characterization of metabolites by LC/MS/MS David D’Argenio, Ph.D., (School of Engineering, UCLA, Los Angeles CA): Pharmacokinetic and
pharmacodynamic modeling of 17-AAG and DMAG, zebularine, FdC
75
Steven Metallo, Ph.D. (Georgetown University): c-myc inhibitors. Martin R Austwick, Ph.D. (University College London): Pharmacokinetics of photodynamic therapeutics
Melanie Flint, Ph.D.Research Assistant Professor
Dr. Blair Jobe (Esophageal Diagnostics and Therapeutic Endoscopy Division of Thoracic and Foregut Surgery, the Heart, Lung, and Esophageal Surgery Institute, University of Pittsburgh Medical Center): Using laser capture micro-dissection to enable identification of protein expression in esophageal cancer patient samples
Dr. Larry Maxwell (Walter Reid Medical Center, Washington, DC): Validating protecomics findings in endometrial cancer using Western blot techniques
Dr. Jennifer Grandis (Department of Otolaryngology, University of Pittsburgh): Head and Neck Spore Cancer Grant
Peter Friedman, Ph.D. Professor
Dr. Maria Kurnikova (Carnegie-Mellon University) Dr. Larry Brunton (University of California at San Diego) Mark Von Zastrow (University of California at San Francisco) Dr. Simon Watkins (Department of Cell Biology, University of Pittsburgh) Dr. Harry Blair (Department of Pathology , University of Pittsburgh) Dr. Timothy Billiar (Department of Surgery, University of Pittsburgh)
William Furey, Ph.D. Professor
Dr. Mulchand Patel (Department of Biochemistry, State University of New York at Buffalo): Human pyruvate dehydrogenase
Dr. F. Jordan (Department of Chemistry, Rutgers University): E. coli pyruvate dehydrogenase multi-enzyme complex component structures
Dr. Guillermo Calero (Department of Structural Biology, University of Pittsburgh): Production of pyruvate dehydrogenase
Dr. Angela Gronenborn (Department of Structural Biology, University of Pittsburgh): X-ray structural studies of cyanovirin-N and BOA
Dr. Michael Palladino (Department of Pharmacology & Chemical Biology, University of Pittsburgh): Structural studies of triosephosphate isomerase
Ferrucio Galbiati, Ph.D. Associate Professor
Dr. Thomas Kensler (Department of Pharmacology & Chemical Biology, University of Pittsburgh): Caveolin-1 and Nrf2
Dr. Donald DeFranco (Department of Pharmacology & Chemical Biology, University of Pittsburgh): Nuclear receptor and caveolin-1
Dr. Patrick Pagano (Department of Pharmacology & Chemical Biology, University of Pittsburgh): Caveolin-1 and Nox
76
Pamela Hershberger, Ph.D. Research Assistant Professor
Drs. Suresh Ramalingam and Taofeek Owonikoko: Evaluation of novel HDAC inhibitor-based therapeutic combinations in NSCLC cells
Dr. Carol Feghali Bostwick (Department of Medicine, University of Pittsburgh): Role of estrogen signaling in dermal fibrosis
Cytochroma, Inc. (Ontario, Canada): Evaluation of CTAA (CYP24 specific inhibitor) on vitamin D anti-tumor activity in lung cancer
Endece, Inc. (Madison, WI): Determining the structure function relationships of NDC compounds in lung cancer models
Drs. Len Appleman and Lily Shah (Department of Medicine, University of Pittsburgh): Potentiation of gemcitabine/cisplatin activity by ABT-888 in bladder cancer
Jing Hu, Ph.D. Associate Professor
Dr. Paul Robbins (Department of Molecular Genetics and Biochemistry, University of Pittsburgh): Sirt1 on elF2α sumoylation and mRNA translation.
Dr. Stergios Moschos (University of Pittsburgh Cancer Institute): Regulation of Aurora B and Plk1 by Ubc9. Dr. Shihfan Kuan (Department of Pathology, University of Pittsburgh): Expresion profiles of several Wnt
pathway components and regulators in human colon cancer tissue.
Edwin Jackson, Ph.D. Professor
Pat Kochanek, M.D. (Department of Critical Care Medicine, University of Pittsburgh): Adenosine in traumatic brain injury
Ed Dixon, Ph.D. (Department of Critical Care Medicine, University of Pittsburgh): Adenosine in brain dysfunction
Elieser Gorelik, Ph.D. (Department of Pathology, University of Pittsburgh): Role of adenosine in cancer Gerard Apodaca, Ph.D. (Department of Medicine, University of Pittsburgh): Role of adenosine in bladder
function Derek W. Gilroy, Ph.D. (Division of Medicine, University College London): Role of adenosine in
inflammation Stevan P. Tofovic, M.D., Ph.D.; Center for Clinical Pharmacology, University of Pittsburgh): Estradiol
metabolites in renal disease Raghvendra K. Dubey, Ph.D. (Department of Obstetrics and Gynecology, University Hospital Zurich):
Vascular biology of estradiol metabolites Lisa Satlin, M.D. (Division of Pediatric Nephrology, Mount Sinai School of Medicine): Purine metabolomics Virginia Miller, Ph.D. (Department of Surgery, Mayo Clinic): Estradiol Metabolomics
Yu Jiang, Ph.D. Associate Professor
Dr. Zhao (Nanjing Medical School, China): Clinical-related studies Dr. Bai (Southern Medical University, China): mTOR signaling Dr. Ferro-Novick (University of California, San Diego): Autophagy-related study Dr. Wang (Department of Orthopedic Surgery, University of Pittsburgh) Dr. Freeman (Department of Pharmacology & Chemical Biology, University of Pittsburgh)
77
Dr. Singh (Department of Pharmacology & Chemical Biology, University of Pittsburgh) Dr. Shiva (Department of Pharmacology & Chemical Biology, University of Pittsburgh)
Edwin Levitan, Ph.D. Professor
John Horn (Department of Neurobiology, University of Pittsburgh): Dopamine neuron project Elias Aizenman (Department of Neurobiology, University of Pittsburgh): Potassium channels and apoptosis David Deitcher (Cornell) and Randy Hewes (Oklahoma): Neuropeptide release in Drosophila Glenn Fishman (NYU): Cardiac potassium channel expression
Patrick Pagano, Ph.D. Professor
P. Michael Bauer (Department of Surgery, University of Pittsburgh) Aaron Barchowsky (Department of Environmental and Occupational Health, University of Pittsburgh) Robin Gandley (Magee Women’s Hospital)Jeffrey Isenberg (Department of Medicine, University of Pittsburgh) Xiang Gao (Department of Pharmaceutical Sciences, University of Pittsburgh) Song Li (Department of Pharmaceutical Sciences, University of Pittsburgh) Carlos Camacho (Department of Computational Biology, University of Pittsburgh) Guangjie Cheng (Emory University) Phil Palade (University of Arkansas) Xiao-Ping Yang (Henry Ford Hospital) William Beierwaltes (Henry Ford Hospital)
Michael Palladino, Ph.D. Associate Professor
Marc Blondel (Faculté de médecine de l’UBO/CHU & CNRS): Collaborate to use yeast and fly models of mitochondrial disease for drug discovery
Wayne VanVoorhies (New Mexico State): Respiration and metabolic studies of Drosophila mutants Ron Wetzel (University of Pittsburgh): The study of TPI aggregation and involvement in amyloidopathies J. Timothy Greenamyre (University of Pittsburgh): The study of oxidative stress and mitochondrial
pathogenesis in myopathy Andrew Van DeMark (University of Pittsburgh): The study of TPI protein pathogenic structure
Guillermo Romero, Ph.D. Associate Professor
Susan Amara (University of Pittsburgh) Meir Aridor (Department of Cell Biology, University of Pittsburgh): Role of PLD in vesicular traffic Tomas Kirchhausen (Harvard Medical School, Cell Biology): Endocytic transport.
James Roppolo, Ph.D. Research Assistant Professor
Seong-Gi Kim (Department of Radiology, University of Pittsburgh): fMRI brain imaging studies Changfeng Tai (Department of Urology, University of Pittsburgh): Neurourology and pharmacology studies Lori Birder (Department of Medicine, University of Pittsburgh): Neurourology and pharmacology studies
78
Francisco Schopfer, Ph.D. Research Associate Professor
Eugene Chen (University of Michigan): Study of the mechanisms of PPARgamma activation by nitrated fatty acids
Anna Lisa Levonen (University of Kuopio, Finland): Study of the activation of phase 2 genes by nitroalkenes, mainly focusing on KEAP/Nrf2 pathway
Sruti Shiva, Ph.D. Assistant Professor
Jeffrey Isenberg (Department of Medicine, University of Pittsburgh) Mark Gladwin (Department of Medicine, University of Pittsburgh) Anje Cauwels (University of Ghent, Belgium)Tienush Rassaf (University Hospital Aachen, Germany) William Frazier (Washington University, St. Louis, MO)
Jill Siegfried, Ph.D. Professor
Phoutone Keohavong (Department of Environmental and Occupational Health, University of Pittsburgh): Kras and p53 mutations in airway mucosa
Jennifer Grandis (Department of Otolaryngology, University of Pittsburgh): GPCR-EGFR interactions Joseph Pilewski (Department of Medicine, University of Pittsburgh): HGF in airway biology Stephen Shapiro (Department of Medicine, University of Pittsburgh): Inflammation in lung cancer David Sidransky (Johns Hopkins University): Methylation marker for lung cancer risk
Shivendra Singh, Ph.D. Professor
Jennifer Grandis (Department of Otolaryngology, University of Pittsburgh): Prevention of head and neck cancer with dietary and CAM agents
Per Basse (Department of Immunology, University of Pittsburgh): Augmentation of NK cell activity by dietary agents
Charles Brown (Department of Surgical Oncology, University of Pittsburgh): Prevention of melanoma by sulforaphane
Robert Sobol, Ph.D. Associate Professor
Hideho Okada (Department of Neurological Surgery, University of Pittsburgh): Investigating Dicer in glioma Patricia Opresko (Department of Environmental and Occupational Health, University of Pittsburgh): Convergent roles of DNA PolB and WRN in genome stability
Barry Gold (Department of Pharmaceutical Science, University of Pittsburgh): Investigations of novel DNA damaging agents
Hussein Tawbi (Department of Medicine, University of Pittsburgh): Temozolomide resistance in melanoma Robert Ferrel (Department of human genetics): Development of a lymphatic-inclusive tissue system for the
study of progression, treatment and unique biomarkers of melanoma Robert Branch (Department of Medicine, University of Pittsburgh): Mutations of PolB in cancer
79
Deric Park (Department of Neurological Surgery, University of Pittsburgh): DNA repair status of glioma stem like cells
Andrew Stewart (Department of Medicine, University of Pittsburgh): Potential role of DNA repair genes in diabetes
Emanuela Taioli (SUNY Downstate): Methylation and DNA Repair defects in cancer Simone Heyliger (Hampton University): Pesticides and genome instability Bruce Demple (Harvard School of Public Health): Pol B in repair of oxidative lesions Guy Poirier (Université Laval): Poly-ADP-ribose modified proteins in cancer Mark Bedford (MD Anderson): Methylation of base excision repair protein. Michael Wyatt (University of Southern California): Reactive nitrogen modulation of DNA repair proteins
Laura Stabile, Ph.D. Research Assistant Professor
Marjorie Romkes and Shama Buch, Department of Medicine, Center for Clinical Pharmacology (Lung SPORE) Stephanie Land and Diana Lenzer (Department of Biostatistics, Lung SPORE, University of Pittsburgh) Phouthone Keovahong (Department of Environmental and Occupational Health, University of Pittsburgh): K-
ras mutation analysis Stephen Belinsky (Lovelace Institute); Methylation Project-DNA from Sputum Ethan Argiris (UPCI, Clinical Trials) Richard Pietras, Edward Garon and Diane Marquez-Garbin (UCLA, Clinical Trials) Jennifer Grandis, Ann Marie Egloff and Sufi Thomas (Department of Otolaryngology, Head and Neck SPORE,
University of Pittsburgh) Sanja Dacic (Department of Pathology, Lung SPORE, University of Pittsburgh,)
Ben Van Houten, Ph.D. Professor
Rita Alevriadou (Ohio State University, Columbus, OH): Role of mitochondrial injury in vascular endothelial cells exposed to shear stress
Marcel Bruchez (Carnegie Mellon University): Developing techniques for imaging single-molecules on DNA and inside living cells
Steve Dwretzky (Knopp Neurosciences, Pittsburgh, PA): Analysis of anti-degenerative compounds on mitochondrial function
Marc Greenberg (Johns Hopkins University, Baltimore, MD): Action mechanism of nucleotide excision repair proteins on endogenous DNA damage
Neil Kad (University of Essex, Colchester, UK): Single-molecule dynamics of DNA repair enzymes Caroline Kisker (University of Wurtzburg, Wurtzburg, Germany): Structure and function of nucleotide
excision repair proteins Steve Kleeberger (NIEHS, Research Triangle Park, NC): Mitochondrial dysfunction in hyperoxia induced
injury Robert London (NIEHS, Research Triangle Park, NC): NMR analysis of UvrB binding to DNA Mike Resnick (NIEHS, Research Triangle Park, NC): Control of oxidative phosphorylation by p53 Ivet Bahar (Department of Computational Biology, University of Pittsburgh): Protein dynamics in DNA
damage recognition Charleen Chu (Department of Pathology, University of Pittsburgh): Effect of Parkin on mitochondrial oxidative
phosphorylation Simon Watkins (Department of Cell Biology, University of Pittsburgh): Visualization of single-molecules of
QDOT labeled DNA repair protein
80
Mike Lotze (Department of Surgery, University of Pittsburgh): Function of HMG-B1 in mitochondrial autophagy and oxidative phosphorylation in cancer cells
Jean Latimer & Steven Grant (Department of Ob/Gyn and Reproductive Science, University of Pittsburgh): Warburg effect in breast cancer cells
James Gnarra (Department of Urology, University of Pittsburgh): Effects of VDL and Hif1 alpha on oxidative phosphorylation and glycolysis in kidney cells
Laura Niedernhofer (Department of Microbiology and Molecular Genetics, University of Pittsburgh): Role of ERCC1 in mitochondrial function and endogenous DNA damage
Tony Graves and Ed Prochownik (Department of Pediatrics, University of Pittsburgh): Function of Myc expression on oxidative phosphorylation in cancer cells
Jean-Pierre Vilardaga, Ph.D. Associate Professor
Harvard University, Endocrine Unit Harvard University, Center for Systems Biology University of Wuerzburg (Germany), Institute of Pharmacology University of Barcelona (Spain), Department of Pathology and Experimental Therapeutics University of Santiago de Compostela (Spain), Department of Pharmacology
Andreas Vogt, Ph.D. Research Assistant Professor
Billy Day (Department of Pharmaceutical Sciences, University of Pittsburgh), Scott Nelson and Dennis Curran (Department of Chemistry, University of Pittsburgh): Novel microtubule-perturbing agents
Billy Day (Department of Pharmaceutical Sciences, University of Pittsburgh): Inhibitors of cytoplasmic dynei Kazonuri Koide (Department of Chemistry, University of Pittsburgh): Chemical approaches for the discovery
of new cancer therapeutic targets Neil Hukriede (Department of Microbiology and Molecular Genetics, University of Pittsburgh): Utilizing small
molecule screens to delineate embryonic signaling mechanisms Michael Tsang (Department of Microbiology and Molecular Genetics, University of Pittsburgh): Small
molecule zebrafish screens for regulators of Fgf signaling Xiao-Ming Yin (Department of Pathology, University of Pittsburgh): Modulators of autophagy Ruth Perez (Department of Neurology, University of Pittsburgh): Inhibitors of Abeta aggregation Charleen Chu (Department of Pathology, University of Pittsburgh): Autophagy in dopaminergic cell death Patrick Moore (Department of Microbiology and Molecular Genetics, University of Pittsburgh): Inhibitors of
Merkel cell carcinoma Garth Powis (MD Anderson): Cancer drugs active against stress signaling pathways Jana Patton-Vogt (Duquesne University): Inhibitors of the glycerophosphoinositol transporter, GIT1 Keren Hulkower (Platypus LLC, Madison, WI): Development of HTS migration assays Scott Keefer (Thermo Fisher Cellomics): Development of zebrafish toxicity assay Tao Zhang (Vanderbilt University): High content screening for zebrafish cardiomyocyte development
Daniela Volonte-Galbiati, Ph.D. Research Assistant Professor
Y. P. Peter Di (Department of Environmental and Occupational Health, University of Pittsburgh)
81
Q. Jane Wang, Ph.D. Associate Professor
Billy Day (Department of Pharmaceutical Sciences, University of Pittsburgh) Adam Glick (Penn State University) J. Frederic Mushinski (National Cancer Institute) Peter M. Blumberg (National Cancer Institute)
Lin Zhang, Ph.D. Assistant Professor
Xiao-Ming Yin (Department of Pathology, University of Pittsburgh): Studying apoptosis induced by proteasome inhibitor
Cary Wu (Department of Pathology, University of Pittsburgh): Studying apoptosis caused by chance in extracellular matrix
Tao Cheng (Department of Radiation Oncology, University of Pittsburgh): Studies of PUMA-knockout mice Robert Schoen (Department of Medicine, University of Pittsburgh): Studying chemoprevention of colon cancer
by NSAIDs Wei Zhou (Emory University): Developing molecular markers of lung cancer Chuanshu Huang (New York University): Studying apoptotis induced by anti-cancer drugs Jim Herman (Johns Hopkins University): Studying DNA methylation in lung cancer Gerry Zambetti (St. Jude’s Children’s Hospital): Studies of PUMA-knockout mice Chinese Academy of Medical Sciences: Studying esophageal cancer drug response
Entrepreneurial Activities
None.
Awards and Honors
Bruce Freeman, Ph.D. Professor and Chair Elected Fellow, American Association for the Advancement of Science
Donald DeFranco, Ph.D.Professor Finalist, Excellence in Medical Education Awards in both “Course Director” and “Lecturer” categoriesFinalist, Distinguished Mentor Award, Biomedical Graduate Student Association Nominee, Chancellor’s Distinguished Teaching Award
William C. deGroat, Ph.D. Distinguished Professor University of Pittsburgh Innovator Award
Peter Friedman, Ph.D. Professor Visiting Professor, CCNY Chemistry Department, February 2012
82
Tija Jacob, Ph.D.Assistant Professor Selected as University of Pittsburgh School of Medicine applicant for Pew Grant, 2011.
Thomas Kensler, Ph.D. ProfessorOxygen Club of California, Jarrow Formulas Health Science Prize, 2012 (shared with Masayuki Yamamoto, University of Tsukuba)
Francisco Schopfer, Ph.D. Research Associate Professor University of Pitttsburgh, 2012 Innovator Award
Robert Sobol, Ph.D. Associate Professor University of Pitttsburgh, 2012 Innovator Award 2011 UPCI Junior Scholar Award in Basic Science Cancer Research, 2012
Bennett Van Houten, Ph.D. Proessor Provost Inaugural Lecture, Richard M. Cyert, Chair of Molecular Oncology, University of Pittsburgh, 2012
Lin Zhang, Ph.D. Associate Professor University of Pitttsburgh, 2011 Innovator Award
Invited Talks
Bruce Freeman, Ph.D. Professor and Chair
“Fishing for Electrophiles and Snagging Moby Dick.” University of Alabama at Birmingham, Department of Pathology, Birmingham, AL, January 27, 2011.
“Pulling New Drug Candidates from the Fires of Inflammation.” Georgetown University, Stephen and Mary Krop Lectureship in Pharmacology, Washington, DC, April 1, 2011.
“Redox-Derived Anti-Inflammatory Mediators.” Cardiovascular Research Training Program, University of Virginia,Charlottesville, VA, April 21, 2011.
“Drug Development in Redox Pathobiology: Perspectives and Pathways.” Free Radicals in Brazil 2011, Águas de São Pedro, State of São Paulo, Brazil, August 21, 2011.
“Formation and Signaling Actions of Electrophilic Fatty Acid Derivatives.” 12th International Conference on Bioactive Lipids in Cancer, Inflammation and Related Disease, Seattle, WA, September 21, 2011.
“Electrophilic Fatty Acid Signaling Mediators: New Hammers in the Redox Toolbox.” South East Lipid Research Conference, Pine Mountain, GA, October 6, 2011.
83
“Formation and Signaling Actions of Electrophilic Nitro- and Keto-Fatty Acid Derivatives.” Molecular and Cellular Bases of Redox Signaling and Oxidative Stress: Implications in Biomedicine, Baeza, Spain, November 3, 2011.
“Redox-Derived Anti-Inflammatory Signaling Mediators.” Gordon Research Conference, Ventura, CA, February 5, 2012.
“Electrophilic Fatty Acid Transduction of Redox Signaling Reactions.” Medical University of South Carolina, Charlestown, SC, March 19, 2012.
“Redox-Derived Anti-Inflammatory Mediators.” 33rd Naito Conference on Oxygen Biology: Hypoxia, Oxidative Stress and Diseases, Sapporo, Japan, June 29, 2012.
“Formation and Signaling Actions of Electrophilic Fatty Acid Derivatives.” 7th International Conference on the Biology, Chemistry and Therapeutic Application of Nitric Oxide, Edinburg, Scotland, July 25, 2012.
“The Fires of Inflammation Forge New Drugs.” Keynote Speaker, Burnett School of Biomedical Sciences, College of Medicine, University of Central Florida, Orlando, FL, August 16, 2012.
“Pulling New Drug Candidates from the Fires of Inflammation.” Wright State University, Dayton, OH, November 29, 2012.
Daniel Altschuler, Ph.D. Associate Professor
ENDO Meeting, July 5, 2011.
Cell Signaling Technology, Massachusetts, April 26, 2012.
Gordon Research Conference, Italy, May 20, 2012.
Palaniappa Arjunan, Ph.D. Research Instructor
“Structural Features of the Dihydrolipoamide Dehydrogenase E3 Component from the E.coli Pyruvate Dehydrogenase Complex.” American Crystallographic Assocation Meeting, Boston, MA, July 2012.
Dr. Alessandro Bisello Associate Professor
“Scaffolding and Remodeling: EBP50/NHERF1 in the Vasculature.” Dartmouth University, April 2012.
Donald DeFranco, Ph.D. Professor
Department of Microbiology, University of Virginia School of Medicine, April 2012.
Department of Pharmacology, University of Pennsylvania School of Medicine, November 2012.
84
W. Chet de Groat, Ph.D. Distinguished Professor
“Urinary Incontinence: From Basic Science to Clincal Practice.” European Urological Association Meeting. Joint symposium sponsored by INComb-TRUST-SEA, Stockholm, Sweden, June 8-11, 2011.
Science of Neuromodulation. IN: Hands-on Neuromodulation. Symposium sponsored by Beaumont Hospital, Dearborn, Michigan, September 30-October 1, 2011.
Organization of Neural Switching Circuitry in the Pons and Mesencephalon that Controls Storage and Voiding Functions of the Lower Urinary Tract. IN: 64th annual meeting of the Japanese Autonomic Nervous System Society, Akita, Japan, October 28, 2011.
The Neurourology of Pelvic Organs: Peripheral and Spinal Control. In: 1st International Neuro-Urology Meeting, Swiss Continence Foundation, Zurich, Switzerland, June 29, 2012.
Peter Friedman, Ph.D. Professor
Endocrine Society, Boston, MA, June 2011
CCNY Chemistry Department, February 2012.
William Furey, Ph.D. Professor
“Structural Features of the Dihydrolilpoamide Dehydrogenase E3 Component from the E. coli Pyruvate Dehydrogenase Complex.” American Crystallographic Association Meeting, Boston, MA, July 2012.
Eun-Ryeong Hahm, Ph.D. Research Instructor
SNUCRI Symposium, May 2012.
Jing Hu, Ph.D. Assistant Professor
HDAC2 in gene regulation: Role of sumoylation-promoting activity. The Seventh Pittsburgh Ubiquitin-Proteasome Meeting. August 22, 2011.
The SUMO Pathway: Attractive Upcoming Cancer Targets? Georgia Health Science University Cancer Center. October 23, 2012.
The SUMO Pathway: Attractive Upcoming Cancer Targets? Texas Tech University Health Sciences Center School of Medicine Cancer Center. December 5, 2012.
85
Edwin Jackson, Ph.D. Professor
Jackson, E.K.: The 2’,3’-cAMP-Adenosine Pathway in Acute Kidney Injury. Presented to the Acute Kidney Injury Symposium, University of Pittsburgh, Pittsburgh, PA, September 9, 2011.
Jackson, E.K., Cheng, D., Tofovic, S.P., and Mi, Z.: Adenosine A1 receptors enhance renal sympathetic neurotransmission via postjunctional coindicent signaling. Presented to the 65th Annual Fall Conference and Scientific Sessions of the Council for High Blood Pressure Research, Orlando, Florida, September 22, 2011.
Jackson, E.K.: Discovery of the 2’,3’-cAMP-Adenosine Pathway: Does This Mechanism Protect Organs From Injury? Presented to the Department of Pharmacology, University of Tennessee, Memphis, TN, January 25, 2012.
Jackson, E.K.: Endogenous Adenosine Contributes to Renal Sympathetic Neurotransmission Via Postjunctional A1-Receptor-Mediated Coincident Signaling. Amercian Journal of Physiology Renal Physiology Podcast. Recorded January 27, 2012.
Jackson, E.K., and Gillespie, D.G.: Extracellular guanosine regulates extracellular adenosine levels. Presented to the 66th Annual Fall Conference and Scientific Sessions of the Council for High Blood Pressure Research, Washington, DC, September 21, 2012.
Tija Jacob, Ph.D.Assistant Professor
Senior Vice Chancellor’s Research Seminar 12/7/2012 “GABAA Receptor Trafficking: Dynamic Inhibition in CNS Health and Disease”
Yu Jiang, Ph.D. Assistant Professor
“The role of Rheb in mTOR regulation” (2012). 2nd International Bone Biology Symposium, Shihezhi, China.
“A novel crosstalk between Bcl-2 and mTOR and its implication in tumorigenesis” (2012). 2nd International Tumor Microenvironment Symposium, Tianjing, China.
“The role of FKBP38 in tumor suppression” (2012). University of Pittsburgh Cancer Institution, Pittsburgh, PA.
“The role of Npr1 kinase in nitrogen discriminating pathway” (2012). Yeast Genetics Meeting at Princeton, NJ.
“Nutrient signaling in yeast” (2012) Keynote speaker at International Signal Transduction Symposium, Guangzhou, China.
Thomas Kensler, Ph.D. Professor
Oxidants and Antioxidants in Biology, Alba, Italy
Oxygen Biology: Hypoxia, Oxidative Stress and Diseases, Sapporo, Japan
86
Symposium on “Global Challenges: Environmental Changes”, AACR Frontiers in Cancer Prevention Meeting, Anaheim, CA School of Pharmacy Distinguished Lectureship, Ohio State University, Columbus, OH
School of Pharmacy, University of Illinois Chicago, Chicago, IL
University of Pittsburgh Cancer Institute, Pittsburgh, PA
Food and Drug Administration, Center for Food Safety and Applied Nutrition, Laurel, MD
Southern California Chapter, Society of Toxicology Annual Meeting, Carlsbad, CA
Medical College of Wisconsin Cancer Center, Milwaukee, WI
Department of Structural and Cellular Biology, Tulane University, New Orleans, LA
Nicholas Khoo, Ph.D. Research Assistant Professor
Therapeutic Strategies for Obesity-induced Type 2 Diabetes using Electrophilic Fatty Acids. Presented at South East Lipid Research Conference; Pine Mountain, Georgia, October 2011.
Inhibition of obesity-induced pulmonary arterial hypertension by nitro-fatty acids. Presented at PACCM Basic & Translational Research in Lung Disease Conference; Pittsburgh, PA, December 2012.
Joan M. Lakoski, Ph.D. Professor
Invited Speaker, “The Art of Being a Successful Mentee and Mentor,” Creighton University School of Medicine, Omaha, NE, February 3, 2011
Invited Speaker, “Career Success through Mentoring,” Creighton University School of Medicine, Omaha, NE, February 3, 2011
Invited Speaker, “Career Success through Mentoring: Moving Your Career from Good to Great,” Creighton University School of Medicine, Omaha, NE, February 3, 2011
Invited Speaker with Dr. Robert J. Milner, “Strategies for Building Effective Research Mentoring Relationships”, Postdoc Retreat, University of Wisconsin, Milwaukee, WI, April 4, 2012.
Invited Speaker with Dr. Robert J. Milner, “Know Your Kangaroo: The Pathway to Independence Award (K99/R00)”, Postdoc Retreat, University of Wisconsin, Milwaukee, WI, April 4, 2012.
Invited Speaker with Dr. Robert J. Milner, “Crafting a Competitive Career Development Proposal” Arteriosclerosis Thrombosis and Vascular Biology (ATVB) 2013 Scientific Sessions, American Heart Association, Chicago, IL, April 17, 2012.
87
Invited Speaker with Dr. Robert J. Milner, “The Inside Story on Decision Making by an NIH Study Section Panel” Arteriosclerosis Thrombosis and Vascular Biology (ATVB) 2013 Scientific Sessions, American Heart Association, Chicago, IL, April 18, 2012.
Invited Speaker with Dr. Robert J. Milner, “Grant Writing Workshop” Thomas Jefferson University, Philadelphia, PA, May 7, 2012. Workshop: “Crafting Effective Specific Aims”Workshop: “The Inside Story on a NIH Review Panel”
Invited Speaker with Dr. Robert J. Milner, “Grant Writing Workshop” Thomas Jefferson University, Philadelphia, PA, May 7, 2012.
Invited Speaker with Dr. Robert J. Milner, “Essential Strategies for Writing Successful Grants”, Memorial Sloan-Kettering Cancer Center, New York, NY, June 1, 2012. Workshop: “Finding the Right Funding”Workshop: “An Inside Look at Grant Reviews” Workshop: “Structure of a Proposal” Workshop: “Writing Your Specific Aims”
Invited Speaker with Dr. Robert J. Milner , “Know Your NRSA”, Grant Writing Conference, New York University, New York, NY, June 20, 2012.
Invited Speaker, “Mentoring For Success: Finding a Mentor and Becoming an Effective Mentor”, How to Secure Promotion and Tenure Workshop, The Endocrine Society, Houston, TX, June 22, 2012.
Invited Speaker, “An Introduction to Ks – A Dynamic Opportunity to Build Your Research Program”, Career Development Workshop, The Endocrine Society Annual Meeting, Houston, TX, June 25, 2012.
Invited Speaker, “Navigating Through the University and Academic Health Center”, Career Mapping Workshops, Early Career Women Faculty Professional Development Seminar, American Association of Medical Colleges (AAMC), Potomac, MD, July 7, 2012.
Invited Speaker with Dr. Robert J. Milner, “Writing Effective Specific Aims”, Grant Writing Workshop, 2012 Gilliam Fellows Meeting, Howard Hughes Medical Institute, Chevy Chase, MD, August 9, 2012.
Invited Speaker with Dr. Robert J. Milner, “NIH Career Development Award General Workshop: Steps to a Competitive Application (including a mock study section)”, Memorial Sloan-Kettering Cancer Center, New York, NY, September 5, 2012. Workshop: “Steps to a Competitive Application” Workshop: “Know Your NRSA”Workshop: “Know Your K” Workshop: “Know Your Kangaroo”
Invited Speaker, “Creating a Dynamic Research Mentoring Relationship: Advising Students and Postdocs”, Faculty Development Program to Enhance Teaching Skills, Penn State College of Medicine, Hershey, PA, September 14, 2012.
Invited Speaker, “Career Planning in Uncertain Times: Utilizing Keystone Habits for Success and Satisfaction”, Postdoctoral Office, Penn State College of Medicine, Hershey, PA, September 14, 2012.
88
Invited Speaker, “Ethics in Research: A Brief Odyssey”, Early Stage Investigators Workshop, The Endocrine Society, San Francisco, CA, September 29, 2012.
Invited Speaker, “Career Path or Career Death: Point/Counter Point”, Group on Women in Medicine and Science (GWIMS)/ Group on Faculty Affairs (GFA)/Women Executives in Science & Healthcare (WESH), Association of American Medical Colleges (AAMC) Annual Meeting, San Francisco, CA, November 4, 2012.
Invited Speaker with Dr. Robert J. Milner, “NIH Career Development Award General Workshop: Steps to a Competitive Application”, University of Massachusetts Medical School, Shrewsbury, MA, November 14, 2012. Workshop: “Know Your K” Workshop: “Know Your Kangaroo”
Invited Speaker with Dr. Robert J. Milner, “NIH Career Development Award General Workshop: Steps to a Competitive Application (including a mock study section)”, Office of Academic Affairs, Columbia University School of Medicine, New York, NY, November 30, 2012. Workshop: “Keys to a Successful K99/R00 Career Development Application” Workshop: “Crafting Effective Specific Aims”
Adrian Lee, Ph.D. Professor
“Rationale for targeting IGF-IR in cancer” 93rd Annual Endocrine Society Meeting, Boston, MA, 2011.
“Setting up a lab” 93rd Annual Endocrine Society Meeting, Boston, MA, 2011.
“IRSs in mammary gland biology”, University of Medicine and Dentistry of New Jersey, NJ, 2011.
“IGFIR and ROS action in breast cancer”, Medical University of South Carolina, SC, 2011.
“Structural rearrangements in breast cancer”. DOD Era of Hope Meeting, Florida, 2011.
“Structural rearrangements in DNA repair genes”. 22nd Breast Cancer Think Tank, Cancun, Mexico, 2012.
“Recent failures of anti-IGFIR cancer therapies: A teaching moment for personalized biomarker directed clinical trials”. Pathology Grand Rounds, University of Iowa, Iowa, 2012.
“Tumor heterogeneity and evolution: An Achilles heel for precision medicine?” RiMED symposium, Palermo, Italy, 2012.
“Role of endocrine hormones in breast cancer” Koc University, Istanbul, Turkey, 2012.
“Recent failures of anti-IGFIR cancer therapies: A teaching moment for personalized biomarker directed clinical trials”. Eppley Cancer Center, Omaha, NE, 2012.
“Recent failures of anti-IGFIR cancer therapies: A teaching moment for personalized biomarker directed clinical trials”. Cedars-Sinai Hospital, Los Angeles, CA, 2012.
89
Carola Neumann, M.D. Visiting Associate Professor
Klinik I fuer Innere Medizine, Cologne, Dr. Michael Hallek: “The peroxidase Prdx1 regulates specifically redox-signaling,” March 2012.
Stony Brook University, Dept. of Pathology, Dr. Ken Shroyer: “The peroxidase Prdx1 regulates specifically redox-signaling,” March 2012.
Steffi Oesterreich, Ph.D. Professor
“Role of Epigenetics in Endocrine resistant Breast Cancer”, 93rd Annual Endocrine meeting, Boston, 2011.
“Nuclear Receptors in Ovarian Cancer as therapeutic targets – time to reconsider?” 3rd International Symposium on Ovarian cancer. Pittsburgh, May 2012
“Mechanism of Endocrine Resistance” McArdle Cancer Center, Madison, WI, Oct 2012
“SRC1 - Bridging hormone response in breast and bone” Think Tank, Cancun, Mexico, 2012.
Patrick Pagano, Ph.D. Professor
Universidad Nacional Autonoma de Mexico, Mexican and Western Pharmacological Society Meeting, “Nox Inhibitor Discovery: A Multidisciplinary Approach to Taming the Adventitia”. May 19, 2011, Mexico City, Mexico.
Fifth Annual Ri.MED Scientific Symposium, Fondazione Ri.MED, “Janus Faces of NADPH Oxidase, Glimpses Past and Future”. October 24, 2011, Palermo, Italy.
University of Mississippi Medical Center, Department of Physiology, “The Vascular Media: Nexus of Oxidative Stress and Inflammation”, Jackson, MS, May 9, 2012.
University of Mississippi Medical Center, Center, Center for Excellence in Cardiovascular-Renal Research, “Targeting Oxidative Stress, Bullseye Nox”, Jackson, MS, May 10, 2012.
Laboratorio de Biologia Vascular, Facultad de Ciencias Médicas, Universidad de Cuyo, “Adventitial Control of Vascular Cell Differentiation & Disease via ROS”, Mendoza, Argentina, November 28, 2012
Francisco Schopfer, Ph.D. Research Associate Professor
Invited Speaker, Institute for Environmental Medicine, University of Pennsylvania, 2012.
Invited Speaker Oxygen Club of California World Congress 2012, Alba, Italy.
90
Sruti Shiva, Ph.D. Assistant Professor
“Mitochondrial ROS increase platelet activation in Sickle Cell disease.” Society for Free Radical Research International Meeting, London, UK, September 2012.
Jill Siegfried, Ph.D. Professor
“Progress and Plans for the Pittsburgh Lung Screening Study.” Loveland Respiratory Research Institute, February 15, 2011.
“Aromatase Inhibitors.” Annual Targeted Therapies of Lung Cancer Meeting, Santa Monica, CA, February 23-26, 2011.
Invited Speaker, National Lung Cancer Partnership annual meeting, Lung Cancer: Navigating the New Frontier, “Targeting the MET Signaling Pathway”, June 3, 2011, Chicago, IL.
Invited Speaker, 12th International Workshop on Scleroderma Research, “Biomarkers of Cancer Risk and Molecular Targets for Early Intervention/estrogen Signaling in Women”, July 24-27, 2011, Cambridge, United Kingdom.
National Cancer Institute Translational Science Meeting: From Molecular Information to Cancer Medicine, July 28-29, 2011, Washington, D.C.
Invited Speaker, Lung SPORE Comprehensive Research Center, H. Lee Moffitt Cancer Center, “The Role of the Estrogen Pathway in Lung Cancer”, Tampa, FL December 6-8, 2011.
AACR-IASLC Molecular Origins of Lung Cancer, January 18-11, 2012, San Diego, CA.
Department of Defense United States Army Medical Research and Materiel Command Congressionally Directed Medical Research Programs Peer Review Meeting, January 18-20, 2012, Reston, VA.
Invited Speaker, 11th Annual Targeted Therapies of Lung Cancer meeting, “Aromatase Inhibitors”, February 22-25, 2012, Santa Monica, CA.
Lung Cancer Workshop IX, Application of High Resolution CT Imaging Data to Lung Cancer Drug Development: Measuring Progress. May 3-4, 2012, Hyattsville, MD
Invited Speaker, Provost’s Inaugural Lecture, “Thirty Years of Lung Cancer Research: How Far Have We Come?,” May 22, 2012, University of Pittsburgh, Pittsburgh, PA
National Lung Cancer Partnership10th annual meeting, “From Bench to Bedside: Bringing Research Advances to the Clinic, June 1, 2012, Chicago, IL
Invited Speaker, Roswell Park Cancer Institute Science Retreat, “Evidence for Estrogen Signaling in Non-small Cell Lung Cancer, July 20, 2012, Buffalo, NY
Chicago Multidisciplinary Symposium in Thoracic Oncology, September 6-8, 2012, Chicago, IL
91
Shivendra Singh, Ph.D. Professor
International Conference on Frontiers in Carcinogenesis and Cancer Prevention: Scientific Advances and Public Health Initiatives, Bangalore, India. Title: Prostate Cancer Prevention by Dietary Agents, February 16, 2011.
International Conference on Recent Advances in Cancer Research: Bench to Bedside, Central University of Gujrat, Gandhinagar, India. Title: Prostate Cancer Prevention by Isothiocyanates, February 19, 2011.
Marlene and Stewart Greenebaum Cancer center, University of Maryland, Baltimore, MD. Title: Cancer Prevention with Dietary Isothiocyanates, May 15, 2011.
3rd China-US Forum on Frontiers of Cancer Research and 1st International Symposium on Stem Cell and Regenerative Medicine, Zengzhou, China. Title: Proteomics-based Discovery of Biomarkers for Dietary Chemopreventive Agents, August 21, 2011.
International Conference on Natural Products and Cancer Targets: Progress and Promise, Zengzhou, China. Title: Chemoprevention with Garlic Constituent Diallyl Trisulfide, August 25, 2011.
Institute of Materia Medica, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China. Title: Molecular Mechanism of Mammary Cancer Prevention with Benzyl Isothiocyanate, August 26, 2011.
Distinguished Speaker, Texas Tech University Health Sciences Center, Amarillo, TX. Title: Cancer Prevention by Isothiocyanates, October 14, 2011.
International Symposium on Recent Advances in Cancer Research: Therapeutics to Chemoprevention, Central University of Gujrat, Gandhinagar, India. Title: Molecular Mechanisms of Mammary Cancer Prevention with Benzyl Isothiocyanate, February 9, 2012.
Session Speaker, 4th International Conference on Drug Discovery & Therapy, Dubai, UAE. Title: Prostate Cancer Prevention with Bioactive Food Components, February 14, 2012.
Integrative Medicine Program at the University of Texas M. D. Anderson Cancer Center, Houston, TX. Title: Bioactive Food Components and Cancer Chemoprevention, March 20, 2012.
Seoul National University Cancer Research Institute Symposium, Innovative Approaches to Explore Novel Druggable Targets, Seoul, Korea. Title: Molecular Targets of Mammary Cancer Prevention with Dietary Benzyl Isothiocyanate, May 16, 2012.
Robert Sobol, Ph.D. Associate Professor
Insights into base excision repair and response to chemotherapy. DNA Damage and Repair Symposium, Abbott Laboratories, Inc., July 27-28, 2011.
Exploiting NAD-biosynthesis defects in glioma for tumor specific responses. FASEB SUMMER Research Conference – NAD Metabolism and Signaling, Lucca, Italy, September 4-8, 2011.
92
Insights into base excision repair and response to chemotherapy. Molecular Mutagenesis & DNA Repair Unit, Istituto Nazionale Ricerca Cancro; Genoa, Italy, September 9, 2011.
Insights into base excision repair and response to chemotherapy. Institute of Toxicology (Institut für Toxikologie), Universitätsmedizin Mainz; Mainz, Germany, September 12, 2011.
Base excision repair and NAD+ Biosynthesis crosstalk - Understanding and exploiting PARP activation-induced cellular energy modulation. Université Laval, Quebec City, Canada.
DNA repair and NAD+ Biosynthesis crosstalk: Understanding and exploiting PARP activation-induced cellular energy modulation. Department of Biochemistry, Carver College of Medicine, University of Iowa, February 8, 2012.
DNA repair and NAD+ Biosynthesis crosstalk: Understanding and exploiting PARP activation-induced cellular energy modulation. Department of Pharmacology and Pharmaceutical Sciences, University of Southern California, March 23, 2012.
XRCC1 independent recruitment and function of DNA Polß in response to DNA damage and PARP activation. Gordon Research Conference; DNA Damage, Mutation & Cancer, Ventura, California. Session Chair: Role of PARP in Genomic Stability and Cancer Chemotherapy, March 25-29, 2012.
The interplay of PARP1, Polß and XRCC1 in response to DNA Damage. Laboratory of Molecular Pharmacology, Center For Cancer Research, National Cancer Institute, NIH, May 11, 2012.
The interplay of PARP1, Polß and XRCC1 in response to DNA Damage. UNT Health Science Center; College of Pharmacy, May 29, 2012.
Base excision repair, PARP and NAD+ biosynthesis crosstalk in response to DNA damage. 3rd Erling Seeberg Symposium, Trondheim and Ørland, Norway, June 2012.
Exploring the Polß/XRCC1 interface and the regulation of base excision repair. Mutagenesis GRC 2012, Salve Regina College, Newport, RI, August 2012.
The interaction and regulation of Polß, XRCC1 and PARP1 in response to DNA damage in tumor cells. EMS 43rd Annual Meeting, Bellevue, WA, September 9, 2012.
Coordination of DNA polymerase beta, XRCC1 and PARP1 in DNA repair and cell metabolism. NIEHS, Laboratory of Structural Biology Annual Retreat, Chapel Hill, North Carolina, September 17, 2012.
PARP1 - A mediator of genotoxin response pathway crosstalk. University of South Alabama, Mitchell Cancer Institute, Mobile, Alabama, November 8, 2012.
Ben Van Houten, Ph.D. Professor
Genetic Mechanisms of aging and genome maintenance: “Watching bacterial nucleotide excision repair: one molecule at a time” June 26-30 June 2011, Alyeska Hotel Girdwood.
GRC Genetic Toxicology: "Alterations in mitochondrial dynamics affects cell cycle control and genome stability" July 10-15 2011, Ciocco Hotel and Resort, Lucca, Barga Italy.
93
International Melanoma Working Group Meeting: “Metabolic flexibility in melanoma cells: refocusing the Warburg lens”, March 25-27, 2012, Lansdowne, VA
Dr. Jan Vijg, Department of Genetics, Albert Einstein College of Medicine, Yeshiva University, New York, NY, April 4, 2012.
Dr. Tom Begley, College of Nanoscale Science and Engineering, University at Albany, SUNY, Albany, NY, May 4, 2012
6th DNA repair Workshop, “Watching DNA repair one molecule at a time: the use of single molecule techniques to investigate nucleotide excision repair. “ Smolenice Castle, Slovakia, June 3-7, 2012
73rd Harden Conference - Machines on genes II - The central dogma at the interface of biology, chemistry and physics. “The xeroderma pigmentosum group E mutation (K244E) in DDB2 of a UV-damaged DNA-binding protein (UV-DDB) results in DNA sliding and loss of damage binding specificity” St Anne's College, Oxford, UK, August 19-23, 2012
Dr. Antoine van Oijen, University of Groningen, Holland, Aug. 31, 2012
43rd Annual Meeting of the Environmental Mutagen Society. “Watching nucleotide excision repair, one molecule at a time”, Bellevue, Washington Sept 8-12, 2012.
Dr. Knox Van Dyke, Department of Biochemistry, West Virginia University, Morgantown, WV, November 13, 2012. Jean-Pierre Vilardaga, Ph.D. Associate Professor
“Emerging Signaling Properties of the Parathyroid Hormone Receptor: A Family B GPCR Paradigm.” Annual Congress: GPCR in Drug Discovery, Berlin, Germany, March 2011.
Endocrine Grand Round and Endocrine-Metabolism Noon Conferences. University of California at San Francisco and VAMC, 2011.
“Novel Signaling Properties of GPCR.” GPCR in Drug Discovery, 10th Annual Congress, Berlin, Germany, 2012.
Q. Jane Wang, Ph.D.Associate Professor
Center for Aging Research, Basic Medicine Institute, Beijing University , 2012.
FASEB Summer Research Conference, “Lipid Signaling Pathways in Cancer”, Steamboat Springs, CO, 2012.
Institute of Molecular Biology, Three Gorges University Medical College, Yichang, Hubei province, China, 2012.
New Drug Discovery and Development Center, Zhujiang Hospital, Southern Medical University, Guangzhou, Guangdong province, China, 2012.
94
Department of Cell Biology, Southern Medical University, Guangzhou, Guangdong province, China, 2012.
Lin Zhang, Ph.D. Associate Professor
Invited Speaker, “Inhibition of Autophagy through Beclin 1 Cleavage in Chemotherapy Response and Cancer Cell Metabolism” in First Regional Translational Research in Mitochondria, Aging, and Disease Symposia, Pittsburgh, PA, Oct. 31st, 2011.
Invited Seminar, College of Life Sciences, Sichuan University, Chengdu, Sichuan, China, May 16th, 2011.
Invited Seminar, Cancer Institute, The Chinese Academy of Medical Sciences (CAMS)/Peking Union Medical College (PUMC), Beijing, China, May 10th, 2011.
Invited Seminar, Medical College of Wisconsin, Milwaukee, WI, March 23rd, 2011.
Invited Speaker and Session Chair, in Second Tianjin Forum on Tumor Microenvironment, June 22nd-24th, 2012 Nankai University, Tianjin, P.R. China
“Role of PUMA in targeted anticancer therapies” Chengdu Institute of Biology, Chinese Academy of Sciences, June 19th, 2012.
108
Teaching Awards
Donald DeFranco, Ph.D. Professor
Sheldon Adler Award for Innovation in Medical Education
Joan Lakoski, Ph.D. Professor
Member, Academy of Master Educators, 2009-2014
Post-doctoral Fellows
Researchers Title LabImad Al Ghouleh Postdoctoral Associate PaganoVeronica Alonso Postdoctoral Associate FriedmanMarie Lue Antony Postdoctoral Associate SinghJuan Ardura Postdoctoral Associate FriedmanDebra Artim Research Associate DegroatMichelle Barbi De Moura Postdoctoral Associate Van HoutenChandra Sekha Bathula Postdoctoral Associate LeePatricia Boley Postdoctoral Associate KenslerDavid Boone Postdoctoral Associate LeeSylvain Bougoin Postdoctoral Associate VilardagaMegan Brady Postdoctoral Associate JacobMonica Buchanan Postdoctoral Associate ZhangBonita Chan Visiting Research Associate LeeKumar Chandra Kuntal Visiting Research Associate SinghKrishnamoorth Chandrasekhar Research Associate FureyDionysios Chartoumpekis Postdoctoral Associate KenslerDongshi Chen Postdoctoral Associate ZhangDongmei Cheng Research Associate JacksonLesley Colgan Postdoctoral Associate LevitanGabor Csanyi Visiting Research Associate PaganoDavide Del Prete Postdoctoral Associate AltschulerLoreto Egana Basic Science Fellows PaganoQingming Fang Visiting Research Associate Sobol Jr.Susan Farabaugh Postdoctoral Associate LeeMarco Fazzari Postdoctoral Scholar FreemanTimothy Feinstein Postdoctoral Associate VilardagaElise Fouquerel Postdoctoral Associate Sobol Jr.Amy Furda Postdoctoral Associate Van HoutenChenxi Gao Postdoctoral Associate HuJianxia Guo Research Associate EisemanRyan Hartmaier Postdoctoral Associate LeeDaniel Hochbaum Visiting Research Associate LeeSu Hyeong Kim Research Associate SinghCarolyn Kitchens Postdoctoral Associate Sobol Jr.
109
Researchers Title LabJoomin Lee Postdoctoral Associate SinghHua Li Postdoctoral Associate ZhangJianfeng Li Research Associate Sobol Jr.Zhongmin Liu Visiting Research Associate GalbiatiYe Peng Postdoctoral Associate Van HoutenRui Peng Basic Science Fellows ZhangWei Qian Postdoctoral Associate Van HoutenAndres Rodriguez Visiting Research Associate PaganoSanghamitra Sahoo Visiting Research Associate PaganoKozue Sakao Postdoctoral Associate SinghAnuradha Sehrawat Visiting Research Associate SinghMatthew Sikora Postdoctoral Associate OesterreichJing Sun Postdoctoral Scholar ZhangManuj Tandon Research Associate WangJingshan Tong Postdoctoral Associate ZhangKristal Tucker Postdoctoral Scholar LevitanJonathan Verrier Postdoctoral Scholar JacksonDario Vitturi Iglesias Postdoctoral Associate FreemanAvani Vyas Postdoctoral Associate SinghPeng Wang Research Instructor ZhangHong Wang Postdoctoral Associate Van HoutenRebecca Watters Postdoctoral Associate OesterreichVanessa Wehbi Postdoctoral Associate VilardagaSteven Woodcock Research Associate FreemanGonghong Yan Research Associate JiangLi Yang Postdoctoral Associate KenslerYongbei Yu Research Associate DegroatXiulin Zhang Research Associate DegroatXuefeng Zhang Postdoctoral Associate AltschulerQiuhong Zhang Research Associate HershbergerLiyong Zhang Research Associate WangChao-Ming Zhou Research Associate LevitanHuafei Zou Research Associate Galbiati
111
Current Faculty
Primary Faculty Name Academic Title
Daniel AltschulerPalaniappa ArjunanAlessandro BiselloDinara BulgariAlicia CelottoEugenia Cifuentes-PaganoMarsha ColeW. Chet de GroatDonald DefrancoJulie EisemanMelanie FlintBruce FreemanPeter FriedmanWilliam FureyFerruccio GalbiatiStacy GelhausEun-Ryeong HahmPamera HershbergerJing HuYi HuangEdwin JacksonTija JacobYu JiangThomas KenslerNicholas KhooJoan LakoskiAdrian LeeEdwin LevitanZhaohui LiuTatyana MamonovaCarola NeumannYuen-Keng NgSteffi OesterreichPatrick PaganoMichael PalladinoGuillermo RomeroJames RoppoloFrancisco SchopferSruti ShivaJill SiegfriedShivendra SinghRobert Sobol, Jr.Gyun Jee SongLaura StabileBen Van HoutenJean-Pierre Vilardaga
Associate ProfessorResearch InstructorAssociate ProfessorReesarch Assistant ProfessorResearch Assistant ProfessorResearch InstructorResearch Assistant ProfessorDistinguished Professor ProfessorResearch Associate ProfessorResearch Assistant ProfessorProfessor and ChairmanProfessorProfessorAssociate ProfessorResearch Assistant ProfessorResearch InstructorResearch Assistant ProfessorAssistant ProfessorResearch Assistant ProfessorProfessorAssistant ProfessorAssociate ProfessorProfessorResearch Assistant ProfessorProfessorProfessorProfessorResearch InstructorResearch InstructorVisiting Associate ProfessorResearch InstructorProfessorProfessorAssociate ProfessorAssociate ProfessorResearch Assistant ProfessorResearch Assocaite ProfessorAssistant ProfessorProfessorProfessorAssociate ProfessorResearch Assistant ProfessorResearch Assistant ProfessorProfessorAssociate Professor
112
NameDaniela Volonte-GalbiatiNobunao WakabayashiBin WangQ. Jane WangLin Zhang
Academic TitleResearch Assistant ProfessorResearch Assistant ProfessorResearch Assistant ProfessorAssociate ProfessorAssociate Professor
Secondary Faculty NameSusan Amara
PositionProfessor
Primary DepartmentNeurobiology
Carolyn AndersonChristopher BakkenistAaron BarchowskyP. Michael BauerLori BirderRobert BranchClifton CallawayJun ChenNancy DavidsonJohn FernstromMitchell FinkGerald GebhartJennifer GrandisGregg HomanicsKaikobad IraniJeffrey IsenbergDaniel JohnsonValerian KaganAnthony KanaiEric KelleyThomas KleymanJohn LazoYong LeeChester MathisMark NicholsJerome ParnessJames PerelMichael PezzoneBruce PittRichard SteinmanDandan SunChangfeng TaiPei TangMargaret TarpeySufi ThomasGonzalo TorresZhou WangWen XieYan XuNaoki Yoshimura
ProfessorAssistant ProfessorProfessorAssistant ProfessorAssociate ProfessorProfessorProfessorProfessorProfessorProfessorProfessorProfessorDistinguished ProfessorProfessorAssociate ProfessorAssociate ProfessorProfessorProfessorAssociate ProfessorInstructorProfessorProfessor EmeritusProfessorProfessorLecturerVisiting ProfessorProfessor EmeritusAssociate ProfessorProfessorAssociate ProfessorProfessorAssistant ProfessorProfessorProfessorAssistant ProfessorAssociate ProfessorProfessorProfessorProfessorProfessor
RadiologyRadiation OncologyEnv. Occup. HealthSurgeryMedicineMedicineEmergency MedicineNeurologyMedicinePsychiatry
AnesthesiologyOtolaryngologyAnesthesiologyMedicineMedicineMedicineEnv. Occup. HealthMedicineAnesthesiologyMedicine
SurgeryRadiology
AnesthesiologyPsychiatryMedicineEnv. Occup. HealthMedicineNeurologyUrologyAnes. & Struct. Biol.AnesthesiologyOtolaryngologyNeurobiologyUrologyPharm. SciencesAnesthesthesiologyUrology
113
New Faculty
Carola Neumann, M.D., Visiting Associate Professor
Membership in Professional Societies
Bruce Freeman, Ph.D. Professor and Chair American Association for the Advancement of Science American Chemical Society American Heart Association American Physiological Society American Society for Cell and Molecular Biology American Thoracic Society Biochemical Society Society for Free Radical Biology and Medicine
Daniel Altschuler, Ph.D. Associate Professor The Endocrine Society
Palaniappa Arjunan, Ph.D. Research Instructor American Crystallographic Association Pittsburgh Diffraction Society
Paul Baker, Ph.D. Research Assistant Professor American Diabetes Association Society for Free Radical Biology and Medicine
Dr. Alessandro Bisello Associate Professor American Society for Bone & Mineral Research Endocrine Society American Heart Association
Dinara Bulgari, Ph.D. Research Instructor Society for Neuroscience
Alicia Celotto, Ph.D. Research Assistant Professor Genetics Society of America Society of Neuroscience ASPET
114
M. Eugenia Cifuentes-Pagano, Ph.D. Instructor American Cancer Society
Marsha Cole, Ph.D. Research Instructor American Chemical Society Society for Free Radical Biology and Medicine
Donald DeFranco, Ph.D. Professor American Association for the Advancement of Science (AAAS) Endocrine Society Society for Neuroscience
W. Chet de Groat, Ph.D. Distinguished Professor Rho Chi Pharmaceutical Honor Society Philadelphia Physiological Society American Association for the Advancement of Science Sigma Xi American Society for Pharmacology and Experimental Therapeutics Society for Neuroscience Pittsburgh Neuroscience Society New York Academy of Sciences Urodynamics Society International Brain Research Organization American Gastroenterological Association International Medical Society of Paraplegia Society for Basic Urologic Research Mid-Atlantic Pharmacology Society American Motility Society International Continence Society The American Autonomic Society The Dana Alliance for Brain Initiatives International Society for Autonomic Neuroscience (Member of Executive Committee) International Spinal Cord Society
Julie Eiseman, Ph.D. Research Associate Professor American Association for Cancer Research FASEB American Association for the Advancement of ScienceSociety of Toxicology Women in Cancer Reseach (now part ofAACR) SPIE
Melanie Flint, Ph.D. Research Assistant Professor Society of Toxicology
115
American Society for Cancer Research Psychoneuroimmunology Research Society
Peter Friedman, Ph.D. Professor American Physiological Society American Society for Biochemistry and Molecular Biology American Society for Bone and Mineral Research American Society of Pharmacology & Experimental Therapeutics Endocrine Society Salt & Water Club Society of General Physiologists British Society for Endocrinology
William Furey, Ph.D. Professor American Crystallographic Association Pittsburgh Diffraction Society New York Academy of Sciences American Association for the Advancement of Science
Ferruccio Galbiati, Ph.D. Associate Professor American Society of Pharmacology & Experimental Therapeutics American Society of Cell Biology American Physiological Society American Association for Cancer Research Stacy Gelhaus, Ph.D. Research Assistant Professor American Association for the Advancement of Science American Society for Mass Spectrometry American Chemical Society National Postdoctoral Association
Eun-Ryeong Hahm, Ph.D. Research Instructor American Association for Cancer Research
Pamela Hershberger, Ph.D. Research Assistant Professor American Association for Cancer Research National Lung Cancer Partnership
Jing Hu, Ph.D. Assistant Professor American Association for Cancer Research
116
Yi Huang, M.D., Ph.D. Research Assistant Professor American Association for Cancer Research
Edwin Jackson, Ph.D. Professor American Heart Association American Society for Pharmacology and Experimental Therapeutics Council for High Blood Pressure Research
Tija Jacob, Ph.D.Assistant Professor American Society for Pharmacology and Experimental Therapeutics Society of General Physiologists Society for Neuroscience
Yu Jiang, M.D., Ph.D. Associate Professor American Society for Microbiology American Society for Pharmacology & Experimental Therapeutics American Society of Genetics
Thomas Kensler, Ph.D. Professor American Association for the Advancement of Science American Association for Cancer Research Society of Toxicology American Society for Pharmacology and Experimental Therapeutics Oxygen Society American Chemical Society: Division of Chemical Toxicology
Nicholas Khoo, Ph.D. Research Assistant Professor Society for Free Radical Biology and Medicine Biomedical Engineering Society South East Lipid Research
Joan Lakoski, Ph.D. Professor American Association for the Advancement of Science American Society for Pharmacology & Experimental Therapeutics The Endocrine Society International Society for Developmental Neuroscience International Society of Neuroendocrinology Serotonin Club Sigma Xi Society for Neuroscience American Endocrine Society Women Executives in Science and Healthcare Society for Executive Leadership in Medicine
117
National Postdoctoral Association Association for Clinical Research Training Society for Clinical and Translational Science
Adrian Lee, Ph.D. Professor American Association for Cancer Research International Society of IGF Research The Endocrine Society American Association for the Advancement of Science American Society for Molecular Biology and Molecular Biology
Edwin Levitan, Ph.D. Professor Society for Neuroscience Biophysical Society AHA basic science council Society of General Physiologists
Carola Neumann, M.D. Visiting Associate Professor Society for Free Radical Biology and Medicine American Association for Cancer Research
Steffi Oesterreich, Ph.D. Professor American Association for Cancer Research The Endocrine Society Women in Endocrinology Women in Cancer Research American Society for Biochemistry and Molecular Biology American Society for Pharmacology and Experimental Therapeutics
Patrick Pagano, Ph.D. Professor Basic Science Council Circulation Council Council for High Blood Pressure Research American Physiological Society American Association for the Advancement of Science Society for Free Radical Biology and Medicine International Society for Free Radical Research
Michael Palladino, Ph.D. Associate Professor Genetics Society of America Society for Neuroscience American Society for Pharmacology and Experimental Therapeutics Pittsburgh Neuroscience Society
118
Guillermo Romero, Ph.D. Associate Professor American Society for Pharmacology and Experimental Therapeutics American Diabetes Association American Society of Cell Biology
James Roppolo, Ph.D. Research Assistant Professor American Association for the Advancement of Science Society for Neuroscience The New York Academy of Sciences
Francisco Schopfer, Ph.D. Research Associate Professor American Heart Association Society for Free Radical Biology and Medicine
Sruti Shiva, Ph.D. Assistant Professor Society for Free Radical Biology (Elected to Council 2010) Nitric Oxide
Jill Siegfried, Ph.D. Professor American Association for Cancer Research American Association for the Advancement of Science International Association for the Study of Lung CancerAmerican Society for Pharmacology and Experimental Therapeutics Society for Executive Leadership in Academic Medicine National Lung Cancer Partnership
Shivendra Singh, Ph.D. Professor American Society for Pharmacology and Experimental Therapeutics American Society for Biochemistry and Molecular Biology Society of Toxicology International Society for the Study of Xenobiotics American Association for Cancer Research
Robert Sobol, Ph.D. Associate Professor American Association for the Advancement of Science American Association for Cancer Research American Society for Microbiology American Society for Cell Biology Environmental Mutagen Society American Chemical Society International Society for Cell & Gene Therapy of Cancer American Society for Pharmacology and Experimental Therapeutics
119
Gyun Jee Song, Ph.D. Research Assistant Professor American Heart Association
Laura Stabile, Ph.D. Research Assistant Professor National Lung Cancer Partnership American Association for Cancer Research Association for Women in Science
Bennett Van Houten, Ph.D. Professor Environmental Mutagen Society American Association for Cancer Research American Chemical Society American Society for Pharmacology and Experimental Therapeutics
Jean-Pierre Vilardaga, Ph.D. Associate Professor Endocrine Society Biophysical Society American Society for Pharmacology and Experimental Therapeutics American Society for Bone and Mineral Research
Andreas Vogt, Ph.D. Research Assistant Professor American Association for Cancer Research American Chemical Society Deutsche Pharmazeutische Gesellschaft Society of Biomolecular Screening
Daniela Volonte, Ph.D. Research Assistant Professor American Society of Cell Biology American Association for Cancer Research
Nobunao Wakabayashi, Ph.D. Research Assistant Professor Japan Society for Bioscience, Biotechnology and Agrochemistry The Molecular Biology Society of Japan The Japan Biochemical Society American Association for Cancer Research
Bin Wang, Ph.D.Research Assistant ProfessorAmerican Society for Bone and Mineral Research
Q. Jane Wang, Ph.D. Associate Professor American society for Pharmacology and Experimental Therapeutics
120
American Association for Cancer Research American Association for the Advancement of Science
Dong Xiao, Ph.D. Research Instructor American Association for Cancer Research
Lin Zhang, Ph.D. Associate Professor American Association for Cancer Research American Association for the Advancement of Science American Society for Pharmacology and Experimental Therapeutics
122
Bruce Freeman, Ph.D. Professor and Chair
Rudolph V, TK Rudolph, FJ Schopfer, G Bonacci, SR Woodcock, MP Cole, PRS Baker, R Ramani and BAFreeman. Endogenous generation and protective effects of nitro-fatty acids in a murine model of focal cardiac ischaemia and reperfusion. Cardiovascular Research 85:155-166, 2010.
Khoo NKH, V Rudolph, MP Cole, F Golin-Bisello, FJ Schopfer, SR Woodcock, C Batthyany and BAFreeman. Activation of vascular endothelial nitric oxide synthase and heme oxygenase-1 expression by electrophilic nitro-fatty acids. Free Radical Biology & Medicine 48:230-239, 2010.
Kelley EE, NK Khoo, NJ Hundley, UZ Malik, BA Freeman and MM Tarpey. Hydrogen peroxide is the major oxidant product of xanthine oxidase. Free Radic Biol Med 48:493-498, 2010.
Borniquel S, EÅ Jansson, MP Cole, BA Freeman and JO Lundberg. Nitrated oleic acid up-regulates PPARγ and attenuates experimental inflammatory bowel disease. Free Radic Biol Med 48:499-505, 2010.
Groeger AL and BA Freeman. Signaling actions of electrophiles: Anti-inflammatory therapeutic candidates. Molecular Interventions 10:39-50, 2010.
Rudolph V, RP Andríe, TK Rudolph, K Friedrichs, A Klinke, B Hirsch-Hoffmann, AP Schwoerer, D Lau, XM Fu, K Klingel, K Sydow, M Didíe, A Seniuk, E-C von Leitner, K Szoecs, JW Schrickel, H Treede, U Wenzel, T Lewalter, G Nickenig, W-H Zimmermann, T Meinertz, RH Böger, H Reichenspurner, BA Freeman, T Eschenhagen, H Ehmke, SL Hazen, S Willems and S Baldus. Myeloperoxidase acts as a profibrotic mediator of atrial fibrillation. Nature Medicine 16:470-474, 2010.
Groeger AL, C Cipollina, MP Cole, SR Woodcock, G Bonacci, TK Rudolph, V Rudolph, BA Freeman and FJ Schopfer. Cyclooxygenase-2 generates anti-inflammatory mediators from omega-3 fatty acids. Nature Chemical Biology 6:433-441, 2010.
Khoo NKH and BA Freeman. Electrophilic nitro-fatty acids: Anti-inflammatory mediators in the vascular compartment. Current Opinion in Pharmacology 10:179-184, 2010.
Schopfer FJ, MP Cole, AL Groeger, C-S Chen, NKH Khoo, SR Woodcock, F Golin-Bisello, UN Motanya, Y Li, J Zhang, MT Garcia-Barrio, TK Rudolph, V Rudolph, G Bonacci, PRS Baker, HE Xu, CI Batthyany, YE Chen, TM Hallis and BA Freeman. Covalent peroxisome proliferator-activated receptor γ adduction by nitro-fatty acids: Selective ligand activity and anti-diabetic signaling actions. Journal of Biological Chemistry 285:12321-12333, 2010.
Sculptoreanu A, FA Kullman, DE Artim, FA Bazley, F Schopfer, S Woodcock, BA Freeman and WC de Groat. Nitro-oleic acid inhibits firing and activates TRPV1- and TRPA1-mediated inward currents in dorsal root ganglion neurons from adult male rats. Journal of Pharmacology and Experimental Therapeutics 333:883-895, 2010.
Koenitzer JR and BA Freeman. Redox signaling in inflammation: Interactions of endogenous electrophiles and mitochondria in cardiovascular disease. Annals of the New York Academy of Sciences 1203:45-52, 2010.
Rudolph TK, V Rudolph, MM Edreira, MP Cole, G Bonacci, FJ Schopfer, SR Woodcock, A Franek, M Pekarova, NKH Khoo, AH Hasty, S Baldus and BA Freeman. Nitro-fatty acids reduce atherosclerosis in apoplipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol 30:938-945, 2010.
Zhang J, L Villacorta, L Chang, Z Fan, M Hamblin, T Zhu, CS Chen, MP Cole, FJ Schopfer, CX Deng, MT Garcia-Barrio, Y-H Feng, BA Freeman and YE Chen. Nitro-oleic acid inhibits angiotensin II-induced hypertension. Circ Res 107:540-548, 2010.
123
Zhang J, L Villacorta, L Chang, Z Fan, M Hamblin, T Zhu, CS Chen, MP Cole, FJ Schopfer, CX Deng, MT Garcia-Barrio, Y-H Feng, BA Freeman and YE Chen. Nitro-oleic acid inhibits angiotensin II-induced hypertension. Circ Res 107:540-548, 2010.
Tsujita T, L Li, H Nakajima, N Iwamoto, Y Nakajima-Takagi, K Ohashi, K Kawakami, Y Kumagai, BAFreeman, M Yamamoto and M Kobayashi. Nitro-fatty acids and cyclopentenone prostaglandins share strategies to activate the Keap1-Nrf2 system: A study using green fluorescent protein transgenic zebrafish. Genes to Cells 16:46-57, 2011.
Bonacci G, FJ Schopfer, CI Batthyany, TK Rudolph, V Rudolph, NKH Khoo and BA Freeman. Electrophilic fatty acids regulate matrix metalloproteinase activity and expression. Journal of Biological Chemistry 18:16074-16081, 2011.
Kansanen E, G Bonacci, FJ Schopfer, SM Kuosmanen, KI Tong, H Leinonen, SR Woodcock, M Yamamoto, C Carlberg, S Ylä-Herttuala, BA Freeman and A-L Levonen. Electrophilic nitro-fatty acids activate NRF2 by a KEAP1 cysteine 151-independent mechanism. Journal of Biological Chemistry 286:14019-14027, 2011.
Malik UZ, NJ Hundley, G Romero, R Radi, BA Freeman, MM Tarpey and EE Kelley. Febuxostat inhibition of endothelial-bound XO: Implications for targeting vascular ROS production. Free Radical Biology and Medicine 51:179-184, 2011.
Bonacci G, EK Asciutto, SR Woodcock, SR Salvatore, BA Freeman and FJ Schopfer. Gas-phase fragmentation analysis of nitro-fatty acids. J Am Soc Mass Spectrom 22:1534-1551, 2011.
Artim DE, F Bazely, SL Daugherty, A Sculptoreanu, KB Koronowski, FJ Schopfer, SR Woodcock, BA Freeman and WC de Groat. Nitro-oleic acid targets transient receptor potential (TRP) channels in capsaicin sensitive afferent nerves of rat urinary bladder. Exp Neurol 232:90-99, 2011.
Guo CJ, FJ Schopfer, L Gonzales, P Wang, BA Freeman and AJ Gow. Atypical PKCzeta transduces electrophilic fatty acid signaling in pulmonary epithelial cells. Nitric Oxide 25:366-372, 2011.
Alastalo TP, M Li, V de Jesus Perez, D Pham, H Sawada, JK Wang, M Koskenvuo, L Wang, BA Freeman,HY Chang and M Rabinovitch. Disruption of PPARγ/β-catenin-mediated regulation of apelin impairs BMP-induced mouse and human pulmonary arterial EC survival. J Clin Invest 121:3735-3746, 2011.
Schopfer FJ, C Cipollina and BA Freeman. Formation and signaling actions of electrophilic lipids. Chem Rev 111:5997-6021, 2011.
Al Ghouleh I, NKH Khoo, UG Knaus, KK Griendling, RM Touyz, VJ Thannickal, A Barchowsky, WM Nauseef, EE Kelley, PM Bauer, V Darley-Usmar, S Shiva, E Cifuentes-Pagano, BA Freeman, MT Gladwin and PJ Pagano. Oxidases and peroxidases in cardiovascular and lung disease: New concepts in reactive oxygen species signaling. Free Radic Biol Med 51:1271-1288, 2011.
Shores DR, DG Binion, BA Freeman and PRS Baker. New insights into the role of fatty acids in the pathogenesis and resolution of inflammatory bowel disease. Inflamm Bowel Dis 17:2192-2204, 2011.
Fukuto JM, SJ Carrington, DJ Tantillo, JG Harrison, LJ Ignarro, BA Freeman, A Chen and DA Wink. Small molecule signaling agents: The integrated chemistry and biochemistry of nitrogen oxides, oxides of carbon, dioxygen, hydrogen sulfide and their derived species. Chemical Research in Toxicology 25:769-793, 2012.
Khoo NK, N Cantu-Medellin, JE Devlin, CM St. Croix, SC Watkins, AM Fleming, HC Champion, RP Mason, BA Freeman and EE Kelley. Obesity-induced tissue free radical generation: An in vivo immuno-spin trapping study. Free Radic Biol Med 52:2312-2319, 2012.
124
Nishida M, T Sawa, N Kitajima, K Ono, H Inoue, H Ihara, H Motohashi, M Yamamoto, M Suematsu, H. Kurose, A van der Vliet, BA Freeman, T Shibata, K Uchida, Y Kumagai and T Akaike. Hydrogen sulfide anion regulates redox signaling via electrophile sulfhydration. Nature Chemical Biology 8:714-724, 2012.
Rothstein SN, JE Kay, FJ Schopfer, BA Freeman and SR Little. A retrospective mathematical analysis of controlled release design and experimentation. Molecular Pharmaceutics 9:3003-3011, 2012.
Bonacci G, PRS Baker, SR Salvatore, D Shores, NKH Khoo, JR Koenitzer, DA Vitturi, SR Woodcock, F Golin-Bisello, MP Cole, S Watkins, C St. Croix, CI Batthyany, BA Freeman and FJ Schopfer. Conjugated linoleic acid is a preferential substrate for fatty acid nitration. Journal of Biological Chemistry 287:44071-44082, 2012.
Daniel Altschuler, Ph.D. Associate Professor
Zuo H, Gandhi M, Edreira MM, Hochbaum D, Nimgaonkar VL, Zhang P, DiPaola J, Evdokimova V, Altschuler DL and Nikiforov YE. Downregulation of Rap1GAP through promoter hypermethylation and loss of heterozygosity promotes invasion and progression of thyroid tumors. Cancer Research 70:1389-1397, 2010.
Hochbaum D, G Barila, F Ribeiro-Neto and DL Altschuler. Radixin assembles cAMP effectors Epac and PKA into a functional cAMP compartment: Role in cAMP-dependent cell proliferation. J Biol Chem 286:859-866, 2011.
Wang B, Means CK, Yang Y, Mamonova T, Bisello A, Altschuler DL, Scott JD, Friedman PA. Ezrin-anchored protein kinase A coordinates phosphorylation-dependent disassembly of a NHERF1 ternary complex to regulate hormone-sensitive phosphate transport (2012) J Biol Chem. Jul 13;287(29): 24148-63.
Palaniappa Arjunan, Ph.D.Research Instructor
Nemeria N, Arjunan P, Chandrasekhar K, Mossad M, Tittmann K, Furey W and Jordan F. Communication between thiamin cofactors in the Escherichia coli pyruvate dehyudrogenase complex E1 component active centers: Evidence for a direct pathway between the 4’-aminopyrimidine N1’ atoms. J Biol Chem 285:11197-11209, 2010.
Paul Baker, Ph.D. Research Assistant Professor
Rudolph V, TK Rudolph, FJ Schopfer, G Bonacci, SR Woodcock, MP Cole, PRS Baker, R Ramani and BA Freeman. Endogenous generation and protective effects of nitro-fatty acids in a murine model of focal cardiac ischaemia and reperfusion. Cardiovascular Research 85:155-166, 2010.
Schopfer FJ, MP Cole, AL Groeger, C-S Chen, NKH Khoo, SR Woodcock, F Golin-Bisello, UN Motanya, Y Li, J Zhang, MT Garcia-Barrio, TK Rudolph, V Rudolph, G Bonacci, PRS Baker, HE Xu, CI Batthyany, YE Chen, TM Hallis and BA Freeman. Covalent peroxisome proliferator-activated receptor γ adduction by nitro-fatty acids: Selective ligand activity and anti-diabetic signaling actions. Journal of Biological Chemistry 285:12321-12333, 2010.
Schopfer FJ, Cole MP, Groeger AL, Chen CS, Khoo NK, Woodcock SR, Golin-Bisello F, Motanya UN, Li Y, Zhang J, Garcia-Barrio MT, Rudolph TK, Rudolph V, Bonacci G, Baker PRS, Xu HE, Batthyany CI, Chen YE, Hallis TM and Freeman BA. Covalent peroxisome proliferator-activated receptor gamma binding by nitro-fatty acids: Endogenous ligands act as selective modulators. J Biol Chem 285:12321-12333, 2010.
125
Shores DR, DG Binion, BA Freeman and PRS Baker. New insights into the role of fatty acids in the pathogenesis and resolution of inflammatory bowel disease. Inflamm Bowel Dis, 2011 [In Press].
Dr. Alessandro Bisello Associate Professor
Horwitz MJ, Tedesco MB, Garcia-Ocana A, Sereika SM, Prebehala L, Bisello A, Hollis BW, Gundberg CM and Stewart AF. Parathyroid hormone-related protein for the treatment of postmenopausal osteoporosis: Defining the maximal tolerable dose. J Clin Endocrinol Metabl 95:1279-1287, 2010.
Song GJ, S Barrick, KL Leslie, B Sicari, NM Fiaschi-Taesch and A Bisello. EBP50 inhibits the anti-mitogenic action of the parathyroid hormone type 1 receptor in vascular smooth muscle cells. J Mol Cell Cardiol 49:1012-1021, 2010.
Murage EN, G Gao, A Bisello and JM Ahn. Development of potent glucagon-like peptide-1 agonists with high enzyme stability via introductions of multiple lactam bridges. J Med Chem 53:6412-6420, 2010.
Horwitz MJ, Tedesco MB, Sereika SM, Prebehala L, Gundberg CM, Hollis BW, Bisello A, Garcia-Ocaña A, Carneiro RM, Stewart AF. A seven day continuous infusion of PTH or PTHrP suppresses bone formation and uncouples bone turnover. J Bone Miner Res. 2011 26(9):2287-97
Williams K, Abanquah D, Joshi-Gokhale S, Otero A, Lin H, Guthalu NK, Zhang X, Mozar A, Bisello A, Stewart AF, Garcia-Ocaña A, Vasavada RC. Systemic and acute administration of parathyroid hormone-related peptide(1-36) stimulates endogenous beta cell proliferation while preserving function in adult mice.Diabetologia. 2011 54(11):2867-77.
Alonso V, Magyar CE, Wang B, Bisello A, Friedman PA. Ubiquitination-deubiquitination balance dictates ligand-stimulated PTHR sorting. J Bone Miner Res. 2011 26(12):2923-34.
Song GJ, Barrick S, Leslie KL, Bauer PM, Alonso V, Friedman PA, Fiaschi-Taesch NM, Bisello A. The Scaffolding Protein EBP50 Promotes Vascular Smooth Muscle Cell Proliferation and Neointima Formation by Regulating Skp2 and p21cip1. Arterioscler Thromb Vasc Biol. 2012 32(1):33-41.
Wang B, Means CK, Yang Y, Mamonova T, Bisello A, Altschuler DL, Scott JD, Friedman PA. Ezrin-anchored protein kinase A coordinates phosphorylation-dependent disassembly of a NHERF1 ternary complex to regulate hormone-sensitive phosphate transport. J Biol Chem. 2012 287(29):24148-63.
Gustavo Bonacci, Ph.D. Research Instructor
Rudolph TK, V Rudolph, MM Edreira, MP Cole, G Bonacci, FJ Schopfer, SR Woodcock, A Franek, M Pekarova, NK Khoo, AH Hasty, S Baldus and BA Freeman. Arterioscler Thromb Vasc Biol 30:938-945, 2010. Groeger AL, C Cipollina, MP Cole, SR Woodcock, G Bonacci, TK Rudolph, V Rudolph, BA Freeman and FJ Schopfer. Cyclooxygenase-2 generates anti-inflammatory mediators from omega-3 fatty acids. Nat Chem Biol 6:433-441, 2010.
Cáceres LC, GR Bonacci, MC Sánchez and GA Chiabrando. Activated alpha-2 macroglobulin induces matrix metalloproteinase 9 expression by low density lipoprotein receptor-related protein 1 through MAPK-ERK1/2 and NF-kappaB activation in macrophage derived cell lines. J Cell Biochem 15:607-617, 2010.
126
Dinara Bulgari, Ph.D. Research Instructor
Levitan ES and Shakiryanova D. Imaging neuropeptide release and signaling in the Drosophila neuromuscular junction with GFP. Drosophila Neurobiology. A Cold Spring Laboratory Course Manual edited by B Zhang, M Freeman and S Waddell. CSHL Press, 2010.
Shakiryanova D, Zettel GM, Gu T, Hewes RS and Levitan ES. Synaptic neuropeptide release induced by octopamine without Ca2+ entry into the nerve terminal. Proc Natl Acad Sci USA 11:4477-4481, 2011.
Levitan ES and Shakiryanova D. Imaging neuropeptide release in the Drosophila neuromuscular junction (NMJ). Cold Spring Harb Protoc, 2011
Levitan ES and Shakiryanova D, Imaging the Drosophila neuromuscular junction (NMJ): Basic optical principles and equipment. Cold Spring Harb Protoc, 2011.
Eugenia Cifuentes-Pagano, Ph.D. Research Instructor
Ardanaz N, Yang XP, Cifuentes ME, Haurani MJ, Jackson KW, Liao TD, Carretero OA and Pagano PJ. Lack of glutathione peroxidase 1 accelerates cardiac-specificity hypertrophy and dysfunction in angiotensin II hypertension. Hypertension 55:116-123, 2010.
Csanyi, G.*, Cifuentes-Pagano, E.*, Al Ghouleh, I., Ranayhossaini, D.J., Egana, L., Lopes, L.R., Jackson, H.M., Kelley, E.E., and Pagano, P.J. Nox2 B-loop Peptide Inhibits Nox2 but not Nox1 or Nox4 Oxidase. Free Radic Biol Med. 51(6):1116-25. 2011 (*:These authors contributed equally to this work).
Al Ghouleh I, Khoo NK, Knaus UG, Griendling KK, Touyz RM, Thannickal VJ, Barchowsky A, Nauseef WM, Kelley EE, Bauer PM, Darley-Usmar V, Shiva S, Cifuentes-Pagano E, Freeman BA, Gladwin MT, Pagano PJ. Oxidases and peroxidases in cardiovascular and lung disease: new concepts in reactive oxygen species signaling. Free Radic Biol Med. 51(7):1271-88. 2011
Cifuentes-Pagano, E., Csanyi, G., and Pagano, P.J. NADPH oxidase inhibitors: A Decade of Discovery from Nox2ds to HTS. Cell Mol Life Sci. 69(14):2315-25. 2012
Grubisha M., Cifuentes-Pagano, E., Hammes S., and DeFranco, DB. A local paracrine and endocrine network involving TGFβ, Cox-2, ROS and estrogen receptor beta influences reactive stromal cell regulation of prostate cancer cell motility. Mol Endocrinol. 26(6):940-54. 2012 Epub 2012 May 16
Marcie Cole, Ph.D. Research Instructor
Borniquel S, Jansson EǺ, Cole MP, Freeman BA, and Lundberg JO. Nitrated oleic acid up-regulates colonic peroxidsome proliferator-activated receptor gamma and attenuates experimental inflammatory bowel disease. Free Radic Biol Med, 48(4):499-505, 2010.
Schopfer FJ*, Cole MP*, Groeger A*, Chen C-S, Woodcock S, Golin-Bisello F, Motanya UN, , Khoo NH, Rudolph T, Rudolph V, Hallis TM, Bonacci G, Xu HE, Chen YE, Agarwal A, and Freeman BA. Covalent peroxisome proliferator-activated receptor adduction by nitro-fatty acids: Selective Ligand Activity and Anti-Diabetic Signaling Actions. J Biol Chem 285:12321-12333, 2010. *contributed equally
127
Joshi G, Aluise CD, Cole MP, Sultana R, Pierce WM, Vore M, St. Clair DK, and Butterfield DA. Alterations in brain antioxidant enzymes and redox proteomic identification of oxidized brain proteins induced by the anti-cancer drug adriamycin: implications for oxidative stress-mediated chemobrain. Neuroscience 166:796-807, 2010.
Rudolph, TK, Rudolph V, Edreira MM, Bonacci G, Schopfer FJ, Woodcock SR, Cole MP, Khoo NKH, Hasty A, and Freeman BA. Nitro-fatty acids reduce atherosclerosis in apoE-/- mice. Arterioscler Thromb Vasc Biol 30:938-945, 2010.
Groeger AL, Cipollina C, Cole MP, Woodcock SR, Bonacci G, Rudolph TK, Rudolph V, Freeman BAand Schopfer FJ. Cyclooxygenase-2 generates anti-inflammatory mediatorsfrom omega-3 fatty acids. Nat Chem Biol 6:433-441, 2010.
Zhang J, L Villacorta, L Chang, Z Fan, M Hamblin, T Zhu, CS Chen, MP Cole, FJ Schopfer, CX Deng, MT Garcia-Barrio, YH Feng, BA Freeman and YE Chen. Nitro-oleic acid inhibits angiotensis II-induced hypertension. Circ Res 107:540-548, 2010.
Jung S-B, C-S Kim, A Naqvi, T Yamamori, I Mattagajasingh, TA Hoffman, MP Cole, A Jumar, J DeRicco, BH Jeon and K Irani. Histone deacetylase-3 antagonizes aspirin-stimulated endothelial nitric oxide production by reversing aspirin-induced lysine acetylation of endothelial nitric oxide synthase. Circ Res 107:877-887, 2010.
Donald DeFranco, Ph.D. Professor
Caltagarone J, Hamilton RL, Murdoch G, DeFranco DB, Jing Z and Bowser R. Analysis of paxillin and hic-5expression and distribution in the hippocampus of control and Alzheimer’s disease subjects. J Neuropath Exp Neurol 69:356-371, 2010.
Sen A, K O’Malley, Z Wang, GV Raj, DB DeFranco and S Hammes. Paxillin regulates androgen- and epidermal growth factor-induced MAPK signaling and cell proliferation in prostate cancer cells. J Biol Chem 285:28787-28785, 2010.
Grubisha, MJ, Cifuentes ME, Hammes, S, DeFranco DB. A local paracrine and endocrine network involving TGFβ, Cox-2, ROS and estrogen receptor beta impacts reactive stromal cell regulation of prostate cancer cell motility. Mol. Endocrinol. 26, 940-954, 2012.
Sen A, De Castro I, DeFranco DB, Deng F-M, Jonathan Melamed3, Kapur J, Raj GV, Rossi R, Stephen R Hammes SR. (2012) Paxillin regulates prostate cancer proliferation by serving as a liaison between extranuclear kinase signaling and intranuclear transcription. J Clin. Invest. 122, 2469-2481.
Patel VP, DeFranco DB, Chu CT. (2012) Altered transcription factor trafficking in oxidatively stressed neuronal cells. Biochem. Biophys. ACTA 1822, 1773-1782.
W. Chet de Groat, Ph.D. Distinguished Professor
Cheng CL and de Groat WC. The role of 5HT1A receptors in the control of lower urinary tract functions in anesthetized rats. Am J Physiol Renal Physiol, 289: F771-F778, 2010.
128
Saitoh C, Yokoyama H, Chancellor MB, de Groat WC and Yoshimura N. Comparison of voiding function and nociceptive behavior in two rat models of cystitis induced by cyclophosphamide or acetone. Neurourol Urodyn, 29:501-505, 2010.
Kita M, Yunoki T, Takimoto K, Miyazato M, Kita K, de Groat WC, Kakizaki H and Yoshimura N. Effects of bladder outlet obstruction on properties of Ca2+ activated K+ channels in rat bladder. American Journal of Physiology- Regulatory, Integrative and Comparative Physiology 298:R1310-1319, 2010.
Buyuknacar HSG, Gocmen C, de Groat WC, Kumcu EK, Wu H-Y and Onder S. Differential effect of l-cystine in isolated whole bladder preparations from neonatal and adult rats. Journal of Pharmacology and Experimental Therapeutics 333:228-235, 2010.
Hezel MP, de Groat WC and Galbiati F. Caveolin-3 promotes nicotinic acetylcholine receptor clustering and regulates neuromuscular junction activity. Molecular Biology of the Cell, 21: 302-310, 2010.
Sculptoreanu A, Kullmann FA, Artim DE, Bazley FA, Schopfer F, Woodcock S, Freeman BA and de Groat WC. Nitro-oleic acid inhibits firing and activates TRPV1- and TRPA1-mediated inward currents in DRG neurons from adult male rats. Journal of Pharmacology and Experimental Therapeutics 333:883-895 2010.
Tai C-F, Shen B, Chen M, Wang J, Liu H, Roppolo JR and de Groat W.C. Suppression of bladder overactivity by activation of somatic afferent nerves in the foot. BJU Int 107:303-309, 2011.
Chen M, B Shen, J Wang, H Liu, JR Roppolo, WC de Groat and C-F Tai. Influence of naloxone on inhibitory pudendal-to-bladder reflex in cats. Experimental Neurology 224:282-291, 2010.
Kitta T, M Miyazato., MB Chancellor, WC de Groat, K Nonomura and N Yoshimura. α2 –Adrenoceptor blockade potentiates the effect of duloxetine on sneeze induced urethral continence reflex in rats. The Journal of Urology 184:762-768, 2010.
Miyazato M, K Sugaya, S Saito, MB Chancellor, WF Goins, JR Goss, WC de Groat, JC Glorioso and NYoshimura. Suppression of detrusor-sphincter dyssynergia by herpes simplex virus vector dediated gene delivery of glutamic acid decarboxylase in spinal cord injured rats. The Journal of Urology 184:1204-1210, 2010.
Prantil-Baun R, WC de Groat, M Miyazato, MB Chancellor, N Yoshimura and DA Vorp. Adrenergic biomechanical and functional alterations of the female urethra in a rat model of birth trauma. American Journal Physiology Renal Physiology 299: F316-F324, 2010.
Kullmann FA, TR Downs, D Artim, BJ Limberg, M Shah, D Contract, WC de Groat and JS Rosenbaum. Urothelial beta3 adrenergic receptors in the rat bladder. Neurourology and Urodynamics 30:144-150, 2011.
Yu Y and WC de Groat. Effects of stimulation of muscarinic receptors on bladder afferent nerves in the in vitro bladder pelvic afferent nerve preparation of the rat. Brain Research 1361:43-53, 2010.
Limberg BJ, KE Andersson, FA Kullmann, G Burmer, WC de Groat and JS Rosenbaum. Beta-adrenergic receptor subtype expression in myocyte and non-myocyte cells in human female bladder. Cell and Tissue Research 342:295-306, 2010.
129
Zhang X and WC de Groat. Activation of CaMKII and ERK1/2 contributes to the time-dependent TRPV1 channel potentiation response by repeated capsaicin application in rat DRG neurons. American Journal Phyiology Regulatory, Intergrative, Comparative, Physiology 300:R644-R654, 2011.
Tai C, M Chen., B Shen, J Wang, H Liu, JR Roppolo and WC de Groat. Plasticity of urinary bladder reflexes evoked by stimulation of pudendal afferent nerves after chronic spinal cord injury in cats. Experimental Neurology 228:109-117, 2011.
Ta C, B Shen, M Chen, J Wang, J Roppolo and WC de Groat. Prolonged post-stimulation inhibition of bladder activity induced by tibial nerve stimulation in cats. American Journal of Physiology Renal Physiology 300: F385-F392, 2011.
Buyuknacar, H.S.G., Eser, N., Gocman, C., de Groat, W.C., Kumcu, E.K., Ertug, P.U. and Onder, S. Prejunctional facilitatory effect of a thio-alkylation agent N-ethylmaleimide on neurogenic contactions in rat prostate smooth muscle. Neurourology and Urodynamics, 31:579-585, 2012.
Tai, C., Chen, M., Shen, B., Wang, J., Roppolo, J.R., and de Groat, W.C. Inhibition of bladder overactivity by stimulation of feline pudendal nerve using transdermal amplitude modulated signal (TAMS). British Journal of Urology International, 109:782-787. 2012
Tai, C., Shen, B., Wang, J., Liu, H., Subbaroyan, J., Roppolo, J.R. and de Groat W.C. Bladder inhibition by intermittent pudendal nerve stimulation in cat using transdermal amplitude-modulated signal (TAMS). Neurology and Urodynamics, 31:1181-1184, 2012.
Tai, C., Larson, J.A., Ogagan, P.D., Chen, G., Shen, B., Wang, J., Roppolo, J.R. and de Groat W.C. Differential role of opioid receptors in tibial nerve inhibition of nociceptive and non-nociceptive bladder reflexes in cats. Am J Physiol Renal Physiol. 302:F1090-F1097, 2012
Tai, C., P. Ogagan, D., Chen, G., Larson, J., Shen, B., Wang, J., Roppolo, J.R. and de Groat, W. C. Involvement of opioid receptors in inhibition of bladder overactivity induced by foot stimulation in cats. Journal of Urology, 188:1012-1016, 2012.
Yoshikawa, S., Oguchi, T., Funahashi, Y., de Groat, W.C. and Yoshiumra, N. Glycine transporter type 2 (GlyT2) inhibitor ameliorates bladder overactivity and nociceptive behavior in rats. European Urology, 62:704-712, 2012.
Zhang, F., Mally, A.D., Ogagan, P. D., Shen, B., Wang, J., Roppolo, J. R., de Groat, W.C. and Tai, C. Inhibition of bladder overactivity by a combination of tibial neuromodulation and tramadol treatment in cats. American Journal of Physiology – Renal Physiology, 302: F1576-1582, 2012.
Zhang, X., Pietra, C., Lovati, E., and de Groat, W.C. Activation of neurokinin-1 receptors increases the excitability of guinea pig dorsal root ganglion cells. Journal of Pharmacology and Experimental Therapeutics, 343: 44-52, 2012.
Tai, C., Shen, B., Mally, A.D., Zhang, F., Zhao, S., Wang, J., Roppolo, J. R., and de Groat, W.C., Inhibition of micturition reflex by activation of somatic afferents in posterior femoral cutaneous nerve. Journal of Physiology (London) 590: 4945-4955, 2012.
130
Mally, A.D., Zhang, F., Matsuta, Y., Shen, B., Wang, J., Roppolo, J.R., de Groat, W.C. and Tai, C. Combination of foot stimulation and tramadol treatment reverses irritation induced bladder overactivity in cats. Journal of Urology, 188:2426-2432, 2012.
Kitta, T., Chancellor, M.B., de Groat, W.C., Kuno, S., Nonomura, K. and Yoshimura, N. Suppression of bladder overactivity by adenosine A2A receptor antagonist in a rat model of Parkinson Disease. Journal of Urology, 187, 1890-1897, 2012.
Ikeda Y, Zabbarova IV, Birder LA, de Groat WC, McCarthy CJ, Hanna-Mitchell AT, Kanai AJ. Botulinum neurotoxin serotype A suppresses neurotransmitter release from afferent as well as efferent nerves in the urinary bladder. European Urology, 62:1157-1164, 2012
Julie Eiseman, Ph.D. Research Associate Professor
Holleran JL, RA Parise, AE Yellow-Duke, MJ Egorin, JL Eiseman, JM Covey and JH Beumer. Liquid chromatography-tandem mass spectrometric assay for the quantiation in human plasma of the novel indenoisoquinoline topoisomerae I inhibitors, NSC 743400 and NSC 725776. J Pharm Biomed Anal 52:714-720, 2010.
Clausen DM, J Guo, RA Parise, JH Beumer, MJ Egorin, JS Lazo, EV Prochownik and JL Eiseman. In vitro cytotoxicity and in vivo efficacy, pharmacokinetics and metabolism of 10074-G5, a novel small molecular inhibitor of C-Myc/Max dimerization. J Pharmacol Exp Ther 335:715-727, 2010.
Liggett, D, Guo, J, Parise, RA, Beumer, JH, Egorin, MJ, Lazo, JS, Prochownik, EV,. Eiseman, JL In vitro cytotoxicity and in vivo efficacy, pharmacokinetics and metabolism of 10074-G5, a novel small-molecule inhibitor of c-Myc/Max dimerization J Pharmacol Exp Ther. 2010 Dec;335(3):715-27.
Zamboni WC, JL Eiseman, S Strychor, PM Rice, E Joseph, BA Zamboni, MK Donnelly, J Shurer, RA Parise, ME Tonda, NY Yu and PH Basee. Tumor disposition of pegylated liposomal CKD-602 and the reticuloendothelial system in preclinical tumor models. J Liposome Res 21:70-20, 2011.
Beumer JH, JL Eiseman, JA Gilbert, JL Hollrean, AE Yellow-Duke, DM Clausen, DZ D’Argenio, MM Ames, PA Hershberger, RA Parise, L Bai, JM Covey and MJ Egorin. Plasma pharmacokinetics and oral bioavailability of the 3,4,5,6-tetrahydrouridine (THU) prodrug, triacetyl-THU (taTHU) in mice. Cancer Chemother Pharmacol 67:421-430, 2011.
Melanie Flint, Ph.D. Research Assistant Professor
Flint MS, Hood BL, Sun M, Stewart NA, Jones-Laughner J and Conrads TP. A proteomic analysis of the murine liver in response to a combined exposure to psychological stress and 7,12-dimethylbenz(a)anthracene. Journal of Proteome Research 9:509-20, 2010.
Flint MS, McCarty K, Jenkins F, Conrads TP, Sun M and Baum A (2010). Psychological stress accelerates the onset of tumour formation and alters the type and location of tumours in a DMBA mouse carcinogenesis model. Stress and Health, 27: n/a. doi: 10.1002/smi.1343
131
Hood BK, Grahovac J, Flint MS, Sun M, Charro N, Becker D, Wells A and Conrads TP (2010). Proteomic Analysis of Laser Capture Microdissected Melanoma Cells from Skin Organ Cultures. Journal of Proteome Research 9 (7) 3656-63.
Teng PN, Rungruang B, Hood BL, Sun M, Flint MS, Bateman NW, Dhir R, Bhargava R, Richard SD, Edwards RP, Conrads T (2010). Assessment of Buffer Systems for Harvesting Proteins from Tissue Interstitial Fluid for Proteomic Analysis. Journal of Proteome Research. 9 (8):4161-9.
Bateman NW, Sun M, Hood BL, Flint MS, Conrads TP (2010). Defining central themes in breast cancer biology by differential proteomics: conserved regulation of cell spreading and focal adhesion kinase. Journal of Proteome Research 9 (10) 5311-24.
Maxwell GL, Hood BL, Day R, Chandran U, Kirchner D, Bateman NW, Allard J, Miller C, Sun M, Flint MS, Kolli VS, Zahn C, Oliver J, Banerjee S, Litzi T, Parwani A, Sandburg G, Rose S, Becich M, Berchuck A, Kohn E, Risinger JI and Conrads TP (2011). Proteomic Analysis of Stage I Endometrial Cancer Tissue: Identification of Proteins Associated with Oxidative Processes and Inflammation. Gynecologic Oncology. 1;121(3):586-94.
Flint MS, Budiu RA, Teng P, Sun M, Stolz D, Lang M, Vlad AM, Hood BL and Conrads TP (2011). Restraint stress and stress hormones significantly impact T lymphocyte migration and function through specific alterations of the actin cytoskeleton. Brain behavior & Immunity. 25(6):1187-96.
Flint MS, Bovbjerg DH (2012). DNA damage as a result of psychological stress: implications for breast cancer. Breast Cancer Res. 14(5): 320-322.
Flint MS, Baum A, Episcopo B, Knickelbein KZ, Liegey Dougall AJ, Chambers WH, Jenkins FJ (2012). Chronic exposure to stress hormones promotes transformation and tumorigenicity of 3T3 mouse fibroblasts. Stress. 2012 Apr 16. [Epub ahead of print]
Peter Friedman, Ph.D. Professor
Klenk C, T Vetter, A Zurn, JP Vilardaga, PA Friedman, B Wang and MJ Lohse. NHERF1, β-arrestin, and the parathyroid hormone receptor form a ternary signaling complex. J Biol Chem 285:30355-30362, 2010.
Romero G, WB Sneddon, Y Yang, D Wheeler, HC Blair and PA Friedman. Parathyroid hormone receptor directly interacts with disheveled to regulate β-catenin signaling and osteoclastogenesis. J Biol Chem 285:14756-14763, 2010.
Alonso V, JA Ardura, B Wang, WB Sneddon and PA Friedman. A naturally occurring isoforms inhibits parathyroid hormone receptor trafficking and signaling. J BoneMiner Res 26:143-155, 2011.
Mamonova T, B Wang, M Kurnikova and PA Friedman. Modeling and mutation of NHERF1 dimerization domains. Biophys J 98:58a, 2010.
Ardura JA, JP Vilardaga and PA Friedman. Protein-exchange dynamics at GPCR micro-domains: A case study withthe parathyroid hormone receptor. Biophys J 98:432a, 2010.
132
Wang B, JA Ardura, G Romero, Y Yang, RA Hall and PA Friedman. Na/H exchanger regulatory factors control PTH receptor signaling by differential activation of Gα protein subunits. J Biol Chem 285:26976-26986, 2010.
Vilardaga JP, G Romero, PA Friedman and TJ Gardella. Molecular basis of parathyroid hormone receptor signaling and trafficking: A family B GPCR paradigm. Cell Mol Life Sci 68:1-13, 2011.
Wheeler DS, SR Barrick, M Grubisha, AM Brufsky, PA Friedman and G Romero. Direct interaction beween NHERF1 and frizzled regulates β-catenin signaling. Oncogene 30:32-42, 2011.
Friedman PA. Safety Update for Osteoporosis Drugs, Biophosphonates and Atypical Fractures. In Goodman & Gilman’s The Pharmacological Basis of Therapeutics, LL Brunton, JS Lazo and KL Parker, eds, 2010.
Friedman PA. Agents affecting mineral ion homeostasis and bone turnover. In Goodman & Gilman’s The Pharmacological Basis of Therapeutics (Brunton LL, Chaber B and Knollmann B eds), pp. 1275-1306, NY: McGraw Hill, 2011.
Friedman PA. Bisphosphonates: How Long Is Enough (Update). In: Goodman & Gilman's The Pharmacological Basis of Therapeutics (12 ed.), edited by Brunton LL, Chabner BA and Knollmann BC. New York: McGraw-Hill, 2012.
Liu L, Alonso V, Guo L, Tourkova I, Henderson SE, Almarza AJ, Friedman PA, and Blair HC. Na+/H+-exchange regulatory factor-1 (NHERF1) directly regulates osteogenesis. J Biol Chem In press, doi:10.1074/jbc.M112.4227662012.
Wang B, Means CK, Yang Y, Mamonova T, Bisello A, Altschuler DL, Scott JD, and Friedman PA. Ezrin-anchored PKA coordinates phosphorylation-dependent disassembly of a NHERF1 ternary complex to regulate hormone-sensitive phosphate transport. J Biol Chem 287: 24148-24163, 2012.
Mamonova T, Kurnikova M, and Friedman PA. Structural basis for NHERF1 PDZ domain binding affinity. Biochemistry 51: 3110-3120, 2012.
Song GJ, Barrick S, Leslie KL, Bauer PM, Alonso V, Friedman PA, Fiaschi-Taesch N, and Bisello A. The scaffolding protein EBP50 promotes vascular smooth muscle cell proliferation and neointima formation by regulating Skp2 and p21cip1. Arterioscler Thromb Vasc Biol 32: 33-41, 2012.
Friedman PA and Bushinsky DA. Renal Tubular Physiology of Calcium Excretion. In: Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism (8 ed.), edited by Rosen CJ. Washington, DC: American Society for Bone and Mineral Research, 2012.
Wang B, Yang Y, Liu L, Blair HC, and Friedman PA. NHERF1 regulation of PTH-dependent bimodal Pi transport in osteoblasts. Bone 52: 268-277, 2013.
William Furey, Ph.D. Professor
Nemeria N, Arjunan P, Chandrasekhar K, Mossad M, Tittmann K, Furey W and Jordan F. Communication between thiamin cofactors in the Escherichia coli pyruvate dehydrogenase complex E1 component active centers: Evidence for a direct pathway between the 4’-aminopyrimidine N1’ atoms. J Biol Chem 285:11197-11209, 2010.
133
Matei E, A Zheng, W Furey, J Rose, C Aiken and A Gronenborn. Anti-HIV activity of defective cyanovirin-Nmutants is restored by dimerization. J Biol Chem 285:13057-13065, 2010.
Koharudin L, W Furey and A Gronenborn. Novel fold and carbohydrate specificity of the potent anti-HIV cyanobacterial lectin from Oscillatoria agardhii. J Biol Chem 286:1588-1597, 2011.
Ferruccio Galbiati, Ph.D. Associate Professor
Hezel M, de Groat WC and Galbiati F. Caveolin-1 promotes nicotinic acetylcholine receptor clustering and regulates neuromuscular junction activity. Mol Biol Cell 21:302-310, 2010.
Bartholomew J and F Galbiati. Mapping of oxidative stress response elements of the caveolin-1 promoter. Methods Mol Biol 594:409-423, 2010.
Zou, H., Volonte, D. and Galbiati, F. 2012. Interaction of caveolin-1 with Ku70 inhibits Bax-mediated apoptosis. PLoS ONE, 7(6):e39379.
Stacy Gelhaus, Ph.D. Research Assistant Professor
Tracey, Lorraine, Sukhai, Mahadeo, Gelhaus, Stacy, Taylor, Dave. Postdocs: NPA’s Success. Science 336(6089): 1638. June, 2012.
Eun-Ryeong Hahm, Ph.D. Instructor
Hahm ER and SV Singh. Sulforaphane inhibits constitutive and interleukin-6-induced activation of signa transducer and activator of transcription 3 in prostate cancer cells. Cancer Prev Res 3:484-494, 2010.
Xiao D, AA Powolny, M Barbi de Moura, EE Kelley, A Bommareddy, SH Kim, ER Hahm, D Normolle, B Van Houten and SV Singh. Phenethyl isothiocyanate inhibits oxidative phosphorylation to trigger reactive oxygen species-mediated death of human prostate cancer cells. J Biol Chem 285:26558-26569, 2010.
Lee JM, ER Hahm and SV Singh. Withaferin A inhibits activation of signal transducer and activator of transcription 3 in human breast cancer cells. Carcinogenesis 31:1991-1998, 2010.
Powolny AA, Bommareddy A, Hahm ER, Normolle DP, Beumer JH, Nelson JB, Singh SV. Chemopreventative Potential of the Cruciferous Vegetable Constituent Phenethyl Isothiocyanate in a Mouse Model of Prostate Cancer. J Natl Cancer Inst. 103(7):571-84, 2011.
Hahm ER, Lee J, Huang Y, Singh SV. Withaferin a suppresses estrogen receptor-α expression in human breast cancer cells. Mol Carcinog. 50(8):614-24. 2011.
Hahm ER, Singh SV. Bim contributes to phenethyl isothiocyanate-induced apoptosis in breast cancer cells. Mol Carcinog. 2011 Jul 7. .
Hahm ER, Moura MB, Kelley EE, Van Houten B, Shiva S, Singh SV. Withaferin a-induced apoptosis in human breast cancer cells is mediated by reactive oxygen species. PLoS One. 6(8):e23354, 2011.
134
Kim SH, Sehrawat A, Sakao K, Hahm ER, Singh SV. Notch activation by phenethyl isothiocyanate attenuates its inhibitory effect on prostate cancer cell migration. PLoS One. 6(10): e26615, 2011.
Sakao K, Desineni S, Hahm ER, Singh SV. Phenethyl isothiocyanate suppresses inhibitor of apoptosis family protein expression in prostate cancer cells in culture and in vivo. Prostate. 72:1104-1116, 2012.
Xiao D, Bommareddy A, Kim SH, Sehrawat A, Hahm ER, Singh SV. Benzyl isothiocyanate causes FoxO1-mediated autophagic death in human breast cancer cells. PLoS One. 2012;7(3):e32597
Singh SV, Kim SH, Sehrawat A, Arlotti JA, Hahm ER, Sakao K, Beumer JH, Jankowitz RC, Chandra-Kuntal K, Lee J, Powolny AA, Dhir R. Biomarkers of phenethyl isothiocyanate-mediated mammary cancer chemoprevention in a clinically relevant mouse model. J Natl Cancer Inst. 104(16):1228-1239, 2012.
Hahm ER, Singh SV. Withaferin A-induced apoptosis in human breast cancer cells is associated with suppression of inhibitor of apoptosis family protein expression. Cancer Lett. 2012 Aug 27. [Epub ahead of print]
Eun-Ryeong Hahm, Kumar Chandra-Kuntal, Dhimant Desai, Shantu Amin, Shivendra V. Singh. Notch Activation Is Dispensable for D, L-Sulforaphane-Mediated Inhibition of Human Prostate Cancer Cell Migration. PLoSONE 7(9) e44957, 2012.
Pamela Hershberger Research Assistant Professor
Owonikoko TK, Ramalingam S, Kanterewicz B, Balius T, Belani CP and Hershberger PA. Vorinostat increases carbopplatin and paclitaxel activity in non-small cell lung cancer cells. Int J Cancer 126:743-755, 2010.
Horvath HC, Lakatos PA, Kosa BP, Bacsi K, Borka K, Bises G, Nittke T, Hershberger PA, Speer G and Kallay E. Expression of the candidate oncogene CYP24A1 during colorectal tumorigenesis. J Histochem Cytochem 58:277-285, 2010.
Nichols M, Cheng P, Liu Y, Kanterewicz B, Hershberger PA and McCarty KS. Breast cancer derived M543V mutation in helix 12 of estrogen receptor alpha inverts response to estrogen and SERMs. Breast Cancer Res Treat 120:761-768, 2010.
Beumer JH, JL Eiseman, JA Gilbert, J HOlleran, AE Yellow-Duke, DM Clausen, D D’Argenio, MM Ames, PA Hershberger, R Parise, L Bai, JM Covey and MJ Egorin. Plasma pharmacokinetics and oral bioavailability of the 3,4,5,6-tetrahydrouridine (THU) prodrug, triacetyl-THU (taTHU) in mice. CCP 67:421-430, 2011.
Srinivasan M, AV Parwani, PA Hershberger, DE Lenzner and JL Weissfeld. Vitamin D receptor expression and overall survival in non-small cell lung cancer: Prognostic and therapeutic implications. J Steroid Biochem Mol Biol 123:30-36, 2011.
Jing Hu, Ph.D. Assistant Professor
Xu X, Vatsyayan J, Gao C, Bakkenist C and Hu J. Sumoylation of EIF4E activates mRNA translation. EMBO Reports 11:299-304, 2010.
135
Qu Z, Fu J, Yan P, Hu J, Cheng S and Xiao G. Epigenetic repression of PDLIM2: Implications for the biology and treatment of breast cancer. J Biol Chem 285:11786-11792, 2010.
Xu X, J Vatsyayan, C gao, CJ Bakkenist and J Hu. HDAC2 promotes eIF4E Sumoylation and activates mRNA translation gene specifically. J Biol Chem 285:18139-18143, 2010.
Qu Z, J Fu, P Yan, J Hu, S Cheng and G Xiao. Epigenetic repression of PDLIM2: Implications for the biology and treatment of breast cancer. J Biol Chem 285:11786-11792, 2010.
White JS, N Yun, J Hu and CJ Bakkenist. The ATM kinase signaling induced by the low-energy β-particles emitted by (33)P is essential for the suppression of chromosome aberrations and is greater than that induced by the energetic-β-particles emitted by (32)P. Mutat Res 708:28-36, 2011.
White JS, Yue N, Hu J, Bakkenist CJ. The ATM kinase signaling induced by the low-energy β-particles emitted by (33)P is essential for the suppression of chromosome aberrations and is greater than that induced by the energetic β-particles emitted by (32)P. Mutat Res. 2011 Mar 15;708(1-2):28-36.
Yi Huang, M.D., Ph.D. Research Assistant Professor
Hahm ER, Lee J, Huang Y, Singh SV. Withaferin A suppresses estrogen receptor-α expression in human breast cancer cells. Mol. Carcinogenesis, 50(8):614-24, 2011.
Huang Y, Nayak S, Jankowitz R, Davidson NE, Oesterreich S. Epigenetics in breast cancer-what’s new? Breast Cancer Research, Epub ahead of print, 2011.
Huang Y, Vasilatos S, Boric L, Shaw PW, Davidson NE. Inhibitors of histone demethylation and histone deacetylation cooperate in regulating gene expression and inhibiting growth in human breast cancer cells. Breast Cancer Res. Treat., 131:777-89, 2012.
Zhu Q, Huang Y, Marton LJ, Woster PM, Davidson NE, Casero RA. Polyamine analogues modulate gene expression by inhibiting LSD1/KDM1 and altering chromatin structure in human breast cancer cells. Amino Acids, 42:887-98, 2012.
Sharma SK, Hazeldine S, Crowley ML, Hanson A, Beattie R, Varghese S, Senanayake TMD, Hirata A, Hirata F, Huang Y, Wu Y, Steinbergs N, Murray-Stewart T, Bytheway I, Casero RA, Woster PM. Polyamine-based small molecule epigenetic modulators. Med. Chem. Commun., 3:14-21, 2012.
Jin K, Kong X, Shah T, Penet MF, Wildes F, Sgroi DC, Ma XJ, Huang Y, Kallioniemi A, Landberg G, Bieche I, Wu X, Lobie PE, Davidson NE, Bhujwalla ZM, Zhu T, Sukumar S.. HOXB7 renders breast cancer resistant to tamoxifen through activation of the EGFR pathway. Proc Natl Acad Sci USA, 109:2736-41,2012.
Hu D, Zhou Z, Davidson NE, Huang Y, Wan Y. Novel insight into KLF4 proteolytic regulation in estrogen receptor signaling and breast carcinogenesis. J. Biol. Chem., 287:13584-97, 2012.
Zhu Q, Jin L, Casero RA, Davidson NE, Huang Y. Role of ornithine decarboxylase in regulation of estrogen receptor alpha expression and growth in human breast cancer cells. Breast Cancer Res. Treat., 136:57-66, 2012
.
136
Edwin Jackson, Ph.D. Professor
Cheng D, Ren J and Jackson EK. Multidrug resistance protein 4 mediates 3’,5’-cAMP efflux from rat preglomerular vascular smooth muscle cells. Clinical and Experimental Pharmacology and Physiology 37:205-207, 2010.
Haselkorn ML, Shellington D, Jackson EK, Vagni VA, Janesko-Feldman VA, Dubey RK, Gillespie DG, Cheng DG, Bell MJ, Jenkins LW, Homanics GE, Schnermann J and Kochanek P. Adenosine A1 receptor activation as a brake on the microglial response after experimental traumatic brain injury in mice. Journal of Neurotrauma 27:901-910, 2010.
Mandapathil M, B Hildorfer, MJ Szczepanski, M Czyatowaka, M Szajnik, J Ren, S Lang, EK Jackson, EGorelik and TL Whiteside. Generation and accumulation of immunosuppressive adenosine by human CD4+CD25highFoxp3+ regulatory T cells (TREG). Journal of Biological Chemistry 285:7176-7186, 2010.
Dubey RK, M Rosselli, DG Gillespie, Z Mi and EK Jackson. Extracellular 3’,5’-cAMP-adenosine pathway inhibits glomerular mesangial cell growth. Journal of Pharmacology and Experimental Therapeutics 333:808-815, 2010.
Tofovic DS, VP Bilan and EK Jackson. Sitagliptin augments angiotensin II-induced renal vasoconstriction in kidneys from rats with the metabolic syndrome. Clinical and Experimental Pharmacology and Physiology 37:689-691, 2010.
Jackson EK, J Ren, DG Gillespie and RK Dubey. Extracellular 2’,3’-cAMP is a potent inhibitor of preglomerular vascular smooth muscle cell and mesangial cell growth. Hypertension 56:151-158, 2010.
Dubey RK, EK Jackson, DG Gillespie, LC Zacharia, B Imthurn and M Rosselli. Resveratrol blocks the antimitogenic effects of estradiol on human female aortic smooth muscle cells. Journal of Clinical Endocrinology and Metabolism 95: E9-E17, 2010.
Baruscotti I, F Barchiesi, EK Jackson, B Imthurm, R Stiller, S Schaufelberger, M Rosselli and Dubey RK. Estradiol stimulates capillary formation by human endothelial cells: Role of ER-α/β, heme oxygenase-1 and tyrosine kinase. Hypertension 56: 397-404, 2010.
Mandapathil M, MJ Szczepanski, M Szajnik, J Ren, EK Jackson, JT Johnson, E Gorelik, S Lang and TL Whiteside. Adenosine and prostaglandin E2 cooperate in the suppression of immune responses mediated by adaptive regulatory T cells. Journal of Biological Chemistry 285: 27571-27580, 2010.
Tofovic SP, T Jones, VP Bilan, EK Jackson and G Petrusevska. Synergistic therapeutic effects of 2-methoxyestradiol with either sildenafil or bosentan on amelioration of monocrotaline-induced pulmonary hypertension and vascular remodeling. Journal of Cardiovascular Pharmaoclogy 56: 475-483, 2010.
Barchies F, E Lucchinetti, M Zaugg, OO Ogunshola, M Wright, M Meyer, M Rosselli, DG Gillespie., EK Jackson and RK Dubey. Candidate genes for 2-methoxyestradiol-mediated vasoprotective actions in human aortic smooth muscle cells. Hypertension 56: 964-972, 2010.
Cheng D J Ren, DG Gillespie, Z Mi and EK Jackson. Regulation of 3’,5’-cAMP in preglomerular smooth muscle and endothelial cells from genetically-hypertensive rats. Hypertension 56: 1096-1101, 2010.
137
Su Y, EK Jackson and E Gorelik. Receptor desensitization and blockade of the suppressive effects of adenosine and prostaglandin E2 (PGE2) on the cytotoxic activity of human melanoma-infiltrating T lymphocytes. Cancer Immunology and Immunotherapy 60: 111-122, 2011.
Jackson, E.K., Gillespie, D.G., and Dubey, R.K.: 2ʹ-AMP and 3ʹ-AMP inhibit proliferation of preglomerular vascular smooth muscle cells and glomerular mesangial cell growth via A2B receptors. Journal of Pharmacology and Experimental Therapeutics 337: 444-450, 2011. PMC3083111
Robert H. Garman, R.H., Jenkins, L.W., Switzer III, R.C., Bauman, R.A., Tong, L.C., Swauger, P.V., Parks, S.A., Ritzel, D.V., Dixon, C.E., Clark, R.S.B., Bayır, H., Kagan, V., Jackson, E.K., and Kochanek, P.M.: Blast exposure in rats with body shielding is characterized by diffuse axonal injury. Journal of Neurotrama 28: 947-959, 2011.
Jackson, E.K., Ren, J., and Gillespie, D.G.: 2’,3’-cAMP, 3’-AMP and 2’-AMP inhibit human aortic and coronary vascular smooth muscle cell proliferation via A2B receptors. American Journal of Physiology – Heart and Circulatory Physiology 301: H391-H401, 2011. PMC3154660
Verrier, J.D., Exo, J.L., Jackson, T.C., Ren, J., Gillespie, D.G., Dubey, R.K., Kochanek, P.M., and Jackson, E.K.: Expression of the 2’,3’-cAMP-adenosine pathway in astrocytes and microglia. Journal of Neurochemistry 118: 979-987, 2011. PMC3166383
Jackson, E.K., Ren, J., Cheng, D. and Gillespie, D.G.: Extracellular cAMP-adenosine pathways in the mouse kidney. American Journal of Physiology – Renal Physiology 301: F565-F573, 2011. PMC3174555
Cheng, D., Zhu, X., Barchiesi, F., Gillespie, D.G., Dubey, R.K., and Jackson, E.K.: RACK1 regulates cell proliferation by modulating calcium signaling. Hypertension 58: 689-695, 2011. PMC3174333
Jackson, E.K., Cheng, D., Tofovic, S.P., and Mi, Z.: Endogenous adenosine contributes to renal sympathetic neurotransmission via postjunctional A1-receptor-mediated coincident signaling. American Journal of Physiology – Renal Physiology 302: F466-FF76, 2012. PMC3289416
Drabek, T., Janata, A, Jackson, E.K., End, B., Stezoskia, J., Vagnia, V., Janesko-Feldman, K., van Rooijene, N., Samuel A. Tisherman, S.A., Kochanek, P.M.: Microglial depletion using intrahippocampal injection of liposome-encapsulated clodronate in prolonged hypothermic cardiac arrest in rats. Resuscitation 83: 517-526, 2012.
Verrier, J.D., Jackson, T.C., Kochanek, P.M., and Jackson, E.K.: The brain in vivo expresses the 2’,3’-cAMP-adenosine pathway. Journal of Neurochemistry 122: 115-125, 2012. PMC3371318
Mandapathil, M., Szczepanski, M.J., Harasymczuk, M., Ren, J., Cheng, D., Jackson, E.K., Gorelik, E., Jonas T. Johnson, J.T., Lang, S, and Whiteside, T.L.: CD26 expression and adenosine deaminase activity in regulatory T cells (Treg) and CD4+ T effector cells in patients with head and neck squamous cell carcinoma. OncoImmunology 1: 659-669, 2012. PMC3429570
Zhang, L., Franchini, M., Eser, M.W., Jackson, E.K., and Dip, R.: Increased adenosine concentration in bronchoalveolar lavage fluid of horses with lower airway inflammation. The Veterinary Journal 193, 268-270, 2012.
138
Prakasam, H.S., Herrington, H., Ropollo, J., Jackson, E.K., and Apodaca, G.: Modulation of bladder function by adenosine turnover and A1 receptor activation. American Journal of Physiology – Renal Physiology 303: F279-F292, 2012. PMC3404591
Jackson, E.K., Kochanek, S.J., and Gillespie, D.G.: Dipeptidyl peptidase IV regulates proliferation of preglomerular vascular smooth muscle and mesangial cells. Hypertension 60: 757-764, 2012. PMC3422672
Jackson, E.K., and Gillespie, D.G.: Extracellular 2’,3’-cAMP and 3’,5’-cAMP stimulate proliferation of preglomerular vascular endothelial cells and renal epithelial cells. American Journal of Physiology – Renal Physiology 303: F954-F962, 2012. PMC Journal-in Process
Jackson, E.K., Cheng, D., Mi, Z., Verrier, D., Janesko-Fledman, K., and Kochanek, P.M.: Role of A1 receptors in renal sympathetic neurotransmission in the mouse kidney. American Journal of Physiology-Renal Physiology 303, F1000-F1005, 2012
Kochanek, P.M., Verrier, J.D., Wagner, A.K. and Jackson, E.K.: The many roles of adenosine in traumatic brain injury. In: Adenosine: A Key Link Between Metabolism and Central Nervous System Activity, Editied by Detlev Boison and Susan Masino, Springer, New York, pages 307-322, 2012
Tija Jacob, Ph.D.Assistant Professor
Jacob TC, Michels G, Silayeva L, Haydon J, Succol F, Moss SJ. Benzodiazepine treatment induces subtype specific changes in GABA-A receptor trafficking and decreases synaptic inhibition. Proc Natl Acad Sci U S A. 2012 Nov 6;109 (45):18595-600.
Yu Jiang, Ph.D. Associate Professor
Ma D, Bai X, Zou H, Lai Y and Jiang Y. Rheb GTPase controls apoptotis by regulating the interaction of FKP38 with Bcl-2 and Bcl-XL. J Biol Chem 285:8621-8627, 2010.
Li M, L Zhao, J Liu, A Liu, C Jia, D Ma, Y Jiang and X Bai. Multi-mechanisms are involved in reactive oxygen species regulation of mTORC1 signaling. Cell Signaling 22:1469-1476, 2010.
Cao H, S Yu, Z Yao, DL Galson, Y Jiang, X Zhang, J Fan, B Lu, Y Guan, M Luo, Y Lai, Y Zhu, N Kurihara, K Patrene, DG Roodman and G Xiao. Activating transcription factor 4 regulates osteoclast differentiation. J Clin Invest 120:2755-276, 2010.
Yan, G. Lai, Y. and Jiang, Y.* (2012). TOR under stress: Targeting TORC1 by Rho1 GTPase. Cell Cycle 11(18):3384-8.
Jia, C.H., Li, M., Liu, J., Zhao, L., Lin, J., Lai, P.L., Liu, A.L., Zhang, Y., Li, H.Y., Gao, T.M., Jiang, Y. and Bai, X. (2012). IKK- mediates hydrogen peroxide induced cell death through p85 S6 kinase 1. Cell Death Differ. 2012 Sep [Epub ahead of print].
139
Thomas Kensler, Ph.D. Professor
Zhou Q, Chaerkady R, Shaw PG, Kensler TW, Pandey A and Davidson NE. Screening for therapeutic targets of vorinostat by SILAC-based proteomic analysis in human breast cancer cells. Proteomics 10: 1-10, 2010.
Gruber F, Mayer H, Lengauer B, Mlitz V, Sanders JM, Kadl A, Bilban M, de Martin R, Wagner O, Kensler TW, Yamamoto M, Leitinger N. and Tschachler E. Nrf2 regulates the stress response to UVA-oxidized phopholipids in skin cells. FASEBJ 24: 39-48, 2010.
Kensler TW and Wakabayashi N. Nrf2: friend or foe for chemoprevention? Carcinogenesis 31:90-99, 2010.
Kwak M-K and TW Kensler. Targeting Nrf2 signaling for cancer chemoprevention. Toxicol. Appl. Pharmacol.244:66-76, 2010. Kensler TW and N Wakabayashi. Nrf2: friend or foe for chemoprevention? Carcinogenesis 31: 90-99, 2010.
Shin D-H, H-M Park, ,K-A Jeong.,H-G Choi, J-A Kim, D-D Kim, KW Sang, SK Ku, TW Kensler and MK Kwak. The Nrf2-heme oxygenase-1 system modulates cyclosporine A-induced epithelial-mesenchymal transition and renal fibrosis. Free Radical Biol Med 48:1051-1063, 2010.
Shah ZA, R-C Li, AS Ahmad, TW Kensler, M Yamamoto, S Biswal, S Doré. The flavanol (-)-epicatechin prevents stroke damage through the Nrf2/HO1 pathway. J Cerebral Blood Flow & Metab 30:1951-1961, 2010.
Wakabayashi N, SL Slocum, JL Skoko, S Shin and TW Kensler. When NRF2 talks, who’s listening? Antioxidant & Redox Signaling 13:1649-1663, 2010.
Kadl A, AK Meher, A, PR Sharma, MY Lee, AC Doran, SR Johnstone, MR Elliott, G Gruber, J Han, W Chen, T Kensler, KS Ravichandran, BE Isakson, BR Wamhoff and N Leitinger. Identification of a novel macrophage phenotype that develops in response to atherogenic phospholipids via Nrf2. Cir. Res 107: 737-746, 2010.
Yao Y, AMH Brodie, NE Davidson, TW Kensler and A Zhou. Inhibition of estrogen signaling activates the NRF2 pathway in breast cancer. Breast Cancer Res Treatment 124:585-591, 2010.
Wakabayashi N, S Shin, S Slocum, ES Agoston, J Wakabayashi, MK Kwak, V Misra, S Biswal, M Yamamoto and TW Kensler. Regulation of Notch1 signaling by Nrf2: implications for tissue regeneration. Science Signaling 3:ra52, 2010.
Malhotra D, E Portales-Casamar, A Singh, S Srivastava, D Arenillas, C Happel, C Shyr, N Wakabayashi, TW Kensler, WW Wasserman and S Biswal. Global mapping of binding sites for Nrf2 identifies novel targets in cell survival response through ChIP-Seq profiling and network analysis. Nucl Acids Res 38:5718-5734, 2010.
Kensler TW, BD Roebuck, GN Wogan and JDGroopman. Aflatoxin: A 50 year odyssey of mechanistic and translational toxicology. Toxico Sci 120:28-48, 2011.
You A, CW Nam, N Wakabayashi, M Yamamoto, TW Kensler and MW Kwak. Transcription factor Nrf2 maintains the basal expression of Mdm2: an implication of the regulation of p53 signaling by Nrf2. Arch Biochem Biophys 507:356-364, 2011.
140
Egner PA, JG Chen, JB Wang, Y Wu, Y Sun, JH Lu, J Zhu, YH Zhang, YS Chen, MD Friesen, LP Jacobson, AMuñoz, D Ng, GS Qian, YR Zhu, TY Chen, NP Botting, QZ Zhang, JW Fahey, P Talalay, JD Groopman and TW Kensler. Bioavailability of sulforaphane from two broccoli sprout beverages: Results of a short term, cross-over clinical trial in Qidong, China. Cancer Prev Res 4:384-395, 2011.
Kensler, T.W., Groopman, J.D., Egner, P.A., Muñoz, A., Qian, G.S. and Chen, J.G. (2012) Chemoprevention of hepatic cancer in aflatoxin endemic areas. Gu, J.R. (ed) Primary Liver Cancer: Challenges and Perspectives, Springer, New York, pp. 339-365.
Kensler, T.W., Egner, P.A. and Groopman, J.D. (2012) Twenty years of collaboration with the Qidong Liver Cancer Institute: The Johns Hopkins Experience. China Cancer 21:734-745.
Fahey, J.W., Wehage, S.L., Holtzclaw, W.D., Kensler, T.W., Egner, P.A., Shapiro, T.A. and Talalay, P. (2012) Protection of humans by plant glucosinolates: efficiency of conversion of glucosinolates to isothiocyanates by the gastrointestinal microflora. Cancer Prev. Res 5: 603-611.
Weerachayaphorn, J., Mennone, A., Soroka, C.J., Harry, K., Hagey, L.R., Kensler, T.W. and Boyer, J.L. (2012) Nuclear factor-E2-related factor 2 is a major determinant of bile acid homeostasis in the liver and intestine. Am. J. Physiol – Gastro Liver Physiol. 302: G925-G936.
Meher, A.K., Sharma, P.R., Kira, V.A., Yamamoto, M., Kensler, T.W., Yan, Z. and Leitinger, N. (2012) Nrf2 deficiency in myeloid cells is not sufficient to protect mice from HFD-induced adipose tissue inflammation and insulin resistance. Free Rad. Biol. Med. 52: 1708-1715.
Kageyama, Y., Zhang, Z-Y, Roda, R., Fukaya, M., Wakabayashi, J., Wakabayashi, N., Kensler, T.W., Reddy, P.H., Iijima, M. and Sesaki, H. (2012) Mitochondiral division ensures the survival of postmitotic neurons by suppressing oxidative damage. J.Cell Biol. 197: 535-551.
Takaya, K., Suzuki, T., Motohashi, H., Onodera, K., Satomi, S., Kensler, T.W. and Yamamoto, M. (2012) Validation of the multiple sensor mechanism of the Keap1-Nrf2 system. Free Radic. Biol. Med. 53: 817-827.
Kaidery, N.A., Banerjee, R., Yang, L., Smirnova, N.A., Hushpulian, D.M., Liby, K.T., Williams, C.W., Yamamoto, M., Kensler, T.W., Ratan, R.R., Sporn, M.B., Beal, M.F., Gazaryan, I.G. and Thomas, B. (2013) Targeting Nrf2-mediated gene transcription by extremely potent synthetic triterpenoids inhibits dopaminergic neurotoxicity in the MPTP- mouse model of Parkinson’s disease. Antiox. Redox Signaling 18: 139-157.
Kensler, T.W., Egner, P.A., Agyeman, A.S., Visvanathan, K., Groopman, J.D., Chen, J-G., Chen, T-Y., Fahey, J.W., and Talalay, P. (2013) Keap1-Nrf2 signaling: A target for cancer prevention by sulforaphane. Topics in Current Chemistry 329: 163-178.
Nicholas Khoo, Ph.D. Research Assistant Professor
Rudolph TK, Rudolph V, Edreira MM, Cole MP, Bonacci G, Schopfer FJ, Woodcock SR, Franek A, Pekarova M, Khoo NK, Hasty AH, Baldus S and Freeman BA. Nitro-fatty acids reduce atherosclerosis in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol 30:938-945, 2010.
Khoo NK, White CR, Pozzo-Miller L, Zhou F, Constance C, Inoue T, Patel RP and Parks DA. Dietary flavonoid quercetin stimulates vasorelaxation in aortic vessels. Free Radic Biol Med 49:339-347, 2010.
141
Schopfer FJ, Cole MP, Groeger AL, Chen CS, Khoo NK, Woodcock SR, Golin-Bisello F, Motanya UN, Li Y, Zhang J, Garcia-Barrio MT, Rudolph TK, Rudolph V, Bonacci G, Baker PR, Xu HE, Batthyany CI, Chen YE, Hallis TM and Freeman BA. Covalent peroxisome proliferator-activated receptor gamma adduction by nitro-fatty acids: selective ligand activity and anti-diabetic signaling actions. J Biol Chem 285:12321-12333, 2010.
Khoo NK and Freeman BA. Electrophilic nitro-fatty acids: anti-inflammatory mediators in the vascular compartment. Curr Opin Pharmacol 10:179-184, 2010.
Bonacci G, Baker PR, Salvatore SR, Shores D, Khoo NKH, Koenitzer JR, Vitturi DA, Woodcock SR, Golin-Bisello F, Watkins S, St Croix C, Batthyany CI, Freeman BA, Schopfer FJ. Conjugated Linoleic Acid is a Preferential Substrate for Fatty Acid Nitration. J Biol Chem. 2012 Nov 9. [Epub ahead of print] PMID: 23144452. doi: 10.1074/jbc.M112.401356 jbc.M112.401356.
Khoo NKH, Cantu-Medellin N, Devlin JE, St Croix CM, Watkins SC, Fleming AM, Champion HC, Mason RP, Freeman BA, Kelley EE. Obesity-induced tissue free radical generation: An in vivo immunospin trapping study. Free Radic Biol Med, Jun 1;52(11-12):2312-9, 2012. PMID: 22564528
Bonacci G, Schopfer FJ, Batthyany CI, Rudolph TK, Rudolph V, Khoo NKH, Kelley EE, Freeman BA. Electrophilic Fatty acids regulate matrix metalloproteinase activity and expression. J Biol Chem, 286(18):16074-81, 2011.
Joan M. Lakoski, Ph.D. Professor
Lang DH, GS Gerhard, JW Griffith, GP VOgler, DJ Vandenbergh, DA Blizard, JT Stout, JM Lakoski and GE McClearn. Quantative trait loci (QTL) analysis of longevity in C57BL/6J by DBA/2J (BXD) recombinant inbred mice. Aging Clinical and Experimental Research 22:8-19, 2010.
Adrian Lee, Ph.D. Professor
Migliaccio I, MF Wu, CGutierrez, L Malorni, SK Mohsin, DC Allred, SG Hilsenbeck, CK Osborne, H Weiss and AV Lee AV. Nuclear IRS-1 predicts tamoxifen response in patients with early breast cancer. Breast Cancer Res Treat 123:651-660, 2010.
Creighton CJ, X Fu , BT Hennessy, AJ Casa, Y Zhang, AM Gonzalez-Angulo, A Lluch, JW Gray, PH Brown,SG Hilsenbeck, CK Osborne, GB Mills, AV Lee and R Schiff. Proteomic and transcriptomic profiling reveals a link between the PI3K pathway and lower estrogen-receptor (ER) levels and activity in ER+ breast cancer. Breast Cancer Res12:R40, 2010.
Gualberto A, M Dolled-Filhart, M Gustavson, J Christiansen, YF Wang, ML Hixon, J Reynolds, S McDonald,A Ang, DL Rimm, CJ Langer, J Blakely, L Garland, LG Paz-Ares, DD Karp and AV Lee. Molecular analysis of non-small cell lung cancer identifies subsets with different sensitivity to insulin-like growth factor I receptor inhibition. Clin Cancer Res 16:4654-4665, 2010.
Qu Y, J Wang, PS Ray, H Guo, J Huang, M Shin-Sim, BA Bukoye, B Liu, AV Lee, X Lin, P Huang, JW Martens, AE Giuliano, N Zhang, NH Cheng and X Cui. Thioredoxin-like 2 regulates human cancer cell growth and metastasis via redox homeostasis and NF-κB signaling. J Clin Invest 121:212-25, 2011.
142
Becker MA, YH Ibrahim, X Cui, AV Lee and D Yee. The IGF Pathway Regulates ER{alpha} through a S6K1-Dependent Mechanism in Breast Cancer Cells Mol Endocrinol 2011 Feb 3. [Epub ahead of print]
Litzenburger BC, CJ Creighton, A Tsimelzon, BT Chan, SG Hilsenbeck, T Wang, JM Carboni, MM Gottardis,F Huang, JC Chang, MT Lewis, MF Rimawi and AV Lee. High IGF-IR activity in triple-negative breast cancer cell lines and tumorgrafts correlates with sensitivity to anti-IGF-IR therapy. Clin Cancer Res. 2010 Dec 22. [Epub ahead of print]
Potter AS, Casa AJ, Lee AV. Forkhead box A1 (FOXA1) is a key mediator of insulin-like growth factor I (IGF-I) activity. J Cell Biochem. 2012 Jan;113(1):110-21. PMID: 21882221
Dearth RK, Kuiatse I, Wang YF, Liao L, Hilsenbeck SG, Brown PH, Xu J, Lee AV. A moderate elevation of circulating levels of IGF-I does not alter ErbB2 induced mammary tumorigenesis. BMC Cancer. 2011 Aug 25;11(1):377. PMID: 21867536
Casa AJ, Potter AS, Malik S, Lazard Z, Kuiatse I, Kim HT, Tsimelzon A, Creighton CJ, Hilsenbeck SG, Brown PH, Oesterreich S, Lee AV. Estrogen and insulin-like growth factor-I (IGF-I) independently down-regulate critical repressors of breast cancer growth. Breast Cancer Res Treat. 2011 May 4. PMID: 21541704
Hartmaier RJ, Richter AS, Gillihan RM, Sallit JZ, McGuire SE, Wang J, Lee AV, Osborne CK, O'Malley BW, Brown PH, Xu J, Skaar TC, Philips S, Rae JM, Azzouz F, Li L, Hayden J, Henry NL, Nguyen AT, Stearns V, Hayes DF, Flockhart DA, Oesterreich S. A SNP in Steroid Receptor Coactivator-1 Disrupts a GSK3β Phosphorylation Site and Is Associated with Altered Tamoxifen Response in Bone. Mol Endocrinol. 2011 Dec 15. PMID: 2217437
Hampton OA, Miller CA, Koriabine M, Li J, Den Hollander P, Carbone L, Nefedov M, Ten Hallers BF, Lee AV, De Jong PJ, Milosavljevic A. Long-range massively parallel mate pair sequencing detects distinct mutations and similar patterns of structural mutability in two breast cancer cell lines. Cancer Genet. 2011 Aug;204(8):447-57. PMID: 21962895
Wang J, Ray PS, Sim MS, Zhou XZ, Lu KP, Lee AV, Lin X, Bagaria SP, Giuliano AE, Cui X FOXC1 regulates the functions of human basal-like breast cancer cells by activating NF-κB signaling. Oncogene. 2012 Jan 16. doi: 10.1038/onc.2011.635
Edwin Levitan, Ph.D. Professor
Levitan ES and Shakiryanova. Imaging the Drosophila neuromuscular junction (NMJ): Basic optical principle and equipment. Cold Spring Harb Protoc 2010:pdb.top92, 2010.
Levitan ES and Shakiryanova. Imaging neuropeptide release in the Drosphila neuromuscular junction (NMJ). Cold Spring Harb Protol 2010:pdb.prot5529, 2010.
Lloyd TE, Machamer J, O'Hara K, Kim JH, Collins SE, Wong MY, Sahin B, Imlach W, Yang Y, Levitan, E.S., McCabe BD, Kolodkin AL. The p150(Glued) CAP-Gly domain regulates initiation of retrograde transport at synaptic termini. Neuron. 74:344-360, 2012.
Ji H, Tucker KR, Putzier I, Huertas MA, Horn JP, Canavier CC, Levitan ES, Shepard PD. Functional characterization of ether-à-go-go-related gene potassium channels in midbrain dopamine neurons: Impli-cations for a role in depolarization block. Eur J Neurosci 36:2906-2916, 2012.
143
Tucker KR, Huertas MA, Horn JP, Canavier CC, Levitan ES. Pacemaker rate and depolarization block in nigral dopamine neurons: a somatic sodium channel balancing act. J Neurosci 32:14519-14531, 2012.
Colgan LA, Cavolo SL, Commons KG, Levitan, ES. Action potential-independent and pharmacologically unique vesicular serotonin release from dendrites. J Neurosci 32:15737-15746, 2012.
Lloyd TE, Machamer J, O'Hara K, Kim JH, Collins SE, Wong MY, Sahin B, Imlach W, Yang Y, Levitan, E.S., McCabe BD, Kolodkin AL. The p150(Glued) CAP-Gly domain regulates initiation of retrograde transport at synaptic termini. Neuron. 74:344-360, 2012.
Ji H, Tucker KR, Putzier I, Huertas MA, Horn JP, Canavier CC, Levitan ES, Shepard PD. Functional characterization of ether-à-go-go-related gene potassium channels in midbrain dopamine neurons: implications for a role in depolarization block. Eur J Neurosci 36:2906-2916, 2012.
Tucker KR, Huertas MA, Horn JP, Canavier CC, Levitan ES. Pacemaker rate and depolarization block in nigral dopamine neurons: a somatic sodium channel balancing act. J Neurosci 32:14519-14531, 2012.
Colgan LA, Cavolo SL, Commons KG, Levitan, ES. Action potential-independent and pharmacologically unique vesicular serotonin release from dendrites. J Neurosci 32:15737-15746, 2012.
Carola Neumann, M.D. Visiting Associate Professor
Hance MV, Dole K, Gopal U, Bohonowych JE, Jezierska-Drutel A, Neumann CA, Liu H, Garraway IP, Isaacs JS. Secreted Hsp90 is a novel regulator of the epithelial to mesenchymal transition in prostate cancer, 2012, Journal of Biological Chemistry 287 (45):37732-44.
Steffi Oesterreich, Ph.D. Professor
Karmaker S, T Gao, MC Pace, S Oesterreich and CL Smith. Cooperative activation of cyclin D1 and progesterone receptor gene expression by the SRC-3 coactivator and SMRT compressor. Mol Endo 24:1187-1202, 2010.
Malik S, Jiang J, Garee J, Verdin E, Lee AV, O’Malley BW, Zhang W, Belaguli NS, Oesterreich S. Intricate Interplay between HDAC7 and FoxA1 in estrogen-mediated repression of RPRM. Mol Cell Biol. 2010, 30:399-412, PMID 1991772
Garee J and Oesterreich S. SAFB1’s Multiple Functions in Biological Control — Lots Still to Be Done. Review. J Cell Biochem. 2010, 109:312-9, PMID 20014070
Hammerich-Hille S, Tsimelzon A, Kaipparettu BA, Creighton C, Jiang S, Polo JM, Melnick A, Meyer R, Oesterreich S. SAFB1 mediates repression of immune regulators and apoptotic genes in breast cancer cells. JBiol Chem 2010, 285:3608-16, PMID 19901029
Pathiraja TN, Stearns V, Oesterreich S. Epigenetic Regulation in estrogen receptor positive breast cancer – role in treatment response. J Mammary Gland Biol Neoplasia 2010, Epub ahead of print. PMID 20101445
144
Dearth RK, Delgado DA, Hiney JK, Pathiraja T, Oesterreich S, Medina D, Dees LW and Lee AV. Parity-induced reduction in circulating growth hormone (GH) is associated with altered mammary gland signaling in two rat strains: Implications for pregnancy protection from breast cancer. Cancer Prevention Res. 2010, 3:312-21, PMID: 20145191
Oesterreich, S Lee AV, Davidson NE. Is It Time To ReSET the Standard for Estrogen Receptor Testing in Breast Cancer? J Clin Oncol 28:41-1-41-3. 2010. PMID: 20697070
Pathiraja TN, Shetty TB, Jelinek J, He R, Hartmaier R, Margossian AL, Hilsenbeck SG, Issa JP, Oesterreich S. Progesterone receptor isoform-specific promoter methylation — Association of PRA methylation with worse outcome in breast cancer patients. Clin Cancer Res. 17:4177-4186. 2011. PMID: 21459801
Spears M, Oesterreich S, Migliaccio I, Guiterrez C, Hilsenbeck S, Quintayo MA, Pedraza J, Munro AF, Thomas JS, Kerr GR, Jack WJ, Kunkler IH, Cameron DA, Chetty U, Bartlett JM. The p160 ER co-regulators predict outcome in ER negative breast cancer. Breast Cancer Res Treat. 2011 in press. PMID: 21390497
Lachapelle S, Oesterreich S, Lebel M. Loss of the helicase function of the Werner gene product increases perinatal lethality of mice lacking the Scaffold Attachment Factor B1 and inhibits mouse embryonic fibroblast immortalization. Aging. 3:277-290. 2011.
Casa AJ, Potter AS, Malik S, Lazard Z, Kuiatse I, Kim HT, Tsimelzon A, Creighton CJ, Hilsenbeck SG, Brown PH, Oesterreich S, Lee AV. Estrogen and insulin-like growth factor-I (IGF-I) independently down-regulate critical repressors of breast cancer growth. Breast Cancer Res Treat. 2011 in press. PMID: 21541704
Garee JP, Meyer R, Oesterreich S. Co-repressor activity of scaffold attachment factor B1 requires sumoylation. Biochem Biophys Res Commun. 2011 May 20;408(4):516-22. PMID: 21527249
Phillips S, Richter A, Oesterreich S, Rae JM, Flockhart DA, Perumal NB, Skaar TC. Functional characterization of a genetic polymorphism in the promoter of the ESR2 gene. Horm Cancer 2011, OCr 7. In press PMID: 21979797
Hartmaier RJ, Richter AS, Gillihan RM, Sallit JZ, McGuire SE, Wang J, Lee AV, Osborne CK, O'Malley BW, Brown PH, Xu J, Skaar TC, Philips S, Rae JM, Azzouz F, Li L, Hayden J, Henry NL, Nguyen AT, Stearns V, Hayes DF, Flockhart DA, Oesterreich S. A SNP in Steroid Receptor Coactivator-1 Disrupts a GSK3β Phosphorylation Site and Is Associated with Altered Tamoxifen Response in Bone. Mol Endo Dec 2011. PMID: 22174377 Watters RJ, Benos PV, Oesterreich S. To bind or not to bind - FoxA1 determines estrogen receptor action in breast cancer progression. Breast Cancer Res.14(3):312. 2012. PMID: 22713214
Cerne JZ, Zong L, Jelinek J, Hilsenbeck SG, Wang T, Oesterreich S, McGuire SE. BRCA1 promoter methylation status does not predict response to tamoxifen in sporadic breast cancer patients. Breast Cancer Res Treat. 2012 Jun 16. PMID: 22706629
Philips S, Rae JM, Oesterreich S, Hayes DF, Stearns V, Henry NL, Storniolo AM, Flockhart DA, Skaar TC. Whole genome amplification of DNA for genotyping pharmacogenetics candidate genes. Front Pharmacol. 2012;3:54. PMID: 22479249
145
Osmanbeyoglu HU, Hartmaier RJ, Oesterreich S, Lu X. Improving ChIP-seq peak-calling for functional co-regulator binding by integrating multiple sources of biological information. BMC Genomics. 13 Suppl 1:S1. 2012 PMID: 22369349 Watters RJ, Benos PV, Oesterreich S.To bind or not to bind - FoxA1 determines estrogen receptor action inbreast cancer progression. Breast Cancer Res. 2012 Jun 19;14(3):312. PMID: 22713214
Smith CL, Migliaccio I, Chaubal V, Wu MF, Pace MC, Hartmaier R, Jiang S, Edwards DP, Gutiérrez MC, Hilsenbeck SG, Oesterreich S. Elevated expression of the SMRT corepressor in breast cancer is associated with earlier tumor recurrence. Breast Cancer Res Treat 2012. PMID 23015261
Steinman RA, Brufsky AM, Oesterreich S. Zoledronic acid effectivencess against breast cancer metastases – arole for estrogen in the microenvironment? Breast Cancer Res 2012 14(5):213; PMID 23014660.
Patrick Pagano, Ph.D. Professor
Rodriguez AI, Gangopadhyay A, Kelley EE, Pagano PJ, Zuckerbraun BS, Bauer PM. HO-1 and CO decrease platelet-derived growth factor-induced vascular smooth muscle cell migration via inhibition of Nox1. Arterioscler Thromb Vasc Biol. 30(1):98-104, 2010.
Ardanaz N, Yang XP, Cifuentes ME, Haurani MJ, Jackson KW, Liao TD, Carretero OA and Pagano PJ. Lack of glutathione peroxidase 1 accelerates cardiac-specific hypertrophy and dysfunction in angiotensin II hypertension. Hypertension. 2010 Jan;55(1):116-23.
Ren Y, D'Ambrosio MA, Liu R, Pagano PJ, Garvin JL, Carretero OA. Enhanced myogenic response in the afferent arteriole of spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 298:H1769-1775, 2010.
Bauer EM, Qin Y, Miller TW, Bandle RW, Csanyi G, Pagano PJ, Bauer PM, Schnermann J, Roberts DD and Isenberg JS. Thrombospondin-1 supports blood pressure by limiting eNOS activation and endothelial-dependent vasorelaxation. Cardiovasc Res 88:471-481, 2010.
Ren Y, D'Ambrosio MA, Liu R, Pagano PJ, Garvin JL and Carretero OA.. Enhanced myogenic response in the afferent arteriole of spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol 298:H1769-1775, 2010.
Ardanaz N, Yang XP, Cifuentes ME, Haurani MJ, Jackson KW, Liao TD, Carretero OA and Pagano PJ. Lack of glutathione peroxidase-1 accelerates cardiac-specific hypertrophy and dysfunction in angiotensin II hypertension. Hypertension 55:116-123, 2010.
Al Ghouleh I and PJ Pagano. Endosomal ClC-3 and Nox1: Moving marksmen of redox signaling? Arterioscler Thromb Vasc Biol 31:240-242, 2011.
Cascino T, G Csányi, I Al Ghouleh, AC Montezano, RM Touyz, MJ Haurani and PJ Pagano. Adventitia-Derived Hydrogen Peroxide Impairs Relaxation of the Rat Carotid Artery via smooth muscle cell p38 Mitogen-Activated Protein Kinase. Antioxid. Redox Signal. 2010 Dec 2. [Epub ahead of print].
Csányi, G., Yao, M., Al Ghouleh, I., Rodriguez, A.I., Frazziano, G., Xiaojun, H., Kelley, EE., Isenberg, JS., and Pagano, PJ. Thrombospondin-1 Regulates Blood Flow via CD47 Receptor-Mediated Activation of NADPH Oxidase 1. Arterioscler. Thromb. Vasc. Biol. 32:2966-73 (2012).
146
Michael J. Palladino, Ph.D. Associate Professor
Hrizo SL and MJ Palladino. Hsp70 and Hsp90 mediate proteasomal degradation of TPIsugarkill that underlies pathogenesis. Neurobiology of Disease 40:676-683, 2010.
Palladino MJ. Modeling mitochondrial encephalomyopathy in Drosophila. Neurobiology of Disease 40:40-45, 2010.
Burton EA and MJ Palladino. Of fish, flies, worms and men: powerful approaches to neuropsychiatric disease using genetic models. Neurobiology of Disease 40:1-3, 2010.
Celotto AM, W.K. Chiu, W. Van Voorhies, Michael J. Palladino (2011). Modes of Metabolic Compensation during Mitochondrial Disease Using the Drosophila Model of ATP6 Dysfunction. PLoS ONE 6(10): e25823.
Liu, Zhaohui, Alicia M. Celotto, Guillermo Romero, Peter Wipf, Michael J. Palladino (2012). Genetically encoded redox sensor identifies the role of ROS in degenerative and mitochondrial disease pathogenesis. Neurobiology of Disease, 45(1):362-8. PMCID: PMC3225579.
Celotto, Alicia M., Zhaohui Liu, Andrew P. VanDemark, and Michael J. Palladino (2012). A novel Drosophila SOD2 mutant demonstrates a role for mitochondrial ROS in neurodevelopment and disease. Brain and Behavior, 2(4):423-34. PMCID: PMC3432965
Guillermo Romero, Ph.D. Associate Professor
Romero G, Sneddon WB, Yang Y, Wheeler D, Blair HC and Friedman PA. Parathyroid hormone receptor directly interacts with disheveled to regulate β-catenin signaling and osteoclastogenesis. J Biol Med 285:14756-14763, 2010.
Wang B, JA Ardrua, G Romero, Y Yang, RA Hall and PA Friedman. Na/H exchanger regulatory factors control parathyroid hormone receptors signaling by facilitating differential activation of G protein subunits. J Biol Chem 285:16976-16986, 2010.
Wheeler DS, SR Barrick, MJ Grubisha, AM Brufsky, PA Friedman and G Romero. Direct interaction between NHERF1 and Frizzled regulates β-catenin signaling. Oncogene 6:32-42, 2011.
Vilardaga JP, G Romero, PA Friedman and TJ Gardella. Molecular basis of parathyroid hormone receptor signaling and trafficking: A family β GPCR paradigm. Cell Mol Life Sci 68:1-13, 2011.
Malik, U.Z., Hundley, N.J, Romero, G., Radi, R., Freeman, B.A., Tarpey, M.M., and Kelley, E.E. Febuxostat inhibition of endothelial-bound XO: implications for targeting vascular ROS production. Free Radic Biol Med. 51:179-84, 2011. PMID: 21554948
Liu, Z., Celotto, A.M., Romero, G., Wipf, P., and Palladino, M.J. Genetically encoded redox sensor identifies the role of ROS in degenerative and mitochondrial disease pathogenesis. Neurobiol Dis. 2011 Aug 25. [Epub ahead of print]. PMID: 21889980
Samarasinghe, R.A., Di Maio, R., Volonte, D.,Galbiati, F., Lewis, M., Romero, G., and DeFranco, D. B. Nongenomic glucocorticoid receptor action regulates gap junction intercellular communication and neural progenitor cell proliferation PNAS 108:16657-16662, 2011. PMID: 21930911.
147
James Roppolo, Ph.D. Research Assistant Professor
Chen M, B Shen, J Wang, L Hailong, JR Roppolo, WC de Groat and C Tai. Influence of naloxone on inhibitory pudendal-to-bladder reflex in the cats. Experimental Neurology 224:282-291, 2010.
Tai C, B Shen, M Chen, J Wang, H Liu, JR Roppolo and WC de Groat. Suppression of bladder overactivity by activation of somatic afferent nerves in the foot. BJU International 107:303-309, 2010.
Tai C, M Chen, B Shen, J Wang, H Liu, JR Roppolo and WC deGroat. Plasticity of urinary bladder reflexes evoked by stimulation of pudendal afferent nerves after chronic spinal cord injury in cats. Experimental Neurology 228:109-117, 2011. Tai C, M Chen., B Shen, J Wang, H Liu, JR Roppolo and WC de Groat. Plasticity of urinary bladder reflexes evoked by stimulation of pudendal afferent nerves after chronic spinal cord injury in cats. Experimental Neurology 228:109-117, 2011.
Ta C, B Shen, M Chen, J Wang, J Roppolo and WC de Groat. Prolonged post-stimulation inhibition of bladder activity induced by tibial nerve stimulation in cats. American Journal of Physiology Renal Physiology 300: F385-F392, 2011.
Tai, C., Chen, M., Shen, B., Wang, J., Roppolo, J.R., and de Groat, W.C. Inhibition of bladder overactivity by stimulation of feline pudendal nerve using transdermal amplitude modulated signal (TAMS). British Journal of Urology International, 109:782-787. 2012
Tai, C., Shen, B., Wang, J., Liu, H., Subbaroyan, J., Roppolo, J.R. and de Groat W.C. Bladder inhibition by intermittent pudendal nerve stimulation in cat using transdermal amplitude-modulated signal (TAMS). Neurology and Urodynamics, 31:1181-1184, 2012.
Tai, C., Larson, J.A., Ogagan, P.D., Chen, G., Shen, B., Wang, J., Roppolo, J.R. and de Groat W.C. Differential role of opioid receptors in tibial nerve inhibition of nociceptive and non-nociceptive bladder reflexes in cats. Am J Physiol Renal Physiol. 302:F1090-F1097, 2012
Tai, C., P. Ogagan, D., Chen, G., Larson, J., Shen, B., Wang, J., Roppolo, J.R. and de Groat, W. C. Involvement of opioid receptors in inhibition of bladder overactivity induced by foot stimulation in cats. Journal of Urology, 188:1012-1016, 2012.
Zhang, F., Mally, A.D., Ogagan, P. D., Shen, B., Wang, J., Roppolo, J. R., de Groat, W.C. and Tai, C. Inhibition of bladder overactivity by a combination of tibial neuromodulation and tramadol treatment in cats. American Journal of Physiology – Renal Physiology, 302: F1576-1582, 2012.
Tai, C., Shen, B., Mally, A.D., Zhang, F., Zhao, S., Wang, J., Roppolo, J. R., and de Groat, W.C., Inhibition of micturition reflex by activation of somatic afferents in posterior femoral cutaneous nerve. Journal of Physiology (London) 590: 4945-4955, 2012.
Mally, A.D., Zhang, F., Matsuta, Y., Shen, B., Wang, J., Roppolo, J.R., de Groat, W.C. and Tai, C. Combination of foot stimulation and tramadol treatment reverses irritation induced bladder overactivity in cats. Journal of Urology, 188:2426-2432, 2012.
148
Chen G, JA Larson, PD Ogagan, B Shen, J Wang, JR Roppolo, WC de Groat and C T. Post-stimulation inhibitory effect on reflex bladder activity induced by activation of somatic afferent nerves in the foot. Journal of Urology 187:338-343, 2012.
Francisco Schopfer, Ph.D. Research Assistant Professor
Groeger AL, Cipollina C, Cole MP, Woodcock SR, Bonacci G, Rudolph TK, Rudolph V, Freeman BA and Schopfer FJ (2010) Cyclooxygenase-2 generates anti-inflammatory mediators from omega-3 fatty acids. Nature Chemical Biology 6(6):433-441.
Khoo NKH, Rudolph V, Cole MP, Golin-Bisello F, Schopfer FJ, Woodcock SR, Batthyany C and Freeman BA (2010) Activation of vascular endothelial nitric oxide synthase and heme oxygenase-1 expression by electrophilic nitro-fatty acids. Free Radical Biology and Medicine 48(2):230-239.
Rudolph TK, Rudolph V, Edreira MM, Cole MP, Bonacci G, Schopfer FJ, Woodcock SR, Franek A, Pekarova M, Khoo NKH, Hasty AH, Baldus S and Freeman BA (2010) Nitro-Fatty Acids Reduce Atherosclerosis in Apolipoprotein E-Deficient Mice. Arteriosclerosis Thrombosis and Vascular Biology 30(5):938-945.
Rudolph V, Rudolph TK, Schopfer FJ, Bonacci G, Woodcock SR, Cole MP, Baker PRS, Ramani R and Freeman BA (2010) Endogenous generation and protective effects of nitro-fatty acids in a murine model of focal cardiac ischaemia and reperfusion. Cardiovascular Research 85(1):155-166.
Schopfer FJ, Cole MP, Groeger AL, Chen CS, Khoo NKH, Woodcock SR, Golin-Bisello F, Motanya UN, Li Y, Zhang JF, Garcia-Barrio MT, Rudolph TK, Rudolph V, Bonacci G, Baker PRS, Xu HE, Batthyany CI, Chen YE, Hallis TM and Freeman BA (2010) Covalent Peroxisome Proliferator-activated Receptor gamma Adduction by Nitro-fatty Acids: selective ligand activity and anti-diabetic signaling actions. Journal of Biological Chemistry 285(16):12321-12333.
Sculptoreanu A, Kullmann FA, Artim DE, Bazley FA, Schopfer F, Woodcock S, Freeman BA and de Groat WC (2010) Nitro-Oleic Acid Inhibits Firing and Activates TRPV1-and TRPA1-Mediated Inward Currents in Dorsal Root Ganglion Neurons from Adult Male Rats. Journal of Pharmacology and Experimental Therapeutics 333(3):883-895.
Zhang JF, Villacorta L, Chang L, Fan ZZ, Hamblin M, Zhu TQ, Chen CS, Cole MP, Schopfer FJ, Deng CX, Garcia-Barrio MT, Feng YH, Freeman BA and Chen YE (2010) Nitro-Oleic Acid Inhibits Angiotensin II-Induced Hypertension. Circulation Research 107(4):540-U200.
Bonacci G, Asciutto EK, Woodcock SR, Salvatore SR, Freeman BA and Schopfer FJ (2011) Gas-Phase Fragmentation Analysis of Nitro-Fatty Acids. Journal of the American Society for Mass Spectrometry 22(9):1534-1551.
Bonacci G, Schopfer FJ, Batthyany CI, Rudolph TK, Rudolph V, Khoo NKH, Kelley EE and Freeman BA (2011) Electrophilic Fatty Acids Regulate Matrix Metalloproteinase Activity and Expression. Journal of Biological Chemistry 286(18).
Guo CJ, Schopfer FJ, Gonzales L, Wang P, Freeman BA and Gow AJ (2011) Atypical PKC zeta transduces electrophilic fatty acid signaling in pulmonary epithelial cells. Nitric Oxide-Biology and Chemistry 25(3):366-372.
149
Kansanen E, Bonacci G, Schopfer FJ, Kuosmanen SM, Tong KI, Leinonen H, Woodcock SR, Yamamoto M, Carlberg C, Yla-Herttuala S, Freeman BA and Levonen AL (2011) Electrophilic Nitro-fatty Acids Activate NRF2 by a KEAP1 Cysteine 151-independent Mechanism. Journal of Biological Chemistry 286(16):14019-14027.Artim DE, Bazely F, Daugherty SL, Sculptoreanu A, Koronowski KB, Schopfer FJ, Woodcock SR, Freeman BA and de Groat WC (2011) Nitro-oleic acid targets transient receptor potential (TRP) channels in capsaicin sensitive afferent nerves of rat urinary bladder. Exp Neurol 232(1):90-99.
Rothstein SN, Kay JE, Schopfer FJ, Freeman BA and Little SR (2012) A retrospective mathematical analysis of controlled release design and experimentation. Mol Pharm 9(11):3003-3011.
Sruti Shiva, Ph.D. Assistant Professor
Lundberg JO, Gladwin MT, Ahluwalia A, Benjamin N, Bryan NS, Butler A, Cabrales P, Fago A, Feelisch M, Ford PC, Freeman BA, Frenneaux M, Friedman J, Kelm M, Kevil CG, Kim-Shapiro DB, Kozlov AV, Lancaster JR Jr, Lefer DJ, McColl K, McCurry K, Patel RP, Petersson J, Rassaf T, Reutov VP, Richter-Addo GB, Schechter A, Shiva S, Tsuchiya K, van Faassen EE, Webb AJ, Zuckerbraun BS, Zweier JL, Weitzberg E. Nitrate and nitrite in biology, nutrition and therapeutics. Nat Chem Biol. 5(12):865-869, 2010.
Zuckerbraun BS, Shiva S, Ifedigbo E, Mathier MA, Mollen KP, Rao J, Choi JJW, Curtis E, Choi AMK and Gladwin MT. Nitrite potently inhibits hypoxic and inflammatory pulmonary arterial hypertension and smooth muscle proliferation via xanthine oxidoreductase dependent NO generation. Circulation 121(1):98-109, 2010.
Shiva S. Mitochondria as metabolizers and targets of nitrite. Nitric Oxide 22:64-74, 2010.
Basu S, Keszler A, Azarova N, Nwanze N, Perlegas A, Shiva S, Broniowska K, Hogg N and Kim-Shapiro DB. A novel role for cytochrome c: effective catalysis of S-nitrosothiol formation. Free Radic Biol Med. 48:255-263, 2010.
Shiva S, Rassaf T, Patel RP and Gladwin MT. Detecting the nitrite reductase and NO generating properties of heme globins using isolated mitochondria. Cardiovasc Res 89:566-573., 2011.
Frazier EP, Isenberg JS, Shiva S, Zhao L, Schlesinger P, Dimitry J, Abu-Asab MS, Tsokos M, Roberts DD, Frazier WA. Age-dependent regulation of skeletal muscle mitochondria by the thrombospondin-1 receptor CD47. Matrix Biol. 2011 Mar; 30(2):154-61. PMID: 21256215. PMCID: PMC3070423.
Cantu-Medellin N, Vitturi DA, Rodriguez C, Murphy S, Dorman S, Shiva S, Zhou Y, Jia Y, Palmer AF, Patel RP. Effects of T- and R-state stabilization on deoxyhemoglobin-nitrite reactions and stimulation of nitric oxide signaling. Nitric Oxide. 2011Aug 1; 25(2):59-69. PMID: 21277987. PMCID: PMC3115472.
Murillo D, Kamga C, Mo L, Shiva S. Nitrite as a mediator of ischemic preconditioning and cytoprotection. Nitric Oxide. 2011 Aug 1; 25(2):70-80. PMID: 21277988. PMCID: PMC3118399.
Tiso M, Tejero J, Basu S, Azarov I, Wang X, Simplaceanu V, Frizzell S, Jayaraman T, Geary L, Shapiro C, Ho C, Shiva S, Kim-Shapiro DB, Gladwin MT. Human neuroglobin functions as a redox regulated nitrite reductase. J Biol Chem. 2011 May 20; 286(20):18277-89. PMID: 21296891. PMCID: 3093900.
Alef MJ, Vallabhaneni R, Carchman E, Morris SM Jr, Shiva S, Wang Y, Kelley EE, Tarpey MM, Gladwin MT, Tzeng E, Zuckerbraun BS. Nitrite-generated NO circumvents dysregulated arginine/NOS signaling to protect against intimal hyperplasia in Sprague-Dawley rats. J Clin Invest. 2011 Apr 1; 121(4):1646-56. doi: 10.1172/JCI44079. Epub 2011 Mar 23. PMID: 21436585. PMCID: PMC3069768.
150
Al Ghouleh I, Khoo NK, Knaus UG, Griendling KK, Touyz RM, Thannickal VJ, Barchowsky A, Nauseef WM, Kelley EE, Bauer PM, Darley-Usmar V, Shiva S, Cifuentes-Pagano E, Freeman BA, Gladwin MT, Pagano PJ. Oxidases and peroxidases in cardiovascular and lung disease: New concepts in reactive oxygen species signaling. Free Radic Biol Med. 2011 Oct 1; 51(7):1271-88. Epub 2011 Jun 14. PMID: 21722728. PMCID: PMC3205968.
Hahm ER, Moura MB, Kelley EE, Van Houten B, Shiva S, Singh SV. Withaferin a-induced apoptosis in human breast cancer cells is mediated by reactive oxygen species. PLoS One. 2011; 6(8):e23354. PMID: 21853114. PMCID: PMC3154436.
Liu S, Yeh TH, Singh VP, Shiva S, Krauland L, Li H, Zhang P, Kharbanda K, Ritov V, Monga SP, Scott DK, Eagon PK, Behari J. β-catenin is essential for ethanol metabolism and protection against alcohol-mediated liver steatosis in mice. Hepatology. 2012 Mar: 55(3):931-40. PMID: 22031168. PMCID: PMC3288318.
Navina S, Acharya C, DeLany JP, Orlichenko LS, Baty CJ, Shiva S, Durgampudi C, Karlsson JM, Lee K, Bae KT, Furlan A, Behari J, Liu S, McHale T, Nichols L, Papachristou GI, Yadav D, Singh VP. Lipotoxicity causes multisystem organ failure and exacerbates acute pancreatitis in obesity. Sci Transl Med. 2011 Nov 2;3(107):107ra110. PMID: 22049070. PMCID: PMC3321362
Curtis E, Hsu LL, Noguchi A, Geary L, Shiva S. Oxygen regulates tissue nitrite metabolism. Antioxid Redox Signal. Antioxid Redox Signal. 2012 Oct 1; 17(7):951-61. PMID: 22098300. PMCID: PMC3411351.
Willaert A, Khatri S, Callewaert BL, Coucke PJ, Crosby SD, Lee JG, Davis EC, Shiva S, Tsang M, De Paepe A, Urban Z. GLUT10 is required for the development of the cardiovascular system and the notochord and connects mitochondrial function to TGFβ signaling. Hum Mol Genet. 2012 Mar 15; 21(6):1248-59. PMID: 22116938. PMCID: PMC3284116.
Kamga C, Krishnamurthy S, Shiva S. Myoglobin and mitochondria: a relationship bound by oxygen and nitric oxide. Nitric Oxide. 2012 May 15; 26(4):251-8. PMID: 22465476. PMCID: PMC3391710.
Cruz JA, Bauer EM, Rodriguez AI, Gangopadhyay A, Zeineh NS, Wang Y, Shiva S, Champion HC, Bauer PM. Chronic hypoxia induces right heart failure in caveolin-1-/- mice. Am J Physiol Heart Circ Physiol. 2012 Jun 15; 302(12):H2518-27. PMID: 22505641. PMCID: PMC3378264.
Neye N, Enigk F, Shiva S, Habazettl H, Plesnila N, Kuppe H, Gladwin MT, Kuebler WM. Inhalation of NO during myocardial ischemia reduces infarct size and improves cardiac function. Intensive Care Med. 2012 Aug; 38(8):1381-91. PMID: 22653370.
Totzeck M, Hendgen-Cotta UB, Luedike P, Berenbrink M, Klare JP, Steinhoff HJ, Semmler D, Shiva S, Williams D, Kipar A, Gladwin MT, Schrader J, Kelm M, Cossins AR, Rassaf T. Nitrite regulates hypoxic vasodilation via myoglobin-dependent nitric oxide generation. Circulation. 2012 Jul 17; 126(3):325-34. PMID: 22685116. PMCID: PMC3410747.
Ghosh S, Willard B, Comhair SA, Dibello P, Xu W, Shiva S, Aulak KS, Kinter M, Erzurum SC. Disulfide Bond As a Switch for Copper-Zinc Superoxide Dismutase Activity in Asthma. Antioxid Redox Signal. 2012 Sep 7. [Epub ahead of print] PMID: 22867017.
Mo L, Wang Y, Geary L, Corey C, Alef MJ, Beer-Stolz D, Zuckerbraun BS, Shiva S. Nitrite activates AMP kinase to stimulate mitochondrial biogenesis independent of soluble guanylate cyclase. Free Radic Biol Med.2012 Oct 1; 53(7):1440-50. doi: 10.1016/j.freeradbiomed.2012.07.080. PMID: 22892143.
Jill Siegfried, Ph.D. Professor Ostrow KL, Hoque MO, Loyo M, Brait M, Greenberg A, Siegfried JM, Grandis JR, Gaither Davis A, Bigbee WL, Rom W., Sidransky D. Molecular analysis of plasma DNA for the early detection of lung cancer by quantitative Methylation-specific PCR. Clin Cancer Res 2010 16(13):3463-72. PMCID2899894.
151
Stabile LP, Rothstein ME, Keohavong P, Lenzner D, Land S, Gaither-Davis A, Kim KJ, Kaminski N, Siegfried JM. Targeting of both the c-Met and EGFR pathways results in additive inhibition of lung tumorigenesis in transgenic mice. Cancers 2010 2, 2153-70 doi:10.3390/cancers2042153
Zeng X, Hood, BL, Sun M, Conrads TP, Day RS, Weissfeld JL, Siegfried JM, William L. Bigbee WL. Lung cancer serum biomarker discovery using glycoprotein capture and liquid chromatography mass spectrometry. J Proteome Res 2010 9(12):6440-9. PMID20931982.
Novello, S, Brachmer JR, Stabile L, Siegfried JM. Gender-Related Differences in Lung Cancer. In Principles and Practice of Lung Cancer: The Official Reference Text of the IASLC, Lippincott Williams & Wolters Kluwer, 2010, 353-368.
Ostroff, R, Bigbee, WL, Franklin, W, Gold L, Mehan M, Miller Y, Pass HI, Rom W, Siegfried JM, Stewart A, Walker JR, Weissfeld, JL, Williams S, Zichi D, Brody E. Unlocking biomarker discovery: Large scale application of aptamer proteomic technology for early detection of lung cancer. PLos One 2010 5(12):e15003. PMCID2999620.
Siegfried JM. Early changes in pulmonary gene expression following tobacco exposure shed light on the role of estrogen metabolism in lung carcinogenesis. 2010 Cancer Prev Res 3:707-17. PMCID2881174.
Zeng X, Hood BL, Zhao T, Conrads TP, Sun M, Gopalakrishnan V, Grover H, Weissfeld JL, Wilson DO, Siegfried JM, Bigbee WL. Lung Cancer Serum Biomarker Discovery Using Label Free Liquid Chromatography Tandem Mass Spectrometry. 2011. J Thoracic Oncol. 6:725-734. PMCID3104087.
Stabile LP, Dacic S, Land SR, Lenzer D, Dhir R, Aquafondata M, Landreneau RJ, Grandis JR, Siegfried. Combined analysis of estrogen receptor beta-1 and progesterone receptor expression identifies lung cancer patients with poor outcome. 2011 Clin Cancer Res 17:154-64 PMCID3050630.
Dulak, A., Gubish, CT, Stabile, LP, Henry C, and Siegfried JM. HGF-Independent Potentiation of EGFR Action by c-Met. 2011 Oncogene 30(33):3625-35 PMCID3126872.
Keohavong P, Kahkonen B, Kinchington E, Yin J, Jin J, Liu X, Siegfried JM, Di, YP. K-ras mutations in lung tumors from NNK-treated mice with lipopolysaccharide-elicited lung inflammation. 2011 Anticancer Res 31(9):2877-82. PMID 21868532.
Wilson DO, Ryan A, Fuhrman C, Schuchert M, Shapiro S, Siegfried JM, Weissfeld J. Doubling times and CT screen detected lung cancers in the Pittsburgh Lung Screening Study (PLuSS). 2011 Am J. Respir Crit Care Med. 185(1)85-9. PMCID3262038.
Buch SC, Diergaarde B, Nukui T, Day RS, Siegfried JM, Romkes M, Weissfeld JL. Genetic variability in DNA repair and cell cycle control pathway genes and risk of smoking-related lung cancer. 2011 Mol Carcinogenesis Oct 4. Doi:1002/mc.20858. PMCID3289753.
Xu H, Stabile LP, Gubish CT, Gooding WE, Grandis JR, Siegfried JM. Dual blockade of EGFR and c-Met abrogates redundant signaling and proliferation in head and neck carcinoma cells. 2011 Clin Cancer Res. 17(13):4425-38. PMCID3138116.
Horne ZD, Jack R, Gray ZT, Siegfried JM, Wilson DO, Yousem SA, Nason KS, Landreneau RJ, Luketich JD, Schuchert MJ. Increased levels of tumor-infiltrating lymphocytes are associated with improved recurrence-free survival in Stage IA non-small cell lung cancer. 2011 J. Surg. Res. 171(1):1-5. PMID21571304.
Bigbee WL, Gopalakrishnan V, Weissfeld JL, Wilson DO, Dacic S, Siegfried JM, Lokshin AE. A Multiplexed serum biomarker immunoassay panel discriminates clinical lung cancer patients from high-risk individuals found to be cancer-free by CT screening. 2012 J. Thoracic Oncol. 7(4):698-708. PMCID3388353.
Siegfried JM, Gubish CT, Rothstein ME, Henry C, Stabile LP. Combining the multi-targeted tyrosine kinase inhibitor vandetanib with the antiestrogen fulvestrant enhances its antitumor effect in non-small cell lung cancer. J. Thorac Oncol. 2012 7(3):485-95. PMCID3288546.
152
Carr SR, Schuchert MJ, Pennathur A, Wilson DO, Siegfried JM, Luketich JD, Landreneau RJ. Impact of tumor size on outcomes after anatomic lung resection for stage 1A non-small cell lung cancer based on the current staging system. J Thorac Cardiovas Surg. 2012 143(2):390-7. PMID22169444.
Tessema M, Yingling CM, Thomas CL, Klinge DM, Bernauer AM, Liu Y, Dacic S, Siegfried JM, Dahlberg SE, Schiller JH, Belinsky SA. SULF2 methylation is prognostic for lung cancer survival and increases sensitivity to topoisomerase-I inhibitors via induction of ISG15. Oncogene 2011 doi: 10.1038/onc.2011.577 PMCID3307938.
Leng S, Stidley CA, Liu Y, Edlund CK, Willink RP, Han Y, Landi MT, Thun M, Picchi MA, Bruse SE, Crowell RE, Van Den Berg D, Caporaso NE, Amos CI, Siegfried JM, Tesfaigzi Y, Gilliland FD, Belinsky SA. Genetic determinants for promoter hypermethylation in the lungs of smokers: A candidate gene-based study. Cancer Res. 2012 72(3):707-15. PMCID3271143.
Pu J, Gu S, Liu S, Zhu S, Wilson DO, Siegfried JM, Gur D. CT based computerized identification and analysis of human airways: A review. Med Phys 2012, 30(5):2603-16. PMCID:PMC3344883.
Schuchert MJ, Abbas G, Awais O, Pennathur A, Nason KS, Wilson DO, Siegfried JM, Luketich JD, Landreneau R. Anatomic segmentectomy for the solitary pulmonary nodule and early-stage lung cancer. Ann Thorac Surg. 2012 93(6):1780-5. PMID22483652.
Egloff AM, Liu X, Davis AL, Trevelline BK, Vuga M, Siegfried JM, Garndis JR. Elevated gastrin-releasing peptide receptor mRNA expression in buccal mucosa: Association with head and neck Squamous cell carcinoma. Head Neck. 2012 Mar 20. doi:10.1002/hed.22963 [Epub ahead of print]. PMID22431275
Schuchert MJ, Awais O, Abbas G, Horne ZD, Nason KS, Pennathur A, Souza AP, Siegfried JM, Wilson DO, Luketich JD, Landreneau RJ. Influence of age and IB status after resection of mode-negative non-small cell lung cancer. Ann Thorac Surg 2012 93(3):929-35; discussion 935-6. PMID:22364984.
Wheeler S, Siwak DR, Chai R, Lavalle C, Seethala RR, Wang L, Cieply K, Sherer C, Joy C, Mills GC, Argiris, Siegfried JM, Grandis JR, Egloff AM. Tumor epidermal growth factor receptor and EGFR PY1068 are independent prognostic indicators for head and neck squamous cell carcinoma. Clin Cancer Res. 2012 18(8):2278-89. PMID:22351687.
Nolen BM, Langmead CJ, Choi S, Lomakin A, Marrangoni A, Bigbee WL, Weissfeld JL, Wilson, DO, Dacic S, Siegfried JM, Lokshin AE. Serum biomarker profiles as diagnostic tools in lung cancer. Cancer Biomark. 10(1):3-12. PMID:22297547.
Egloff AM, Gaither Davis A, Shuai Y, Land S, Pilewski JM, Luketich JD, Landreneau R, Miller YE, Grandis JR, Siegfried JM. Gastrin-releasing peptide receptor expression in non-cancerous bronchial epithelia is associated with lung cancer: a case-control study. Respir Res. 2012 13:9. PMCID:3305653.
Flores, K, Stidley CA, Mackey AJ, Picchi MA, Stabler SP, Siegfried JM, Byers T., Berwick M, Belinsky SA, Leng S. Sex-specific association of sequence variants in CBS and MTRR with risk for promoter hypermethylation in the lung epithelium of smokers. Carcinogenesis 2012; doi:10.1093/carcin/bgs194.
Hershberger PA, Siegfried JM. Estrogen Receptor Signaling in Lung Cancer. In Cell Signaling & Molecular Targets in Cancer. Springer, 2012,191-210.
Shivendra Singh, Ph.D. Professor
Xiao, D., and Singh, S.V.: Phenethyl isothiocyanate sensitizes androgen-independent human prostate cancer cells to docetaxel-induced apoptosis in vitro and in vivo. Pharm. Res., 27: 722-731, 2010. PMCID: PMC2837783.
153
Hahm ER and Singh SV. Sulforaphane inhibits constitutive and interleukin-6-induced activation of signal transducer and activator of transcription 3 in prostate cancer cells. Cancer Pre. Res 3:484-494, 2010.
Sharma R, Sharma A, Chaudhary P, Pearce V, Vatsyayan R, Singh SV, Awasthi S and Awasthi YC. Role of lipid peroxidation in cellular responses to D,L-sulforaphane, a promising cancer chemopreventive agent. Biochemistry 49:3191-3202, 2010.
Xiao D and Singh SV. p66shc is indispensable for phenethyl isothiocyanate-induced apoptosis in human prostate cancer cells. Cancer Res 70:3150-3158, 2010.
Warin R, Xiao D, Arlotti JA, Bommareddy A and Singh SV. Inhibition of human breast cancer xenograft growth by cruciferous vegetable constituent benzyl isothiocyanate. Mol. Carcinogenesis 49: 500-507, 2010.
Herman-Antosiewicz A, Kim YA, Kim SH, Xiao D and Singh SV. Diallyl trisulfide-induced G2/M phase cell cycle arrest in DU145 cells is associated with delayed nuclear translocation of cyclin-dependent kinase 1. Pharm Res 27:1072-1079, 2010.
Kim SH and Singh SV. p53-Independent apoptosis by benzyl isothiocyanate in human breast cancer cells is mediated by suppression of XIAP expression. Cancer Prev Res 3: 718-726, 2010.
Xiao D, Powolny AA, Barbi de Moura M, Kelley EA, Bommareddy A, Kim S-H, Hahm ER, Normolle D, Van Houten B and Singh SV. Phenethyl isothiocyanate inhibits oxidative phosphorylation to trigger reactive oxygen species-mediated death of human prostate cancer cells. J Biol Chem 285:26558-26569, 2010.
Lee JS, Hahm ER and Singh SV. Withaferin A inhibits activation of signal transducer and activator of transcription 3 in human breast cancer cells. Carcinogenesis 31:1991-1998, 2010.
Chandra-Kuntal K and Singh SV. Diallyl trisulfide inhibits activation of signal transducer and activator of transcription 3 in prostate cancer cells in culture and in vivo. Cancer Prev Res 3:1473-1483, 2010.
Powolny A. and Singh SV. Differential response of normal (PrEC) and cancerous human prostate cells (PC-3) to phenethyl isothiocyanate-mediated changes in expression of antioxidant defense genes. Pharm Res 27:2766-2775, 2010.
Stan SD, Singh SV and Brand RE. Chemoprevention strategies for pancreatic cancer. Nature Rev Gastroenterol Hepatol 7:347-356, 2010.
Chandra-Kuntal, K, and Singh, S.V.: Diallyl trisulfide inhibits activation of signal transducer and activator of transcription 3 in prostate cancer cells in culture and in vivo. Cancer Prev. Res., 3: 1473-1483, 2010.
Leeman-Neill, R.J., Seethala, R.R., Singh, S.V., Freilino, M.L., Bednash, J.S., Thomas, S.M., Panahandeh, M.C., Gooding, W.E., Joyce, S.C., Lingen, M.W., Neill, D.B., and Grandis, J.R.: Inhibition of EGFR-STAT3 signaling with erlotinib prevents carcinogenesis in a chemically-induced mouse model of oral squamous cell carcinoma. Cancer Prev. Res., 4: 230-237, 2011.
Kim, S.H., Nagalingam, A., Saxena, N.K., Singh, S.V., and Sharma, D. Benzyl isothiocyanate inhibits oncogenic actions of leptin in human breast cancer cells by suppressing activation of signal transducer and activator of transcription 3. Carcinogenesis, 32: 359-367, 2011.
154
Xiao, D., Zeng, Y., Prakash, L., Badmaev, V., Majeed, M., and Singh, S.V.: Reactive oxygen species-dependent apoptosis by gugulipid extract of Ayurvedic medicine plant Commiphora mukul in human prostate cancer cells is regulated by c-JUN N-terminal kinase. Mol. Pharmacol., 79: 499-507, 2011.
Powolny, A.A., Bommareddy, A., Hahm, E.R., Normolle, D.P., Beumer, J.H., Nelson, J.B., and Singh, S.V.: Chemopreventative potential of the cruciferous vegetable constituent phenethyl isothiocyanate in a mouse model of prostate cancer. J. Natl. Cancer Inst., 103: 571-584, 2011. .
Powolny, A.A., Singh, S.V., Melov, S., Hubbard, A., and Fisher, A.L.: The garlic constituent Diallyl trisulfide increases the lifespan of C. elegans via skn-1 activation. Exp. Gerontol., 46: 441-452, 2011.
Kim, S.H., Bommareddy, A., and Singh, S.V.: Garlic constituent diallyl trisulfide suppresses X-linked inhibitor of apoptosis protein in prostate cancer cells in culture and in vivo. Cancer Prev. Res., 4: 897-906, 2011.
Sehrawat, A., and Singh, S.V.: Benzyl isothiocyanate inhibits epithelial-mesenchymal transition in cultured and xenografted human breast cancer cells. Cancer Prev. Res., 4: 1107-1117, 2011. .
Hahm, E.R., Huang, Y., and Singh, S.V.: Withaferin A suppresses estrogen receptor-α expression in human breast cancer cells. Mol. Carcinogenesis, 50: 614-624, 2011.
Hahm, E.R., Moura, M.B., Kelley, E.E., Van Houten, B., Shiva, S., and Singh, S.V.: Withaferin A-induced apoptosis in human breast cancer cells is mediated by reactive oxygen species. PLoS ONE, 6: e23354, 2011..Kim, S.H., Sehrawat, A., Sakao, K., Hahm, E.R., and Singh, S.V.: Notch activation by phenethyl isothiocyanate attenuates its inhibitory effect on prostate cancer cell migration. PLoS ONE, 6: e26615, 2011.
Hahm, E.R, and Singh, S.V.: Bim contributes to phenethyl isothiocyanate-induced apoptosis in breast cancer cells. Mol. Carcinogenesis, 51: 465-474, 2012.
Sakao, K., and Singh, S.V.: D,L-Sulforaphane-induced apoptosis in human breast cancer cells is regulated by the adapter protein p66Shc. J. Cell. Biochem., 113: 599-610, 2012. .
Antony, M.L., Kim, S.H., and Singh, S.V.: Critical role of p53 upregulated modulator of apoptosis in benzyl isothiocyanate-induced apoptotic cell death. PLoS ONE, 7(2): e32267, 2012. .
Xiao, D., Bommareddy, A., Kim, S.H., Sehrawat, A., Hahm, E.R., and Singh, S.V.: Benzyl isothiocyanate causes FoxO1-mediated autophagic death in human breast cancer cells. PLoS ONE, 7(3): e32597, 2012.
Sakao, K., Desineni, S., Hahm, E.R., and Singh, S.V.: Phenethyl isothiocyanate suppresses inhibitor of apoptosis family protein expression in prostate cancer cells in culture and in vivo. Prostate, 72: 1104-1116,2012.
Kim, S.H., Sehrawat, A., and Singh, S.V.: Notch2 activation by benzyl isothiocyanate impedes its inhibitory effect on breast cancer cell migration. Breast Cancer Res. Treat., 134: 1067-1079, 2012.
Singh, S.V., Kim, S.H., Sehrawat, A., Arlotti, J.A., Hahm, E.R., Sakao, K., Beumer, J.H., Jankowitz, R.C., Chandra-Kuntal, K., Lee, J., Powolny, A.A., and Dhir, R.: Biomarkers of phenethyl isothiocyanate-mediated mammary cancer chemoprevention in a clinically relevant mouse model. J. Natl. Cancer Inst., 104: 1228-1239, 2012.
155
Hahm, E.R., Chandra-Kuntal, K., Desai, D., Amin, S., and Singh, S.V.: Notch activation is dispensable for D,L-sulforaphane-mediated inhibition of human prostate cancer cell migration. PLoS One, 7: e44957, 2012. .
Lee, J., Sehrawat, A., and Singh, S.V.: Withaferin A causes activation of Notch2 and Notch4 in human breast cancer cells. Breast Cancer Res. Treat., 136: 45-56, 2012.
Sehrawat, A., Arlotti, J.A., Murakami, A., and Singh, S.V.: Zerumbone causes Bax- and Bak-mediated apoptosis in human breast cancer cells and inhibits orthotopic xenograft growth in vivo. Breast Cancer Res. Treat., 136: 429-441, 2012.
Powolny, A.A., Bommareddy, A., and Singh, S.V.: Slow but steady progress in cancer chemoprevention with phenethyl isothiocyanate: fulfilled promises and translational challenges. In: Nutraceuticals and Cancer (Sarkar, F.H., ed.), pp. 231-258, Springer, 2012. Singh, S.V., and Singh K.: Cancer chemoprevention with dietary isothiocyanates mature for clinical translational research. Carcinogenesis, 33: 1833-1842, 2012.
Robert Sobol, Ph.D. Associate Professor
Tang, J, Goellner E, Wang X, Trivedi RN, St. Croix CM, Jelezcova E, Svilar D, Brown AR and Sobol RW. Bioenergetic metaboites regulate base excision repair dependent cell death in response to DNA damage. Molecular Cancer Research 8:67-79, 2010.
Asagoshi K, Liu Y, Masaoka A, Lan L, Prasad R, Horton JK, Brown AR, Wang, X, Sobol RW, Bdour HM, Taylor J-S, Yasui A and Wilson SH. DNA polymerase β-dependent long patch base excision repair in living cells. DNA Repair 9:109-119, 2010.
Jelezcova E, Trivedi RM, Wang X, Tang J, Brown AR, Goellner EM, Schamus S, Fornsaglio JL and Sobol RW. Parp1 activation in mouse embryonic fibroblasts promotes Pol β-dependent cellular hypersensitivity to alklation damage. Mutation Research – Fundamental and Molecular Mechanisms of Mutagenesis 688:57-67, 2010.
Langevin SM, Bunker CH, Stone RA, Grandis JR, Sobol RW and Taioli E. MicroRNA-137 hypermethylation in oral rinses from pateitns with squamous cell carcinoma of the head and neck. Carcinogenesis 31:864-870, 2010.
Pollack, I. F., Hamilton, R.L., Sobol, R.W., Nikiforova, M.N., Nikiforov, Y.E., Lyons-Weiler, M.A., LaFramboise, W.A., Burger, P.C., Brat, D.J., Rosenblum, M.K., Gilles, F.H., Yates, A.J., Zhou, T., Cohen, K.J., Finlay, J.L. and Jakacki, R.I. “Mismatch Repair Deficiency is an Uncommon Mechanismof Alkylator Resistance in Pediatric Malignant Gliomas: A Report from the Children’s Oncology Group”(2010) Pediatric Blood & Cancer, Dec 1;55(6):1066-1071.
Pollack, I. F., Hamilton, R.L., Sobol, R.W., Nikiforova, M.N., Lyons-Weiler, M.A., LaFramboise, W.A., Burger, P.C., Brat, D.J., Rosenblum, M.K., Holmes, E.J., Zhou, T. and Jakacki, R.I. “IDH1 Mutationsare Common in Malignant Gliomas Arising in Adolescents: A Report from the Children’s OncologyGroup” (2011) Child’s Nervous System, Jan; 27(1): 87-94.
Langevin, S.M., Bunker, C.H., Stone, R. A., Seethala, R.R., Grandis, J.R., Sobol, R.W., & Taioli, E “MicroRNA-137 promoter methylation is associated with poorer overall survival in patients with squamous cell carcinoma of the head and neck” (2011) Cancer, Apr 1;117(7):1454-62.
156
Lisovich, A., Chandran, U.R., Lyons-Weiler, M.A., LaFramboise, W.A., Brown, A.R., Jakacki, R.I., Pollack, I.F. and Sobol, R.W. “A novel SNP analysis method to detect copy number alterations with an unbiased reference signal directly from tumor samples” (2011) BMC Medical Genomics, Jan26;4(1):14.
Goellner, E.M., Grimme, B., Brown, A.R., Lin, Y., Wang, X.H., Sugrue, K.F., Mitchell, L., Trivedi, R.N., Tang, J.B., and Sobol, R.W. “Overcoming temozolomide resistance in glioblastoma via dual inhibition of NAD+ biosynthesis and base excision repair” (2011) Cancer Research, Mar 15;71(6):2308-17.
Tang, J.B., Svilar, D., Trivedi, R.N., Wang, X.H., Goellner, E.M., Moore, B., Hamilton, R.L., Banze, L.A., Brown, A.R., and Sobol, R.W. “N-methylpurine DNA glycosylase and DNA Polymerase ß modulate BER inhibitor potentiation of glioma cells to temozolomide” (2011) Neuro-Oncology, May;13(5):471-86.
Yoder K.E., Espeseth A., Wang X.H., Fang, Q., Russo, M.T., Lloyd, R.S., Hazuda, D., Sobol, R.W., and Fishel, R. “The Base Excision Repair Pathway Is Required for Efficient Lentivirus Integration”(2011) PLoS ONE, Mar 23;6(3):e17862.
Kothandapani, A., Dangeti, V.S., Akshada Sawant, Brown, A.R., Banze, L.A., Wang, X.H., Sobol, R.W. and Patrick, S.M., “Novel Role of Base Excision Repair (BER) in Mediating Cisplatin Cytotoxicity”(2011) J. Biol. Chem., Apr 22; 286(16):14564-74.
Li, H., Wang, P., Sun, Q., Ding,W.X., Yin, X.M., Sobol, R.W., Stolz, D.B., Yu, J., and Zhang, L., “Following cytochrome c release, autophagy is inhibited during chemotherapy-induced apoptosis by caspase-8-mediated cleavage of Beclin-1” (2011) Cancer Research, May 15; 71(10): 3625-34.
Mutamba, J.T., Svilar, D., Prasongtanakij, S., Wang, X.H., Lin, Y.C., Dedon, P.C., Sobol, R.W. and Engelward, B.P., “XRCC1 and Base Excision Repair Balance in Response to Nitric Oxide” (2011) DNARepair, 10: 1282-1293; PMID: 22041025; PMCID: N/A.
Svilar, D., Vens, C., and Sobol, R.W. “Quantitative, real-time analysis of base excision repair activity in cell lysates utilizing lesion-specific molecular beacons” (2012) J. Vis Exp., Aug 6;(66), pii: 4168.
Srinivasan, A., Wang, L., Cline, C.J., Sobol, R.W., Xie, X.Q., and Gold,B. “The Identification andCharacterization of Human AP Endonuclease-1 Inhibitors” (2012) Biochemistry, 51(31): 6266-6259.
Svilar, D., Dyavaiah, M., Brown, A.R., Tang, J.B., Li, J., McDonald, P.R., Shun, T.Y., Braganza, A., Wang, X.H., Maniar, S., St Croix, C.M., Lazo, J.S., Pollack, I.F., Begley, T.J., and Sobol, R.W. “Alkylation Sensitivity Screens Reveal a Conserved Cross-species Functionome” (2012) MolecularCancer Research; (10): 1580-1596. Li, J., Luthra, S., Wang, X.H., Chandran, U.R., & Sobol, R.W. “Transcriptional profiling revealselevated Sox2 in DNA Polymerase ß null mouse embryonic fibroblasts” (2012) Am J Cancer Res, 2(6):699-713.
Vens, C., and Sobol, R.W. “Targeting DNA Repair Pathways for Cancer Therapy” in Cell Death Signalingin Cancer Biology and Treatment (2012) Daniel E. Johnson, Editor; Book Series Cell Death in Biology and Diseases, Xiao-Ming Yin, and Zheng Dong, Editors; Springer, New York.
157
Gyun Jee Song, Ph.D. Research Assistant Professor
Song GJ, Barrick S, Leslie KL, Sicari B, Fiaschi-Taesch N and Bisello A. EBP50 inhibits the anti-mitogenic action of the Parathyroid Hormone type 1 Receptor in vascular smooth muscle cells. Journal of Molecular and Cellular Cardiology 2010; 49 (6): 1012-21.
Song GJ, Barrick S, Leslie KL, Bauer PM, Friedman PA, Fiaschi-Taesch N and Bisello A. The scaffolding protein EBP50 promotes VSMC proliferation and neointima formation by regulating p21cip1 stability. Arteriosclerosis, Thrombosis and Vascular Biology 2012;32(1):33-41.
Song GJ, Leslie KL, Barrick S, Bougoin S, Taboas JM and Bisello A. EBP50 promotes focal adhesion turnover and vascular smooth muscle cells migration. Journal of Molecular and Cellular Cardiology 2012: 53(6):809-19.
Laura Stabile, Ph.D. Research Assistant Professor
Stabile LP, Rothstein ME, Keohavong P, Lenzner D, Land SR, Gaither-Davis AL, Kim KJ, Kaminski N, Siegfried JM. Therapeutic targeting of both the c-Met and EGFR pathways results in additive inhibition of lung tumorigenesis in transgenic mice. Cancers. 2010; 2(4), 2153-70.
Novello S, Brahmer JR, Stabile LP, Siegfried JM. Sex-Related Differences in Lung Cancer. Lung Cancer IV. April 2010.
Stabile LP, Dacic S, Land SR, Lenzner D, Dhir R, Aquafondata M, Landreneau RJ, Siegfried JM. Combined analysis of estrogen receptor beta-1 and progesterone receptor expression identifies lung cancer patients with poor outcome. Clin Cancer Res. 2011; Jan 1;17(1):154-64.
Dulak AM, Gubish CT, Stabile LP, Henry C, Siegfried JM. HGF-independent potentiation of EGFR action by c-Met. Oncogene. 2011; Aug 18;30(33):3625-35.
Xu H, Stabile LP, Gubish CT, Gooding WE, Grandis JR, Siegfried JM. Dual blockade of EGFR and c-Met abrogates redundant signaling and proliferation in head and neck carcinoma cells. Clin Cancer Res. 2011 Jul 1;17(13):4425-38.
Siegfried JM, Gubish CT, Rothstein ME, Henry C, Stabile LP. Combining the multi-targeted tyrosine kinase inhibitor vandetanib with the anti-estrogen fulvestrant enhances its anti-tumor effect in non-small cell lung cancer. J Thoracic Oncol. 2012 Mar;7(3):485-95.
Henry C, Lopez-Chavez A, Stabile LP, Siegfried JM. HGF airway over-expression leads to enhanced pulmonary vascularization without induction of VEGF. Current Angiogenesis. 2012;1:52-63.
Stabile LP, Rothstein ME, Cunningham DE, Land SR, Dacic S, Keohavong P, Siegfried JM. Prevention of tobacco carcinogen-induced lung cancer in female mice using antiestrogens. Carcinogenesis. 2012 Nov;33(11):2181-9.
158
Ben Van Houten, Ph.D. Professor
Peng X, Ghosh A, Van Houten B and Greenberg MM. Nucleotide excision repair of a DNA Interstrand cross-link produces single and double strand breaks. Biochemistry 49(1):11-19, 2010.
Boyd WA, Crocker TL, Rodriguez AM, Leung MCK, Lehmann DW, Freedman FH, Van Houten B and Meyer JN. Nucleotide excision repair genes are expressed at low levels and are not detectably inducible in Caenorhabditis elegans somatic tissues, but their function is required for normal adult life after UVC exposure. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis 683(1-2):57-67, 2010.
A. C Haugen, N.A. Di Prospero, J.S. Parker, R.D Fannin, J. Chou, J.N. Meyer, C.Halweg, J.B. Collins, A. Durr, K. Fischbeck, and B. Van Houten. Altered gene expression and DNA damage in peripheral blood cells from Friedreich's Ataxia patients: cellular model of pathology. PLoS Genetics Jan 15;6(1):e1000812, 2010.
Qian W and Van Houten B. Alterations in bioenergetics due to changes in mitochondrial DNA copy number. In: Methods, "Mitochondrial DNA Replication and Repair". William Copeland, Ed. Elsevier, 2010.
Peng Y, Wang H, dos Santos LS, Kisker C and Van Houten B. Nucleotide excision repair form bacteria to humans: structure-function studies. In: Chemical Carcinogenesis, Trevor M. Penning, Springer, Ed. New York, New York, 2010.
N.M.Kad, H. Wang, G. G. Kennedy, D.M. Warshaw, and B. Van Houten. (2010). Collaborative dynamic DNA scanning by nucleotide excision repair proteins investigated by single-molecule fluorescence imaging of quantum dot labeled proteins. Molecular Cell, 37(5):702-13.
M.B. Moura, L. S. Santos and B. Van Houten. (2010). Mitochondrial dysfunction in neurodegenerative diseases and cancer. IN: Environmental and Molecular Mutagenesis, 51(5):391- 405. Special Issue, The Mitochondrial Genome: Dynamics, Mechanisms of Repair, and a Target in Disease and Therapy. David Wilson, Ed. W. Qian and B. Van Houten. (2010). Alterations in bioenergetics due to changes in mitochondrial DNA copy number.. IN: Methods, 51(4)452-457. Special Issue: "Mitochondrial DNA Replication and Repair". William Copeland, Ed. Elsevier.
M. Sander, T.J. Begley, C. Desaintes, A.C. Gavin, R. Pelroy, J. Pothof, Y. Shiloh, D. van Gent, B. Van Houten, M. Yaffe, L. Mullenders. (2010). 3rd US-EU workshop: systems level understanding of DNA damage responses. Mutat Res. 692(1-2):53-60.
X. Xiao, A. A. Powolny, M. Barbi de Moura, A. Bommareddy, E.R. Hahm, B. Van Houten, and S. V. Singh. (2010). Phenethyl isothiocyanate inhibits oxidative phosphorylation to trigger reactive oxygen species-mediated death of human prostate cancer cells. J. Biol. Chem. 285(34):26558-69.
J. A. Graves, K. Rothermund, T. Wang, W. Qian, B. Van Houten, and E. V. Prochownik. (2010). Point mutations in c-Myc uncouple neoplastic transformation from multiple other phenotypes in rat fibroblasts PLoS One 5(10):e13717.
B. Van Houten (2010). Bacterial Nucleotide Excision Repair. IN: “Encyclopedia of Biological Chemistry”, William Lennarz, M. Daniel Lane (Eds.). Academic Press/Elsevier Science. (pp. 134-142.)
159
Y. Peng, H. Wang, L. Santana-Santos, C. Kisker and B. Van Houten. Chapter 13. Nucleotide excision repair from bacteria to humans: structure-function studies. IN: “Chemical Carcinogenesis”, Trevor M. Penning, Springer, Ed. New York, New York. (2011) (pp. 267-296).
D. Simsek, A. Furda, Y. Gao, J. Artus, E. Brunet, A. Hadjantonakis, B. Van Houten, S. Shuman, P. J. McKinnon & M. Jasin. (2011). Crucial roles for DNA ligase III in mitochondria but not in XRCC1-dependent repair. Nature Mar 10;471(7337):245-8.
N. Mazloum, M.A. Stegman, D.L. Croteau, B. Van Houten, N.S. Kwon, Y. Ling, C. Dickinson, A. Venugopal, M.A. Towheed, and C. Nathan. (2011). Identification of a chemical that inhibits the mycobacterial UvrABC complex in nucleotide excision repair. Biochemistry. Mar 1;50(8):1329-35.
R.K. Dagda, A.M. Gusdon, I. Pien, S. Strack , S. Green, C. Li, B. Van Houten, S.J. Cherra 3rd, and C.T. Chu. (2011). Mitochondrially localized PKA reverses mitochondrial pathology and dysfunction in a cellular model of Parkinson's disease. Cell Death Differ. 18(12):1914-23. S. Varum, A.S. Rodrigues, M.B. Moura, O. Momcilovic, C.A. Easley 4th, J. Ramalho-Santos, B. Van Houten , G. Schatten G. (2011). Energy metabolism in human pluripotent stem cells and their differentiated counterparts. PLoS One. 6(6):e20914..
Y. Liu, D. Reeves, K. Kropachev, Y. Cai , S. Ding, M. Kolbanovskiy, A. Kolbanovskiy, J.L. Bolton, S. Broyde, B. Van Houten , N.E. Geacintov. (2011). Probing for DNA damage with β-hairpins: Similarities in incision efficiencies of bulky DNA adducts by prokaryotic and human nucleotide excision repair systems in vitro. DNA Repair (Amst), 10(7):684-96.
D. Tang, R. Kang, K.M. Livesey, G. Kroemer, T.R. Billiar, B. Van Houten , H.J. Zeh 3rd, and M.T. Lotze. (2011). High-mobility group box 1 is essential for mitochondrial quality control. Cell Metab. 13(6):701-11.
E-R. Hahm, M. B Moura, E. E. Kelley, B. Van Houten, S. Shiva, and S.V. Singh (2011). Withaferin A-induced apoptosis in human breast cancer cells is mediated by reactive oxygen species. PLOS One. 6(8):e23354.
A.W. Tann, I. Boldogh, G. Meiss , W. Qian, B. Van Houten, S. Mitra, and B. Szczesny. (2011) Apoptosis induced by persistent single-strand breaks in the mitochondrial genome:Critical role of EXOG (5' EXO/Endonuclease) in their repair. J Biol Chem.286(37):31975-83. D. Green, and B. Van Houten. (2011). Snapshot: Mitochondrial Quality Control. Cell 147(4):950-951.
K. Asagoshia , W. Lehmann , Lucas Santana-Santos, R. Prasada, B. Van Houten and S.H. Wilson. (2011). Single-nucleotide base excision repair DNA polymerase activity in C. elegans in the absence of DNA polymerase β. Nucleic Acids Research 40(2):670-81.
H. M. Roth, J. Römer, V. Grundler , B. Van Houten , C. Kisker, and I. Tessmer. (2012). The nucleotide excision repair helicase XPB regulates Bax1 DNA incision. DNA Repair. 11(3):286-93.
A. M. Gusdon, J.Zhu, B. Van Houten, and C.T. Chu. (2012). ATP13A2 regulates mitochondrial bioenergetics through macroautophagy. Neurobiol Dis. 45(3):962-72.
E.C. Nakajima, and B. Van Houten. (2012). Metabolic Symbiosis in cancer: refocusing the Warburg lens. Mol. Carcinogenesis [Epub ahead of print]
160
B. Pascucci, T. Lemma, E. Iorio, S. Giovannini, B. Vaz, I. Iavarone, A. Calcagnile, L. Narciso, P. Degan, F. Podo, V. Roginskya, B. M. Janjic, B. Van Houten, M.Stefanini, E. Dogliotti and M. D’Errico. (2012). An altered redox balance mediates the hypersensitivity of Cockayne syndrome primary fibroblasts to oxidative stress Aging Cell. Jun;11(3):520-9. Epub 2012 Apr 5 PubMed.
H-Y. Cho, B. Van Houten, X. Wang, L. Miller-DeGraff, J. Fostel, W. Gladwell, L. Perrow, V. Panduri, L. Kobzik, M. Yamamoto, D. A. Bell and S. R. Kleeberger. (2012) Targeted deletion of Nrf2 impairs lung development and oxidant injury in neonatal mice. Antioxidant & Redox Signaling.
X. Liang, M.E. de Vera, W.J. Buchser, A. Romo de Vivar Chavez , P. Loughran, D. Beer-Stolz, P. Basse, T.Wang, B. Van Houten , H.J. Zeh, and M. Lotze M. (2012). Inhibiting systemic autophagy during interleukin 2 immunotherapy promotes long-term Tumor regression. Cancer Res Jun 1;72(11):2791-801. Apr 3. PMCID: PMC3417121 J.H. Zhu, A.M. Gusdon, H. Cimen, B. Van Houten, E. Koc, C.T. Chu. (2012).Impaired mitochondrial biogenesis contributes to depletion of functional mitochondria in chronic MPP(+) toxicity: dual roles for ERK1/2. Cell Death Dis. 2012 May24
J.A. Graves , Y. Wang, S. Sims-Lucas , E. Cherok, K. Rothermund , M.F. Branca, J. Elster, D. Beer-Stolz, B. Van Houten, J. Vockley, E.V. Prochownik. (2012). Mitochondrial Structure, Function and Dynamics Are Temporally Controlled by c-Myc. PLoS One. 2012;7(5):e37699.
A. M. Furda, A. M. Marrangonib, A. Lokshinb , B.Van Houten. (2012). Oxidants and not alkylating agents induce rapid mtDNA loss and mitochondrial dysfunction. DNA Repair 11(8):684-92. Epub 2012 Jul 4.
C.,Furtado, M. Kunrath-Lima, M.A. Rajão, I.C. Mendes, M.B Moura, P.C. Campos, A.M. Macedo, G.R. Franco, S.D.J.Pena, S.M.R.Teixeira, B. Van Houten, and C.R. Machado. (2012). Functional characterization of 8-oxoguanine DNA glycosylase of Trypanosoma cruzi. PLoS One ; 7(8):e42484.
M. Barbi de Moura, G. Vincent, S. L. Fayewicz, N. Bateman, B. Hood, M. Sun, J. Suhan, S. Duensing, Y. Yin, C. Sander, J. M. Kirkwood, D. Becker, T. P. Conrads, B. Van Houten, and S. J Moschos. (2012). Mitochondrial respiration - an important therapeutic target in melanoma PLoS One 7(8):e40690.
J.I. Yeh, A. S. Levine, S. Du, U. Chinte, H. Ghodke, H. Wang, Shi, H, C. L. Hsieh, T. Vu, J. F. Conway, B. Van Houten, and V. Rapić-Otrin (2012). Damaged DNA induced UV-DDB dimerization and its roles on chromatinized DNA repair . Proc. Natl. Acad. Sci. 109(41):E2737-46.
S. Brar, J. Meyer, C. Bortner, B. Van Houten, and W. Martin, II (2012). Mitochondrial DNA-depleted A549 cells are resistant to bleomycin. American Journal of Physiology - Lung Cellular and Molecular Physiology 303(5):L413-24.
A. Furda, A. S. Bess, J. N. Meyer, and B. Van Houten. Analysis of DNA damage and repair in nuclear and mitochondrial DNA of animal cells using quantitative PCR. IN Methods Mol Biol. DNA Repair Protocols, Third Edition. Ed. Lotte Bjergbaek Springer Science+Business Medical LLC, NY, NY 920:111-132, 2012.
N. M. Kad and B. Van Houten. Dynamics of lesion processing by bacterial nucleotide excision repair proteins. IN: Mechanisms of DNA Repair, in the Progress in Molecular Biology and Translational Science series. Ed. Paul Doetsch. Elsevier, Amsterdam, Holland, 110:1-24, 2012.
161
Jean-Pierre Vilardaga, Ph.D. Assocaite Professor
Klenk C, Vetter T, Zuern A, Vilardaga JP, Friedman PA, Lohse MJ. Formation of a ternary complex among NHERF1, -arrestin1, and parathyroid hormone receptor. J. Biol. Chem. 2010: 285:30355-30362.
Feinstein TN, Wehbi VL, Ardura JA, Wheeler DA, Ferrandon S, Gardella TJ, Vilardaga JP*. Retromer terminates the generation of cAMP by internalized PTH-receptors. Nature Chemical Biology 2011: 7: 278-284 (*Corresponding author)
Chen S, Webber MJ, Vilardaga JP, Khatri A, Brown D, Ausiello DA, Bouley R. Visualizing microtubule-dependent vasopressin type 2 receptor trafficking using a new high affinity fluorescent vasopressin ligand. Endocrinology 2011152:3893-3904.
Ardura, JA, Wang B, Watkins SC, Vilardaga JP, Friedman PA. Dynamic NHERF1 association and dissociation regulate PTH receptor trafficking at membrane microdomains J. Biol. Chem 2011 286:35020-35029
Liu Z, Turan S, Wehbi VL, Vilardaga JP, Bastepe M. The extra-long G s variant XL s escapes activation-induced �subcellular redistribution and is able to provide sustained signaling. J. Biol. Chem 2011 286:38558-38569.
Nagao M, Feinstein TN, Ezura Y, Hayata T, Notomi T, Saita Y, Hanyu R, Hemmi H, Izu Y, Takeda S, Wang K, Rittling S, Nakamoto T, Kaneko K, Kurosawa H, Karsenty G, Denhardt DT, Vilardaga JP*, Noda M*. Sympathetic control of bone mass regulated by osteopontin. Proc. Natl. Acad. Sci. USA 2011 108:17767-17772. (*Corresponding author).
Hanyu R, Wehbi VL, Hayata T, Moriya S, Feinstein TN, Yoichi Ezura Y, Nagao M, Saita Y, Hemmi H, NotomiT, Nakamoto T, Schipani E, Takeda S, Kaneko K, Kurosawa H, Karsenty G, Kronenberg KM, Vilardaga JP*,Noda, M*. Anabolic action of PTH regulated by the 2-adrenergic receptor. Proc. Natl. Acad. Sci. USA 2012 109:7433-7438. (*Corresponding author).
Vilardaga JP*, Gardella, TJ, Wehbi VL, Feinstein TN. Non-canonical signaling of the PTH receptor. Trends Pharmacol. Sci. 2012, 33:423-431. (*corresponding author) Andreas Vogt, Ph.D. Research Assistant Professor
Lazo JS, Reese CE, Vogt A, Vollmer LL, Kitchens CA, Gunther E, Graham TH, Hopkins CD and Wipf P. Deconstructing resistance determinants for the antimitotic natural products disorazole C1 and A1. Journal of Pharmacology and Experimental Therapeutics 332:906-911, 2010.
Daniela Volonte, Ph.D. Research Assistant Professor
Huafei Zou, Daniela Volonte and Ferruccio Galbiati. 2012. Interaction of Caveolin-1 with KU70 inhibits Bax-mediated apoptosis. PloS ONE. 2012;7(6):e39379.
162
Nobunao Wakabayashi, Ph.D. Research Assistant Professor
Kensler TW and Wakabayashi N. Nrf2: friend or foe for chemoprevention? Carcinogenesis 31: 90-99, 2010.
Singh A, Bodas M, Wakabayashi N, Bunz F and Biswal S. Gain of Nrf2 function in non-small-cell lung cancer cells conferes radioresistance. Antioxid Redox Signal 13:1627-1637, 2010.
Malhotra D, Portales-Casamar E, Singh A, Srivastava s, Arenillas D, Happel C, Shyr C, Wakabayashi N, Kensler TW, Wasserman WW and Biswal S. Global mapping of binding sites for Nrf2 identifies novel targets in cell survival response through ChIP-Seq profiling and network analysis. Nucleic Acids Res 38:5718-5734, 2010.
The dynamin-related GTPase Opa1 is required for glucose-stimulated ATP production in Pancreatic beta cells. Zhang Z, Wakabayashi N, Wakabayashi J, Tamura Y, Song WJ, Sereda S, Clerc P, Polster BM, Aja SM, Pletnikov MV, Kensler TW, Shirihai OS, Iijima M, Hussain MA, Sesaki H. Mol Biol Cell. 22. 2235-2245, 2011.
Transcription factor Nrf2 maintains the basal expression of Mdm2: An implication of theregulation of p53 signaling by Nrf2.You A., Nam CW, Wakabayashi N., Yamamoto M., Kensler T.W., Kwak M.K. Arch Biochem Biophys. 507, 356-364, 2011.
Mitochondrial division ensures the survival of postmitotic neurons by supressing oxidative damege. Kageyama Y, Zhang Z, Roda R, Fukaya M, Wakabayashi J, Wakabayashi N, Kensler TW, Reddy PH, Iijima M, Sesaki H. J Cell Biol. 197, 535-551, 2012.
Bin Wang, Ph.D. Research Assistant Professor
Wang B, JA Ardura, G Romero, Y Yang, RA Hall and PA Friedman. Na/H exchanger regulatory factors control PTH receptor signaling by facilitating differential activation of G alpha protein subunits. J Biol Chem 285:26976-26986, 2010.
Wang B, CK Means, Y Yang, T Mamonova, A Bisello, DL Altschuler, JD Scott and PA Friedman. Ezrin-anchored PKA coordinates phosphorylation-dependent disassembly of a NHERF1 ternary complex to regulate hormone-sensitive phosphate transport. J Biol Chem 287:24148-24163, 2012.
Wang B, Y Yang, L Liu, HC Blair and PA Friedman. NHERF1 regulation of PTH-dependent bimodal Pi transport in osteoblasts. Bone, 2012, October 7 [Epub ahead of print].
Q. Jane Wang, Ph.D. Assistant Professor
LaValle, C. R., Bravo-Altamirano, K., Giridhar, K. V., Chen, J., Sharlow, E. R., Lazo, J. S., Wipf, P., and Wang, Q. J. Novel protein kinase D inhibitors cause potent arrest in prostate cancer cell growth and motility. BMC Chem Biol, 10: 5, 2010.
Bravo-Altamirano, K., George, K. M., Frantz, M.-C., LaValle, C. R., Tandon, M., Leimgruber, S., Sharlow, E., Lazo, J. S., Wang, Q. J., and *Wipf, P. Synthesis and Structure-Activity Relationships of Benzothienothiazepinone Inhibitors of Protein Kinase D. ACS Med Chem Lett, 2: 154-159, 2011.
163
George, K. M., Frantz, M.-C., Bravo-Altamirano, K., LaValle, C. R., Tandon, M., Leimgruber, S., Sharlow, E., Lazo, J. S., Wang, Q. J., and *Wipf, P. Design, Synthesis, and Biological Evaluation of PKD Inhibitors. Pharmaceutics, 3: 186-228, 2011.
Bernal, P., Bauer, E., Cao, R., Maniar, S., Mosher, M., Chen, J., Wang, Q. J., Glorioso, J. C., Pitt, B. R., Watkins, S., and *St. Croix, C. M. A role for zinc in regulating hypoxia-induced contractile events in pulmonary endothelium. Am J Physiol - Lung Cell Mol Physiol, 300: L874-86, 2011.
Sharlow, E. R., Mustata, G., Close, D., Leimgruber, S., Tandon, M., Reed, R., Shun, T. Y., Wang, Q. J., Wipf, P., and Lazo, J. S. Discovery of diverse small molecule chemotypes with cell-based PKD1 inhibitory activity. PLoS One, 6: e25134, 2011 [Epub ahead of print]
Chen, J., Giridhar, K. V., and Wang, Q. J. A protein kinase C/protein kinase D pathway protects LNCaP prostate cancer cells from phorbol ester-induced apoptosis by promoting ERK1/2 and NF-κB activities.Carcinogenesis, 32: 1198-1206, 2011.
LaValle, C. R., Zhang, L., Xu, S., Eiseman, J. L., and Wang, Q. J. Inducible silencing of protein kinase D3 inhibits secretion of tumor-promoting factors in prostate cancer. Mol Cancer Ther, 11: 1389-99, 2012.
Zou, Z., Zeng, F., Xu, W., Wang, C., Ke, Z., Wang, Q. J., *Deng, F. PKD2 and PKD3 Promote Prostate Cancer Cell Invasion via uPA by Shifting Balance Between NF-κB and HDAC1. J Cell Sci, 2012. [Epub ahead of print]
Guo, J., Clausen, D. M., Beumer, J. H., Parise, R. A., Egorin, M. J., Bravo-Altamirano K, Wipf, P., Sharlow, E. R., Wang, Q. J., and *Eiseman, J. L. In vitro cytotoxicity, pharmacokinetics, tissue distribution and metabolism of small-molecule protein kinase D inhibitors, kb-NB142-70 and kb-NB184-43, in mice bearing human cancer xenografts. Cancer Chemother Pharmacol, 2012. [Epub ahead of print]
Tandon, T., Wang, L., Xu, Q., Xie, X., Wipf, P., Wang, Q. J. A targeted library screen reveals a new inhibitor scaffold for protein kinase D. PLoS One, 2012, 7(9):e44653, Epub 2012 Sep 18.
Lin Zhang, Ph.D. Associate Professor
Dudgeon C., Wang, P., Sun, X., Peng, R., Sun, Q., Yu, J. and Zhang, L. (2010) PUMA induction by FoxO3a mediates the anticancer activities of the broad-range kinase inhibitor UCN-01. Molecular Cancer Therapeutics 9: 2893-2902.
Wang, P., Rajan K. Bista, R.K., Qiu, W., Khalbuss, W.E., Zhang, L., Brand, R.E., and Liu, Y. (2010) An insight into statistical refractive index properties of cell internal structure via lowcoherence statistical amplitude microscopy Optics Express 18: 21950-21958.
Zhang, G., Yanamala, N., Kira L. Lathrop, K. L., Zhang, L., Klein-Seetharaman, J., and Srinivas, H. (2010) Ligand-independent anti-apoptotic function of estrogen receptor beta in lung cancer cells. Molecular Endocrinology 24: 1737-1747.
164
Gurzov. E.N., Germano1, C.M., Cunha1, D.A., Ortis, F., Vanderwinden, J-M., Marchetti, P., Zhang, L., and Eizirik, D.L. (2010) Puma activation contributes to pancreatic beta cell apoptosis induced by pro-inflammatory cytokines and endoplasmic reticulum stress. Journal of Biological Chemistry 285: 19910-19920.
Yu, H., Shen, H., Yuan, Y., Xufeng, R., Hu, X., Zhang, L., Yu, J., Zambetti, G., and Cheng, T. (2010) Deletion of Puma protects hematopoietic stem cells and confers long-term survival in response to high-dose γ-irradiation. Blood 115: 3472-3480.
Qiu, W., Leibowitz, B., Zhang, L., and Yu, J. (2010) Growth factors protect intestinal stem cells from radiation-induced apoptosis by suppressing PUMA through the PI3K/AKT/p53 axis Oncogene 29: 1622–1632.
Gao, J., Senthil, M., Ren, B., Yan, J., Yu, J., Zhang, L., and Yim, J.H. (2010) IRF-1 transcriptionally up-regulates PUMA which mediates the mitochondrial apoptotic pathway in IRF-1 induced apoptosis in cancer cells. Cell Death & Differentiation 17: 699-709.
Wang, P., Bista, R.K., Khalbuss, W.E., Qiu, W., Uttam, S., Staton, K., Zhang, L., Brentnall, T.A., Brand, R.E., and Liu. Y. (2010) Nanoscale nuclear architecture for cancer diagnosis beyond pathology via spatial-domain low-coherence quantitative phase microscopy J. Biomed. Opt. 15: 066028.
Qiu, W., Wang, X., Leibowitz, B., Liu, H., Barker, N., Okada, H., Oue, N., Yasui W., Clevers, H., Schoen, R., Yu, J. and Zhang, L. (2010) Chemoprevention by nonsteroidal anti-inflammatory drugs eliminates oncogenic intestinal stem cells via SMAC-dependent apoptosis Proc. Natl. Acad. Sci. USA. 107: 20027-20032.
Qiu, W.*, Wang, X.*, Leibowitz, B., Yang, W., Zhang, L. and Yu, J. (2011) PUMA-mediated apoptosis drives chemical hepatocarcinogenesis in mice. Hepatology 54:1249-1258.
Xu, Y., Zhou, L., Huang, J., Liu, F., Yu, J., Zhan, Q., Zhang, L. * and Zhao, X. * (2011) Role of Smac in determining the chemotherapeutic response of esophageal squamous cell carcinoma. Clinical Cancer Research. 17: 5412-5422. *corresponding authors.
Dirisina, R., Katzman, R.B., Goretsky, T., Managlia, E., Mittal, N., Williams, D.B., Qiu, W., Yu, J., Chandel, N.S., Zhang, L. and Barrett, T.A. (2011) p53 and PUMA independently regulate intestinal epithelial cell apoptosis in colitis. Gastroentrology 141:1036-1045.
Qiu, W., Wu, B., Wang, X., Buchanan, M., Regueiro, M.D., Hartman, D., Schoen, R.E., Yu, J. and Zhang, L. (2011) PUMA-mediated intestinal epithelial apoptosis contributes to ulcerative colitis in humans and mice. Journal of Clinical Investigation 121:1722-1732. (Cover Story)
Li, H.*, Wang, P.*, Ding, W.X., Yin, X.M., Sun, Q., Stolz, D.B., Yu, J. and Zhang, L. (2011) Following cytochrome c release, autophagy is inhibited during chemotherapy-induced apoptosis by caspase-8-mediated cleavage of Beclin-1. Cancer Research 71: 3625-3634.
Leibowitz, B., Qiu, W., Liu, H., Cheng, T., Zhang, L., and Yu, J. (2011) Uncoupling p53 functions in radiation-induced intestinal damage via PUMA and p21. Molecular Cancer Research 9: 616-625.
Mustata, G., Li, M., Zevola, N., Bakan, A., Zhang, L., Epperly, M., Greenberger, J.S., Yu, J.* and Bahar, I.* (2011) Development of small-molecule PUMA inhibitors for mitigating radiation-induced cell death. Current Topics in Medicinal Chemistry 11: 281-290.
165
Sun, Q., Zheng, X., Zhang, L. and Yu, J. (2011) SMAC modulates chemosensitivity in head and neck cancer cells through the mitochondrial apoptotic pathway Clinical Cancer Research 17: 2361-2372.
Livesey, K.M., Kang, R., Vernon, P., Buchser, W., Loughran, P, Watkins, S.C., Zhang, L., Manfredi, J.J., Zeh, H.J., Li, L., Lotze, M.T., and Tang, D. (2012) p53/HMGB1 complexes regulate autophagy and apoptosis. Cancer Research 72: 1996-2005.
Qiu, W. Liu, H., Sebastini, A., Sun., Q., Wang, H., Zhang, L. and Yu, J. (2012) An apoptosis-independent role of SMAC in tumor suppression. Oncogene 2012 Jul 2. doi: 10.1038/onc.2012.265. [Epub ahead of print]. Bista, R.K., Uttam, S., Hartman, DJ., Qiu, W., Yu, J., Zhang, L., Brand, R.E. and Liu, Y. (2012) Investigation of nuclear nano-morphology marker as a biomarker for cancer risk assessment using a mouse model. J. Biomed. Opt. 17: 066014.
Dudgeon, C., Peng, R., Wang, P., Sebastiani, A., Jian Yu, J., and Zhang, L. (2012) Inhibiting oncogenic signaling by sorafenib activates PUMA via GSK3b and NF-κB to suppress tumor cell growth. Oncogene 2012 Jan 30 [Epub ahead of print].
167
Executive Summary
I. Mission and Goals The principal goal of the Department of Pharmacology and Chemical Biology is the creation of an intellectual and physical environment in which teaching and research in Pharmacology and Chemical Biology are pursued as one common enterprise. The major responsibilities of the Department are to: (1) educate medical students and physicians in the rationale for drug selection; (2) train contemporary pharmacologists; (3) develop new knowledge in the biomedical sciences; and (4) provide information about existing and emerging drugs to members of the University of Pittsburgh Medical Center, the University of Pittsburgh and the general community.
The philosophical approach of the Department is guided by the view that the field of Pharmacology exhibits a unique combination of characteristics that distinguish it from other basic medical sciences. Pharmacology encompasses a broad range of interests extending from the abstract domain of the physical chemistry of ligand-receptor interactions to the therapeutic use of drugs in patients. Thus, Pharmacology has stronger clinical ties than most other basic science disciplines. In the current revised medical curriculum, the faculty inculcates both core pharmacological principles and places them in the context of specific organ systems and bodily functions. Collectively, we provide a key educational experience to both medical and graduate students. Our faculty members also maintain vigorous research programs that are interactive and interdisciplinary. We have bridged with the Department of Chemistry in a unique and constructive manner through our activities in Drug Discovery.
The goals of the Department are:
To be one of the top five NIH funded Departments of Pharmacology in the USA. To define pharmacological research for the 21st century. To improve the presence of Structural Pharmacology and Drug Discovery at the University of
Pittsburgh. To educate premier future basic researchers, physician-scientists and teachers. To enhance the quality of graduate students matriculating and graduating from our PhD program at the
University of Pittsburgh. II. Departmental History and Status: Major growth in the Department of Pharmacology and Chemical Biology occurred within the last 6 years after a new Chair was recruited and new funds were directed to the Department. As evidenced by the tremendous growth in research support funds, faculty publications, numbers of postdoctoral fellows and the number of members on the Graduate Faculty, we have evolved to become one of the top departments in the country based on our extramural research support and our impact on postdoctoral training. Last year we were ranked eighth in the nation for NIH funding among the more than 100 medical school departments and this year we were ranked seventh. Our Department now views its primary peer programs to be the following institutions: Yale University, University of Michigan, University of North Carolina, University of Pennsylvania, University of Virginia, Emory University, University of Texas Southwestern, University of Washington, Washington University, Vanderbilt University and the Johns Hopkins University.
III. Strengths: In January 2006, Dr. Bruce A. Freeman was appointed Chair of the Department of Pharmacology and Chemical Biology to lead our strong cohort of well-funded and nationally recognized pharmacologists, cell and chemical biologists and geneticists. The department has about 50 primary faculty and 35 secondary faculty that contribute to the missions of the department. The members of the Department of Pharmacology and Chemical Biology are highly interactive with frequent co-authorship and co-funding. In spite of challenging times, our faculty
168
members are well-funded, with departmental primary faculty currently receiving $7.4 million in total direct annual costs and $10.3 million in total annual costs. During the past five years, the Department has emphasized cell signaling as an area of excellence. These interests address primary themes such as cancer, cardiovascular,renal and neurobiology. We are now extending this research focus to include two new areas of excellence: drug discovery and structural pharmacology. To supplement our training in these areas, the Department of Pharmacology has an NIH Predoctoral Training Grant in Pharmacological Sciences. The faculty also is well recognized for both their Medical School and Graduate School teaching. The prominence of our faculty members is recognized by their important leadership positions with Centers and Institutes, such as the UPCI, the PINDS, the Drug Discovery Institute, the School of Medicine and the University. IV. Initiatives: The Department will initiate a search for two or three new faculty members. The Department also intends to continue to replace aging equipment, renovate laboratory space and in this context, relocate faculty within thematic areas. We will also continue developing a strong interdisciplinary Drug Discovery Program, The Department will continue to partner with the emerging programs in Computational and Structural Biology as we emphasize Structural Pharmacology in faculty recruiting processes.
SWOT Analysis
Strengths Since John S. Lazo assumed the Chair, the Department of Pharmacology and Chemical Biology has grown from three tenure-stream faculty to 42 faculty of which 22 are either tenured or in the tenure stream. This growth reflects department-initiated recruitment as well as “opportunistic” recruitments in collaboration with the UPCI that have benefited both the UPCI and the University. Thus, six of our current tenure stream faculty members are physically located within the UPCI as well as 9 nontenure stream faculty. One tenure stream faculty member is physically located in the Center for Clinical Pharmacology. Virtually all faculty are well-funded, with the Department currently receiving more than $6.9 million in total direct annual costs, approximately $2.0 million of which is co-credited to the UPCI or the Center for Clinical Pharmacology because the faculty members have appointments and space there. Our research success reflects strong independent investigator-initiated research support, a key factor for the development of future thematic research projects. We have already begun to define areas for interactive intra-institution research teams. During the past five years, the Department has emphasized cellular signaling and communication as an area of excellence. These interests are spread over three existing disease/organ areas: cancer, cardiovascular/renal and neurobiology. We are now intending to complement this research focus on cellular signaling with two new areas of excellence: drug discovery and structural pharmacology.
The members of the Department of Pharmacology and Chemical Biology have extensive interactions with other Basic Science Programs. In particular, strong collaborative relationships exist with School of Medicine faculty studying cellular communication and signaling, including faculty from the Departments of Cell Biology and Physiology, Molecular Microbiology and Genetics, Pathology, Neurobiology, and Immunology. Topics of interest range from protein phosphorylation and dephosphorylation, cell cycle checkpoints, G proteins, receptor biology, cell death, pain, combinatorial chemistry, neurotransmitters, channels and redox signalling. Forceful relationships with clinical elements of the Medical Center also exist. These include strong collaborative projects with the Departments of Medicine, Surgery, Anesthesiology, Critical Care Medicine, Pediatrics, Neurology, Urology, Psychiatry and Pathology. The laboratories of members of the Department of Pharmacology house advanced fellows from several clinical units: Pulmonary Medicine, Medical Oncology, Surgery, Anesthesiology and Critical Care Medicine. Interactions also exist with the key Centers and Institutes within the Medical Center and Main Campus including the CNUP, UPCI and the Vascular Medicine Institute. These activities reflect the strong commitment of the Department of Pharmacology to engage in translational research and to provide a
169
forum for integrative sciences. The Department considers its role in bridging the basic and clinical sciences of UPMC as a core element of its missions related to fundamental investigation and drug discovery.
Active programs reaching out to the Main Campus have also been instituted. Consequently, there are major collaborations between members of the Department of Pharmacology and the Department of Chemistry. Members of the Department also interact with investigators in the Departments of Environmental and Occupational Health, Biological Sciences and Neuroscience, as well as investigators at Carnegie Mellon University, particularly from the National Science Foundation Center for Fluorescence. Because of this multidisciplinary research activity, the Department took a leadership role in the submission of the multimillion dollar Pittsburgh Molecular Target Laboratory application, which is making Pittsburgh an epicenter for academic drug discovery.
The Department of Pharmacology and Chemical Biology was honored that it was selected to receive an NIH Predoctoral Training Grant in Pharmacological Sciences. This was the only new graduate Training Grant for Pharmaceutical Sciences to be awarded by the NIH in 1994. Moreover, our program was one of only a few recently initiated grants to be renewed for a second cycle. The acquisition of this training grant, which supports four students, was a primary goal of the Department for several years and we are proud to have obtained it.
In addition to their splendid research record, the faculty has displayed outstanding teaching records, both in the Medical School and Graduate School courses. We believe this is due primarily to placing special emphasis on quality teaching and limiting the student interactions of those teachers rated less effective by the students. Our faculty members have also assumed important leadership positions with Centers and Institutes, such as the UPCI, the School of Medicine and the University. To summarize, we have created:
� Strong research activities and NIH grant support � Interactive faculty � Interdisciplinary program with the Department of Chemistry � NCI funded Program Project on Drug Discovery � Funded NIH Predoctoral Training Grant in Pharmacological Sciences � NCI funded Specialized Program of Research Excellence in Lung Cancer � Focus on Cell Communication, Drug Discovery and Structural Pharmacology � Outstanding teachers of medical and graduate students, e.g. Professor de Groat, who is a five-time winner of
the School of Medicine “Golden Apple Award”.
Weaknesses During the past five years tenured and non-tenured faculty left the Department. We expect that one of our prized lecturers, Professor de Groat, will select retirement within the next three years. Thus, the Department must continue to recruit new faculty to ensure quality teaching to medical school students and retain the critical mass required to be among the top five programs nationally. The curriculum for medical students is routinely being reviewed so that we can develop more blueprints for teaching pharmacology to medical students. We are encouraged by the medical students who realized the importance of strong pharmacological training not only for scoring highly on board examinations but also for treating patients. A basic introductory lecture series on classical pharmacology is currently deemed essential to the current organ system-based training of medical students.
The Department was criticized in the most recent review of its Training Grant that it had too few junior faculty members. To allow the Department to function effectively and to achieve critical mass, additional faculty will be needed.
170
Currently there are only two program project-type research grants (PO1, P50) within the Department of Pharmacology and Chemical Biology; the national emphasis on specific disease areas lends itself to programmatic efforts and the Department should exploit this. The increased awareness of the productive aspects of linking contemporary chemistry with modern biology also should be an area in which Pharmacology plays a key role. Indeed, we organized a response to an NIH Request for Proposals on Molecular Targets Laboratory, because of the close research links between these two programs. The Department has now focused on Cellular Signaling and Communication as a major theme. We also believe our interest in Drug Discovery and Structural Pharmacology is both timely and institutionally appropriate. In contrast to the Cellular Signaling and Communication, we have not yet reached critical mass in Drug Discovery and Structural Pharmacology. Weplan to fortify these areas by recruiting new faculty members in a manner that would complement the academic mission of the University.
There are limited amounts of pharmaceutical research dollars awarded to the Department of Pharmacology and Chemical Biology. The Department has not placed enough emphasis on obtaining funding from pharmaceutical organizations but rather has paid more attention to Federal dollars. We are now placing more focus on the commercial sector to support research, but intellectual property issues and data sharing are still hurdles.
There continues to be a need for capital investment within the Department of Pharmacology and Chemical Biology to replace aging equipment and to advance our depth in new technological capabilities. The rapid advances in new technologies mandate that new investments be made for our faculty and trainees to maintain our national competitive standing. In summary, we need to:
� Maintain critical mass of faculty � Grow and better integrate space and facilities for research � Limited financial research support from pharmaceutical and biotechnology firms and budget cuts at the
National Institutes of Health
Opportunities During the past four years there has been a remarkable and unprecedented growth in drug discovery and development in the US. Both large pharmaceutical firms and biotechnology companies have invested heavily to exploit this new knowledge. Consequently, major new therapeutic advances directed against important disease groups are now emerging. The completion of the Human Genome Project has increased the number of potential therapeutic targets by more than one order of magnitude. The future challenge will be to identify the drugs that will interact with these emerging biochemical and molecular targets. Because of the unique attributes at the University of Pittsburgh that place Chemistry physically close to Biology, we posit:
� There is a unique opportunity for a few academic institutions to participate and profit from this changing paradigm in drug discovery. The Bayh-Dole Act now allows resourceful Universities the opportunity to replace the income lost from managed care with income derived from its intellectual property. A strong Department of Pharmacology is a vital component of such an activity.
� There will be a significantly increased industrial and academic need for well-trained graduates from Ph.D. granting programs with a concentration in Pharmacological Sciences and Drug Discovery. A strong Department of Pharmacology and Chemical Biology is a vital component of such an activity.
� Important new knowledge and reagents continue to emerge that are relevant to many biological systems and all aspects of cell communication. A strong Department of Pharmacology and Chemical Biology is a vital component of such an activity.
� Advances in Structural Biology and Bioinformatics should make it possible in the near future to optimize small molecules that are more selective and potent towards their molecular targets. The area of Structural Pharmacology will be grown to be a vital component of the Department of Pharmacology.
� Ph.D., D.M.D. and M.D. students will need to become even more cognizant and thoughtful about the highly
171
selective therapies of the future that may be used based on the genetic profile of each patient. A strong Department of Pharmacology is a vital component of such an activity.
The University of Pittsburgh is uniquely situated to participate in defining future research and graduate education in Pharmacology and in recruiting to its campus some of the best students. The University of Pittsburgh’s advantages are:
� A cohort of dedicated faculty members, who are eager to teach graduate students � The presence of strong existing programs in neuroscience, virology, tumor immunology, cancer biology,
developmental biology, structural biology and computational biology � The presence of strong clinical programs � A growing drug discovery enterprise
Barriers One of the greatest threats to the Department of Pharmacology and Chemical Biology would be to lose its vigor and enthusiasm during the current downward trend in NIH funding. Currently the program is nationally identified as a model of growth. This has helped in the recruitment of new faculty. Nonetheless, other institutions have become eager to develop programs in drug discovery and to enhance their pharmacology departments. We believe it is likely that they will seek to recruit our valuable faculty.
Initiative and Implementation Strategies
� To achieve the overall goal of becoming one of the top three Departments of Pharmacology in the next three years, the Department will recruit new faculty members during the next few years. The Department of Pharmacology and Chemical Biology intends to focus on the three defined areas of research interest previously identified: Cellular Communication, Structural Pharmacology and Drug Discovery. In particular, the Department will continue to partner with the new Drug Discovery Institute, providing unique instrumentation, archived chemical libraries and specialized research services and teaching for members of the University and UPMC.
172
Financial Statement
University of Pittsburgh School of Medicine Statement of Revenues and Expenses – June 30, 2012
Hard Money
Self Supporting
Discretionary and
Restricted Research Total RevenueSchool of Medicine - ECU 441,770 441,770Indirect Cost Recovery 2,416,083 2,414,083Direct Grants 6,674,327 6,674,327Endowment Income 77,137 77.137Other Revenue - 20,648 9,087 29,735
Total Revenue 2,857,853 20,648 86,224 6,674,327 9,639,052
ExpenseMedical Faculty Salary 1,902,799 - 144,102 2,061,842 4,108,743Other Faculty Salary - 139,026 714,569 853,595Staff Salary 810,114 5,908 202,970 908,136 1,927,129Medical Faculty Fringes 471,785 - 38,764 487,147 997,696Other Faculty Fringes - - 47,130 228,050 275,179Staff Fringes 246,476 101 53,472 198,246 498,295Subtotal Compensation 3,431,176 6,009 625,464 4,597,990 8,660,637
Other Expense 404,951 32,191 981,837 2,076,337 3,403,721Transfers (intra-department) 53,384 - -151,302 - -105,392Transfers (inter-department) - - - - -Subtotal Other Operating 458,335 38,200 830,535 2,076,337 3,298,329
Stepdown 2,835,269 7,755 - - 2,656,106
Total Expense 6,724,779 45,955 1,455,999 6,674,327 14,690,083
(3,866,926) (25,307) (1,369,775) 0 (5,262,008)
June 30, 2010 Fund Balance 6,518,738Restricted Net Activity Year to Date -982,870Prior Year Settlement Transfer 8,906Current Month end Restricted Fund Balance 5,544,774SOM Quasi Endowment Market value – March 2011 1,834,430Total Available Balances 7,379,204

















































































































































































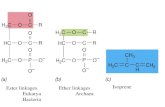





![[Gordon R. Freeman and Phyllis J. Freeman] Stonehenge Archeology](https://static.fdocuments.us/doc/165x107/5571f81e49795991698cacea/gordon-r-freeman-and-phyllis-j-freeman-stonehenge-archeology.jpg)







